ABSTRACT
Background
Pegylated arginine deiminase (ADI-PEG 20) is a metabolism-based strategy that depletes arginine, resulting in tumoral stress and cytotoxicity. Preclinically, ADI-PEG 20 modulates T-cell activity and enhances the therapeutic efficacy of programmed death-1 (PD-1) inhibition.
Methods
A phase 1b study, including a dose-escalation cohort and an expansion cohort, was undertaken to explore the effects of ADI-PEG 20 in combination with pembrolizumab, an anti-PD-1 antibody, for safety, pharmacodynamics, and response. CD3 levels and programmed death-ligand 1 (PD-L1) expression were assessed in paired biopsies collected prior to and after ADI-PEG 20 treatment but before pembrolizumab.
Results
Twenty-five patients, nine in the dose-escalation cohort and sixteen in the expansion cohort, were recruited. Treatment was feasible with adverse events consistent with those known for each agent, except for Grade 3/4 neutropenia which was higher than expected, occurring in 10/25 (40%) patients. Mean arginine levels were suppressed for 1–3 weeks, but increased gradually. CD3+ T cells increased in 10/12 (83.3%) subjects following ADI-PEG 20 treatment, including in three partial responders (p = .02). PD-L1 expression was low and increased in 3/10 (30%) of subjects. Partial responses occurred in 6/25 (24%) heavily pretreated patients, in both argininosuccinate synthetase 1 proficient and deficient subjects.
Conclusions
The immunometabolic combination was safe with the caveat that the incidence of neutropenia might be increased compared with either agent alone. ADI-PEG 20 treatment increased T cell infiltration in the low PD-L1 tumor microenvironment. The recommended phase 2 doses are 36 mg/m2 weekly for ADI-PEG 20 and 200 mg every 3 weeks for pembrolizumab.
Introduction
Arginine deprivation therapy with the amino acid degrading enzyme, pegylated arginine deiminase (ADI-PEG 20), has shown clinical efficacy in a variety of cancers.Citation1–6 Moreover, ADI-PEG 20 potentiated chemotherapy with superior response rates and a side-effect profile mostly consistent with that expected for the chemotherapy alone in pancreatic cancer, non-small cell lung cancer, malignant pleural mesothelioma, and hepatocellular carcinoma.Citation7–9 These promising response rates and good safety profiles of ADI-PEG 20-containing chemotherapy have led to ongoing registration studies in multiple cancers.
Although ADI-PEG 20 has been combined with cytotoxic agents in the clinic, its role has remained unexplored with immunotherapy. Immune checkpoint therapy (ICT) has been approved for a variety of indications, and has revolutionized cancer treatment in recent years.Citation10 Specifically, programmed death-1 (PD-1) inhibition is most effective in some tumor types enriched for programmed death-ligand 1 (PD-L1) expression.Citation11 However, many tumors are low or deficient in tumor immunity, and thus, remain insensitive to ICT. A common strategy is to co-treat with an agent that would modulate tumor immunity, thereby increasing susceptibility to the PD-1/PD-L1 blockade.
Specifically, arginine and citrulline, the degradation product of ADI-PEG 20, are important regulators of immune responses.Citation12 In terms of a tumor suppressive role, abundant extracellular arginine may promote regulatory T cell activity while effector T cell function is inhibited by tumoral arginase-2.Citation13 Moreover, laboratory studies with ADI-PEG 20 reveal complex immunomodulatory effects on T cells: decreasing regulatory T cells and inhibiting CTLA-4 and PD-1 expression on effector T cells.Citation14 Furthermore, ADI-PEG 20 induced PD-L1 expression on cancer cells in vitro, a proinflammatory tumor immune microenvironment in vivo, and the combination with an anti-PD-1/PD-L1 antibody resulted in tumor shrinkage in arginine-auxotrophic murine tumor models compared to either ADI-PEG 20 or the antibody alone.Citation14–16 Though the regulatory mechanism remains unclear, the fact that tumoral PD-L1 is modulated in a STAT3-dependent manner by tetrahydrobiopterin (BH4), the nitric oxide synthase cofactor that converts arginine to nitric oxide, emphasizes the close relationship between arginine metabolism and immunity.Citation17 These data supported the testing of ADI-PEG 20, with its known immunomodulatory activity, in combination with ICT.
