ABSTRACT
The success of checkpoint immunotherapy has created optimism that cancer may be curable. However, not all patients respond, resistance is common and many patients relapse owing to immune escape. We demonstrate that HDAC inhibition not only decreases the trafficking of myeloid-derived suppressor cells (MDSCs) into tumors but also potentiates tumor-associated macrophages (TAMs) to specify anti-tumoral phenotype and bolster T cells activation within the tumor microenvironment (TME).
Novel therapy that promotes anti-tumor immunity shifted the paradigm of cancer treatment. Most of these immunomodulatory strategies have focused on manipulating the adaptive immune system, either by blocking inhibitory pathways with checkpoint blockades, or by targeting activating pathways, as with chimeric antigen receptor T cells or bispecific antibodies.Citation1–3 However, despite these therapies have led to unprecedented successes, immunotherapies for solid tumors, in general, benefit only a subset of patients, as intrinsic or acquired tumor immune tolerance remains a major hurdle.Citation1,Citation4 Given the crucial role of innate immune responses in immunity, harnessing these responses opens up new possibilities for multilayered tumor control.Citation5
Cancers continuously evolve, in part through dynamic and reversible epigenetic changes that confer a fitness advantage. This epigenetic plasticity, a termed used hereinafter in reference to reversible changes in epigenetic marker on DNA, histone and non-histone proteins, as well as the functional consequences of these alterations, is also involved in primary and acquired resistance to various anticancer therapies, through modulation of tumors cell or their microenvironment.Citation6 Epi-drugs might therefore be most effective when used in combination with other treatments and present particularly attractive strategies for sensitizing tumors to a giving therapy and for overcoming acquired resistance mechanisms in a dynamic fashion. Antitumor immunity involves the complex interplay among immune, cancer and stromal cells. Specific DNA-modifying or histone-modifying enzymes and histone chaperones, respectively, contribute to both the immunogenicity of cancer cells and the lineage commitment and/or maturation of immune cells,Citation7 therefore, epi-drugs can potentially be used to modulate antitumor immunity.Citation8
Lysine acetylation is one of the best-studied histone modifications, and it reduces occupancy of DNA, thereby creating a transcriptionally permissive chromatin structure. Lysine acetylation is controlled by the opposing actions of two enzyme families, histone acetyltransferases and histone deacetylases (HDACs). HDAC inhibitor (HDACi) blocks the action of HDACs, which remove acetyl marks from tagged histones to increase global histone acetylation. The rational of employing epigenetic drugs is supported by finding that the increased presence of histone acetylation (e.g., H3K9 or H3K27 acetylation) and active chromatin state are tightly correlated with the function of CD8+ T cells.Citation7 The tumor-infiltrating lymphocytes (TILs) in immunosuppressive TME acquire a dysfunctional chromatin state in advanced tumor stages, which may limit the efficacy of immunotherapies. We had a particular interest for the HDAC inhibitor since its role was reported in the modulation of activation-induced cell death (AICD) of T cells by our group.Citation9 Notably, HDACs drive innate immune-cell mediated inflammation. Broad-spectrum inhibitors of classical HDACs can modulate the TLR-inducible production of different inflammatory mediators from innate immune cells.Citation10 Despite the importance of macrophages in cancer, epigenetic modifications in macrophages remain on the verge of being unraveled. Whether epigenetic modifications to innate cells, particularly macrophages, could modulate the immune responses to cancers in vivo remains to be defined.