Here, we report on the safety and preliminary efficacy of the first clinical trial of ADI-PEG 20 combined with pembrolizumab, and the effects on tumor immunity.
Methods
Objectives
From prior experience, ADI-PEG 20 exhibited acceptable safety profiles when administered at the recommended dose of 36 mg/m2 in combination with specific chemotherapy regimens.Citation7–9 The safety of ADI-PEG 20, however, has not been documented with ICTs. Thus, we conducted an open-label, single-center, phase 1b trial of ADI-PEG 20 plus pembrolizumab with the primary objective to assess safety and tolerability of the combination (ClinicalTrials.gov: NCT03254732). The secondary objectives were to assess the maximum tolerated dose (MTD) for a recommended phase 2 dose, progression-free survival (PFS), overall survival (OS), overall response rate (ORR) by RECIST 1.1 and by Immune-Related Response Criteria (irRC),Citation18 and the effect of ADI-PEG 20 on the expression of PD-L1 and T-cell infiltration.
Subjects
All subjects were recruited at National Cheng Kung University Hospital. Eligible subjects were over 18 years of age, with a confirmed advanced solid tumor that failed prior systemic therapy, measurable disease, archival tumor tissue, ECOG performance status of 0 or 1, adequate hematologic, hepatic, and renal function and an expected survival of at least 3 months. No anti-cancer therapy could be received in the 2 weeks prior to study entry. Additionally, patients with uncontrolled intercurrent illness, expected noncompliance, history of another primary cancer unless unlikely to affect outcome, prior ADI-PEG 20 therapy, prior immunotherapy, and known allergy to ADI-PEG 20 were ineligible.
Study design
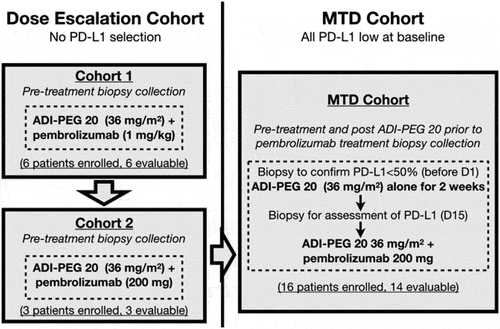
In the dose-escalation cohort, a “3 + 3 + 3” design was applied. The starting dose in cohort 1 was ADI-PEG 20 at 36 mg/m2 weekly, which represented 100% of the typical dose, and 1 mg/kg pembrolizumab every three weeks, which was 50% of the USA FDA approval dose for melanoma.Citation19 The safety of ADI-PEG 20 at this dose has been demonstrated either as monotherapy or in combination with chemotherapy.Citation1–9 The first subject received two drugs on the same day with a week of safety follow up before the next 2 subjects were to be enrolled. Dose-limiting toxicities (DLTs) were assessed during the first cycle. Without any DLT occurring, the enrollment would escalate into cohort 2, with pembrolizumab increased to 200 mg. In all cohorts, if 1/3 had reported a DLT, additional enrollment of 3 patients was required for up to two times. If the DLT rate occurred in 2/3, 3/6, 3/9, or more, the cohort would meet the maximum administered dose and require de-escalation for MTD search.
After dose determination, up to 20 subjects could be enrolled into an expansion MTD cohort with the additional criteria of pre-treatment tissue confirming low PD-L1, defined as ≤49% expression by immunohistochemical (IHC) staining (Dako 22C3 pharmDx). The subjects first received ADI-PEG 20 monotherapy on days 1, 8 and 15. The subjects underwent re-biopsy 24–28 h after the 3rd dose of ADI-PEG 20, followed by administration of pembrolizumab. Subjects then continued on the scheduled regimen. Baseline tomography images for tumor assessment were conducted, and then every 9 weeks while on treatment. Subjects received the combination regimen for up to 24 weeks if the tumor showed stable disease (SD) or better, then ADI-PEG 20 monotherapy after 24 weeks. RECIST 1.1 was used for determining progressive disease (PD) and study drug discontinuation. Subjects with PD might remain on study upon investigators’ decision and if PD was not confirmed in 4 to 6 weeks with repeat imaging.
Safety
All subjects were evaluated for safety by routine physical examination, and adverse event (AE) assessment by the NCI-CTCAE version 4.03. Complete blood counts and serum chemistry were obtained at screening and every third week. Endocrine data related to immune-related AEs were obtained at baseline and at weeks 11 and 23. Monitoring continued for 30 days after the treatment and until AE stabilization or resolution.