Though our work published in Oncogene,Citation11 we report a previously undefined role of HDACi in inducing anti-cancer immunity in syngeneic mouse models (). This effect is mediated through steering TAMs toward an anti-tumor phenotype and blocking the recruitment of MDSCs within tumors, which ultimately elicits a robust T cell-mediated anti-tumoral immune responses
Figure 1. HDAC inhibition converts TAMs into pro-inflammatory macrophages that promote T cell responses to suppresses tumor growth. Low-dose TSA can inhibit the trafficking of MDSC into tumors. HDAC inhibition can also synergize with checkpoint-targeted therapy (i.e., PD-L1 antibody) to promote anti-tumor immune responses that induce tumor regression in syngeneic mouse models of cancer
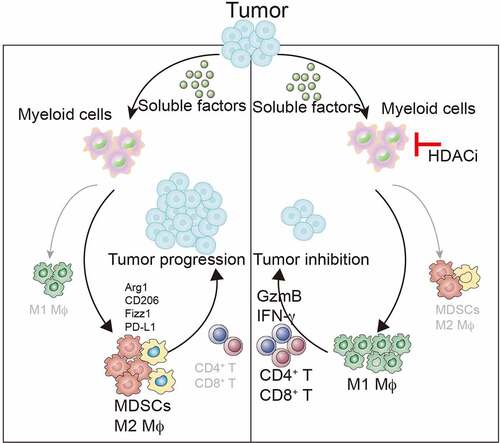
The take-home message from our work is that we reveal an immunostimulatory effect of HDAC inhibition that contrasts with those by strategies of depleting or inhibiting TAMs for cancer therapy, providing a strong rationale for combination therapy in clinical trials.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Li X, Shao C, Shi Y, Han W. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J Hematol Oncol. 2018;11:31.
- Wang Y, Chen M, Wu Z, Tong C, Dai H, Guo Y, Liu Y, Huang J, Lv H, Luo C, et al. CD133-directed CAR T cells for advanced metastasis malignancies: a phase I trial. Oncoimmunology. 2018;7(7):e1440169. doi:10.1080/2162402X.2018.1440169.
- Dai H, Zhang W, Li X, Han Q, Guo Y, Zhang Y, Wang Y, Wang C, Shi F, Zhang Y, et al. Tolerance and efficacy of autologous or donor-derived T cells expressing CD19 chimeric antigen receptors in adult B-ALL with extramedullary leukemia. Oncoimmunology. 2015;4(11):e1027469. doi:10.1080/2162402X.2015.1027469.
- Li X, Song W, Shao C, Shi Y, Han W. Emerging predictors of the response to the blockade of immune checkpoints in cancer therapy. Cell Mol Immunol. 2019;16:28–2. doi:10.1038/s41423-018-0086-z.
- Demaria O, Cornen S, Daeron M, Morel Y, Medzhitov R, Vivier E. Harnessing innate immunity in cancer therapy. Nature. 2019;574:45–56. doi:10.1038/s41586-019-1593-5.
- Easwaran H, Tsai HC, Baylin SB. Cancer epigenetics: tumor heterogeneity, plasticity of stem-like states, and drug resistance. Mol Cell. 2014;54:716–727. doi:10.1016/j.molcel.2014.05.015.
- Pace L, Goudot C, Zueva E, Gueguen P, Burgdorf N, Waterfall JJ, Quivy J-P, Almouzni G, Amigorena S. The epigenetic control of stemness in CD8+T cell fate commitment. Science. 2018;359:177–186. doi:10.1126/science.aah6499.
- Aspeslagh S, Morel D, Soria JC, Postel-Vinay S. Epigenetic modifiers as new immunomodulatory therapies in solid tumours. Ann Oncol. 2018;29:812–824. doi:10.1093/annonc/mdy050.
- Cao K, Wang G, Li W, Zhang L, Wang R, Huang Y, Du L, Jiang J, Wu C, He X, et al. Histone deacetylase inhibitors prevent activation-induced cell death and promote anti-tumor immunity. Oncogene. 2015;34(49):5960–5970. doi:10.1038/onc.2015.46.
- Brogdon JL, Xu Y, Szabo SJ, An S, Buxton F, Cohen D, Huang Q. Histone deacetylase activities are required for innate immune cell control of Th1 but not Th2 effector cell function. Blood. 2007;109:1123–1130. doi:10.1182/blood-2006-04-019711.
- Li X, Su X, Liu R, Pan Y, Fang J, Cao L, Feng C, Shang Q, Chen Y, Shao C, et al. HDAC inhibition potentiates anti-tumor activity of macrophages and enhances anti-PD-L1-mediated tumor suppression. Oncogene. 2021;40(10):1836–1850. doi:10.1038/s41388-020-01636-x.