DLTs were defined as severe AEs that could be attributed to the study treatment during the first 21 days. These events included prolonged grade 4 neutropenia, febrile neutropenia with temperature up to 38.5°C or higher, grade 3–4 anemia or grade 4 thrombocytopenia that required transfusion therapy, grade 3–4 nonhematologic toxicity with the exception of alopecia, controllable gastrointestinal symptoms, rash, and fatigue, or any other asymptomatic laboratory evaluation unless clinically significant. In addition, grade 2 immune-related toxicity and any resultant DLT would be monitored. If a subject did not receive the qualifying doses for assessment due to reasons other than toxicity, they would be non-evaluable and replaced.
Pharmacodynamic and immunogenicity evaluations
Blood samples were taken before each dose of ADI-PEG 20 for weeks 1–3, and then every other week from weeks 5–23 to analyze arginine and citrulline levels and for immunogenicity analyses. An arginine level below 10 μM post ADI-PEG 20 dosing was defined as arginine suppression.Citation9
Immunohistochemical staining
Argininosuccinate synthetase 1 (ASS1) staining and reporting followed the previous protocol.Citation5,Citation20 Immune phenotyping was performed for CD3 (Cat#A0452, Dako/Agilent, Santa Clara, CA, USA) and PD-L1 (Cat#13684S, Cell Signaling, Danvers, MA, USA), staining were conducted as described previously.Citation21
Statistical consideration
Categorical variables were summarized with frequency and percentage. The survival data were summarized with median and 95% confidence interval (CI). Continuous variables were summarized with mean and median. Two-tailed paired t tests were used for data analysis with a significance level of 0.05, and were calculated by Prism 7 (GraphPad Software, San Diego, CA, USA).
Results
Patient enrollment and drug administration
Patient enrollment was between July 2017 and August 2018, and the data cut off was 11 July 2019. The subjects were heavily pretreated for various malignancies with a median of 3 prior lines of chemotherapy (ranging from 1 to 6; ). Nine subjects were enrolled in the dose-escalation portion, including six in cohort 1 and three in cohort 2, and all subjects were evaluable. There was one subject only in cohort 1 reported for DLT. From cohort 2, we defined the MTD to be 36 mg/m2 ADI-PEG 20 and 200 mg of pembrolizumab, and enrolled an additional sixteen patients in an expanded MTD cohort (). The mean/median number of treatments was 15/12 and 5/4, respectively, for ADI-PEG 20 and pembrolizumab.
Table 1. Patient characteristics
Toxicities
Of the non-hematological AEs reported, most were mild (). Fatigue occurred in 12/25 (48%) subjects, and was all grades 1–2. Pyrexia occurred in 7/25 (28%) subjects, with two reported as grade 3. Other AEs, such as arthralgia and pruritus, were less frequent. For grades 3–4 AEs, 14 episodes of neutropenia occurred in 10/25 (40%) subjects (). Among them, eight received colony stimulating factors, but only one required longer support up to 5 days. Three subjects had febrile neutropenia requiring a week of antibiotics and colony stimulating factor support. Six subjects required drug interruption according to the treatment schedule. Notably, pembrolizumab was discontinued in subject D004 due to concurrent development of grade 3 hepatitis, which was regarded a DLT that required corticosteroid management.
Table 2. Summary of adverse events
Table 3. List of all neutropenia events
Responses
Regarding the ORR, 6 of 25 (24%) enrolled and 6 of 23 (26.1%) per protocol evaluable subjects had a partial response (PR): 2 in the dose-escalation cohort 1; and 4 in the MTD cohort (). By irRC, the same 6 subjects had a PR ( & Supplementary Table S1). SD was observed as the best response of tumor measurement in 7/25 (28%) enrolled and 7/23 (30.4%) per protocol evaluable subjects. Together, the overall disease control rate was 13/25 (52%) enrolled and 13/23 (56.5%) per protocol evaluable subjects. Notably, two subjects had initial PD followed by SD (), which was sustained until the sixth month before PD in one subject (B008), while the other subject withdrew in the fourth month due to a new bone metastasis (B022).
Table 4. Summary of responses and survival
Figure 2. The swimmer plot shows the treatment response (RECIST 1.1) for subjects, noted with immune-related response criteria (irRC) listed aside. the plot is sorted by cohort and time on treatment. Note the subject B029 was a censored event as he was lost to follow up
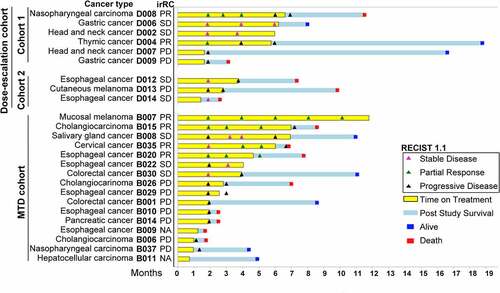
Figure 3. The spider plot shows measurement of target lesion of each subjects over time. all the measurements followed RECIST 1.1 criteria
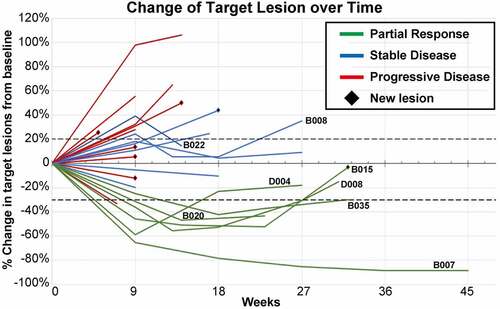
Thirteen subjects were reported for mortality during the post-treatment follow up. The median OS was 8.5 months (95% CI, 6.6~ not reached) for all subjects with 10/23 censored, and 7.8 months (95% CI, 2.5~ not reached) for the MTD cohort with 6/14 censored. The median PFS was 1.9 months (95% CI, 1.9 ~ 6) by RECIST and 3.9 months (95% CI, 1.9 ~ 6.7) by irRC for all subjects, and 2.9 (95% CI, 1.9 ~ 7.2) by irRC for the MTD cohort.
Pharmacodynamics and immunogenicity
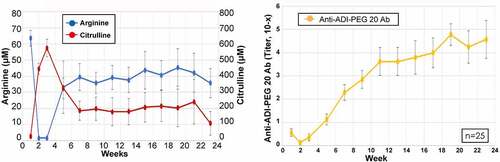
Mean arginine levels were undetectable for weeks 1–3, and remained suppressed to ~50% for the 6 months of testing (). In contrast, citrulline levels peaked early on, and did not return to baseline levels. There was a gradual increase in levels of anti-ADI-PEG 20 antibodies, reaching a plateau by week 17. For 5 of 6 subjects with a PR (D008, B007, B015, B020, B035), the duration of arginine suppression was only 3–4 weeks, with a concomitant rise in antibody levels (Supplementary Figure S1). Nevertheless, the responses persisted, ranging from 6 to 37 weeks (). In the sixth subject with PR (D004), arginine depletion was prolonged for 3–4 months and the response persisted for 10 weeks. Two other subjects with SD, B008 and D012, also exhibited prolonged arginine depletion (data not shown).
Figure 4. Pharmacodynamics and immunogenicity related to experimental drugs was measured. the level of arginine/citrulline level (left panel) and the anti-ADI-PEG 20 antibody titer (right panel) by time is summarized in dot-line plots. each dot represents mean levels of 25 patients with 25% and 75% percentiles (±SEM)
The median ASS1 staining at the baseline was 65% positive and the mean was 54%. PRs were observed in subjects with varying degrees of ASS1 expression (>95, 0, <5, 70, 40 and 40%), with no clear correlation. This was similarly noted in SD subjects (Supplementary Table S1). In the 14 available matched samples, ASS1 expression was variably altered after ADI-PEG 20 injection (P > .05, ).
Figure 5. The paired immunohistochemistry results in tissue at screening and at week 3 biopsy are quantified in the dot-line plots (left panels). the subjects with response were labeled in red color. note that in CD3, there are four (33.3%) increase by >10%. representative CD3 and PD-L1 IHC images are from subject B015, a partial responder (right panels)
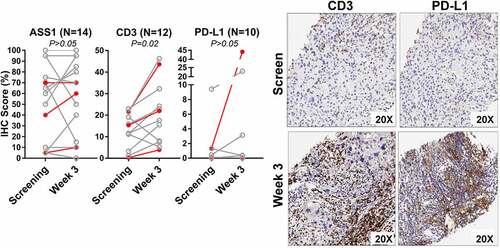
Increased CD3+ cells infiltration (paired tumor biopsies)
Twelve-paired samples were available to evaluate CD3+ T cells. The percentage of CD3+ T cells increased post-ADI-PEG 20 treatment in the 3 responders with assessable tumor tissue (B007, B015, and B035). In the non-responders, the percentage of CD3 + T cells increased in 7 subjects post-ADI-PEG 20 treatment (, Supplementary Table S1). Overall, ADI-PEG 20 treatment increased CD3+ T cell levels in 10/12 (83.3%, P = .02) patients.
PD-L1 levels at baseline were evaluated for 6 subjects from the dose-escalation cohort, which was absent (0-<1%) in 4 and low (1-<50%) in 2. Among them, only one PR was observed in the subject with the highest expression at 25% (D004). Paired tumor tissue samples were evaluated for PD-L1 expression in 10 subjects from the MTD cohort; PD-L1 expression increased in 3 subjects (30%, ).
Discussion
This is the first clinical report on the safety and tolerability of ADI-PEG 20 combined with ICT. Arginine depletion with ADI-PEG 20 and pembrolizumab was safe, and increased tumoral CD3+ T cells in the heavily pretreated subjects. Such T cell infiltration has been shown to correlate with responses, especially with ICT.Citation22 The immune effect was supported by possible pseudoprogression in two cases with initial PD to SD by irRC ().Citation18
The combination was well tolerated. Notably, among the thirteen fatalities, including the five subjects that died within three months (B006, B009, B010, B014, and D014; ), all were tumor-related and none were linked to drug AEs. Concerning grade 3–4 AEs, neutropenia occurred in 40% (n = 10/25), including the 3 subjects experiencing grade 3 febrile neutropenia. These side effects are uncommon with immune checkpoint inhibitors, as noted in the package insert for pembrolizumab.Citation23,Citation24 Similarly, hematologic AEs are infrequently observed with ADI-PEG 20 monotherapy, but are more common when combined with chemotherapy.Citation5,Citation8,Citation9 Thus, based on our phase 1b study, we observed an increase in neutropenic AEs when ADI-PEG20 was combined with pembrolizumab. Interestingly, the neutropenia was more evident in the dose escalation cohort subjects, where both drugs were combined at the beginning, compared to the MTD subjects, where the first pembrolizumab injection was staggered 16 days after the first dose of ADI-PEG 20 (). Moreover, most events occurred in the first 5 weeks during peak arginine depletion. In subject D004 with multiple episodes of neutropenia, prolonged depletion of arginine was also noted. Notably, while arginine deprivation appears not to impact neutrophil activation as measured by chemotaxis, phagocytosis and generation of ROS, both increased apoptosis and cytokine dysregulation may account for the higher rates of neutropenia in the context of arginine suppression and PD1 blockade.Citation16,Citation25–27 Nonetheless, the precise mechanism for these synergistic AEs remains unknown, but should be anticipated and studied further in future combination trials.
Consistent with previous studies, arginine levels decreased promptly with a corresponding increase in citrulline levels ().Citation5,Citation28 The arginine depletion was maintained for 2 months compared to at least 4 months in clinical trials of ADI-PEG 20 with chemotherapy.Citation7–9 However, as the trough levels were sustained for several weeks before peaking of the drug antibody titer, this provides a window of opportunity for T cell infiltration and response to ICT.Citation29 As noted in multiple prior ADI-PEG 20 studies, although blood arginine levels increase with the appearance of anti-ADI-PEG 20 antibodies, the blood draws were performed weekly immediately before ADI-PEG 20 dosing, therefore reflecting static rather than dynamic changes in amino acid levels. Moreover, the concentration of arginine and its metabolites within the cancer microenvironment following ADI-PEG 20 therapy remains unclear and requires further study. Nevertheless, our data translate the earlier preclinical data that arginine modulation impacts both PD-L1 expression and T cells distribution,Citation14 and validate observations of altered T cell localization in a recent ADI-PEG 20 triplet chemotherapy study in patients with mesothelioma.Citation16
Objective responses were observed in subjects with thymic cancer, nasopharyngeal carcinoma, cholangiocarcinoma, mucosal melanoma, esophageal cancer, and cervical cancer, with the latter three cancer types designated by the FDA for treatment with pembrolizumab. These patients did not receive prior ICTs because the reimbursement program by the Taiwan Health Insurance Administration initiated from mid-2019. Although the objective responses may be attributed to pembrolizumab alone, with response rates ranging from 8 to 30% in published studies, a contributory role of ADI-PEG 20 cannot be excluded.Citation30–35 Arguably, responses were not correlated with the degree of ASS1 expression, however our study was small overall and included subjects with a wide variety of cancers. Though patient selection with ASS1 deficiency resulted in significantly improved outcome for ADI-PEG 20 in mesothelioma,Citation5 ASS1-independent responses have been described when combined with chemotherapy.Citation8,Citation9 This suggests the capacity of tumor cells to replete arginine may be compromised or insufficient to maintain cell viability in the context of multimodality chemotherapy. Alternatively, although expressed by the tumor, ASS1 may be nonfunctional.Citation36 Nonetheless, it appears that ADI-PEG 20 has multiple anti-tumor mechanisms, by acting directly on certain tumors that are arginine deficient as well as indirectly via an immune effect.
PD-L1 was increased in 3 of 10 paired tumoral samples, including 1 of the 2 subjects with a PR. Specific chemotherapies have been documented to upregulate PD-L1 expression in various preclinical models and also to synergize with ICT.Citation37 In a head and neck cancer study, cisplatin modulated tumoral PD-L1 expression, notably converting 9/13 subjects from negative to positive PD-L1 status, providing a rationale for the ICT combination.Citation38,Citation39 Further studies of the mechanisms underlying PD-L1 induction by ADI-PEG 20 are needed in specific tumor types.
Since arginine depletion is known to impact T cell anti-tumor activity as well as the viability of urea cycle deficient tumor cells, the benefits of ADI-PEG 20 in the context of tumor immunity have been questioned. Specifically, L-arginine has crucial roles in anti-tumor T cell proliferation, differentiation, and survival.Citation40 For instance, in a phase II clinical trial of azacitidine and vorinostat in patients with acute myeloid leukemia (AML), cancer antigens failed to enhance anti-tumor immunity because of exhausted T cells in a low arginine environment generated by AML blast arginase activity.Citation41 In our study, the increased T cells in the sequential tumor biopsies indicate that ADI-PEG 20 has less impact on T cell anti-tumor activity. Indeed, it has been shown that T cells recycle the citrulline by-product of ADI-PEG 20 via an amino acid transporter to regenerate endogenous arginine.Citation42 Moreover, recent work by Lee and colleagues identified urea cycle dysregulation in tumors, including ASS1 deficiency, as enhancing tumorigenicity but importantly also increasing susceptibility to ICT.Citation43 This was attributed to higher presentation of neoantigens, due to accumulation of pyrimidine-rich transversion mutational bias that was a stronger predictor of ICT than tumor mutational burden. Collectively, given the increased tumoral PD-L1 expression with ADI-PEG 20 therapy, the combination with pembrolizumab seeks to bypass T cell exhaustion and supports further clinical studies of this novel immunometabolic strategy. Nevertheless, although all three responders with assessable tissue displayed a variably increased number of CD3+ T cells, it remains unanswered if the altered immunity was associated with the response. Given that tumor infiltrating lymphocytes are often related to prognosis and ICT treatment,Citation44 our results are limited by the small sample size in this study, and larger trials are needed to more fully these aspects.
In conclusion, the combination of ADI-PEG 20 and pembrolizumab in heavily pretreated subjects was feasible. Objective responses were observed, but also with an apparent higher incidence of neutropenia that was manageable. The translational biopsies confirmed an immunomodulatory effect of ADI-PEG 20, with statistically significant T cell infiltration and evidence for PD-L1 induction. These conclusions, however, are based on a small and heterogeneous group of subjects, and further clinical trials are planned in patients enriched for defined arginine auxotrophic cancers.
Authors’ contribution:
K-Y.C.: Conceptualization, Project administration, Writing - Original Draft, Investigation. N-J.C., S-Y.W., C-J.Y., S-H.C., Y-M.Y.: Investigation. C-F.L., J.G., S.K.S., A.O.K., J.M.B., S.S.Y.: Methodology, Resources. X.F., K.W., A.J.: Formal analysis. J.S.B., B-W.W.: Funding acquisition, Conceptualization, Methodology, Writing - Review and Editing, Visualization. P.W.S.: Conceptualization, Writing - Review & Editing. L-T.C.: Conceptualization, Project administration, Writing - Review and Editing, Supervision.
Ethics approval and consent to participate:
This study was conducted following international standards consistent with the International Council for Harmonisation E6 Guideline for Good Clinical Practice, and was performed in accordance with the Declaration of Helsinki. The study protocol was approved by the ethics committees at National Cheng Kung University Hospital (B-BR-105060). Studies of the tissue sample was also approved by the ethics committees at MD Anderson (PA130291).
Trial registration number:
ClinicalTrials.gov: NCT03254732
Declaration of competing interest:
K-Y.C. reports non-financial support from Polaris Pharmaceuticals, personal fees from MSD, outside the submitted work; S-Y.W. reports personal fees and non-financial support from Novartis, personal fees from Sanofi, Merck Sharp & Dohme, Boehringer Ingelheim, Merck KGaA, outside the submitted work; C-F.L. reports grants from Polaris, outside the submitted work; X.F. reports personal fees from Polaris Pharmaceuticals, outside the submitted work; A.J. reports personal fees from Polaris Pharmaceuticals, during the conduct of the study; personal fees from Polaris Pharmaceuticals, outside the submitted work; J.S.B. reports other from Polaris Pharmaceuticals, Inc., during the conduct of the study; in addition, J.S.B. has a patent Methods of treatment with arginine deiminase issued. P.W.S. reports grants from Polaris Group, personal fees from BMS, non-financial support from Merck, during the conduct of the study; J.G. reports personal fees from ARMO Biosciences, AstraZeneca, CRISPR Therapeutics, Jounce, NEKTAR, Pfizer, Janssen, Polaris, Symphogen, outside the submitted work; S.K.S. reports personal fees and other from Janssen Oncology, Apricity Health, AstraZeneca, Bristol-Myers Squibb, personal fees from Polaris, Dendreon, Amgen, Bayer, Dava Oncology, Cancer Now, MEDACorp, Parker Institute for Cancer Immunotherapy, Society for Immunotherapy of Cancer, outside the submitted work; L-T.C. reports grants from Polaris, during the conduct of the study; personal fees from MSD, outside the submitted work; N-J.C., C-J.Y., S-H.C., Y-M.Y., K.W., B-W.W., A.O.K., J.M.B., S.S.Y. have nothing to disclose.
Supplemental Material
Download ()Acknowledgments
The authors express sincere appreciation to all participants in this clinical trial and to their families. We acknowledge all the effort from clinical investigators, statisticians, clinical research associates, and administrative staff who have contributed to the trial.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- Ascierto PA, Scala S, Castello G, Daponte A, Simeone E, Ottaiano A, Beneduce G, De Rosa V, Izzo F, Melucci MT, et al. Pegylated arginine deiminase treatment of patients with metastatic melanoma: results from phase I and II studies. J Clin Oncol. 2005;23(30):7660–10. doi:10.1200/JCO.2005.02.0933
- Feun LG, Marini A, Walker G, Elgart G, Moffat F, Rodgers SE, Wu CJ, You M, Wangpaichitr M, Kuo MT, et al. Negative argininosuccinate synthetase expression in melanoma tumours may predict clinical benefit from arginine-depleting therapy with pegylated arginine deiminase. Br J Cancer. 2012;106(9):1481–1485. doi:10.1038/bjc.2012.106
- Glazer ES, Piccirillo M, Albino V,Di Giacomo R, Palaia R, Mastro AA, Beneduce G, Castello G, De Rosa V, Petrillo A, et al. Phase II study of pegylated arginine deiminase for nonresectable and metastatic hepatocellular carcinoma. J Clin Oncol. 2010;28(13):2220–2226. doi:10.1200/JCO.2009.26.7765
- Izzo F, Marra P, Beneduce G, Castello G, Vallone P, De Rosa V, Cremona F, Ensor CM, Holtsberg FW, Bomalaski JS, et al. Pegylated arginine deiminase treatment of patients with unresectable hepatocellular carcinoma: results from phase I/II studies. J Clin Oncol. 2004;22(10):1815–1822. doi:10.1200/JCO.2004.11.120
- Szlosarek PW, Steele JP, Nolan L, Gilligan D, Taylor P, Spicer J, Lind M, Mitra S, Shamash J, Phillips MM, et al. Arginine deprivation with pegylated arginine deiminase in patients with argininosuccinate synthetase 1-deficient malignant pleural mesothelioma: a randomized clinical trial. JAMA Oncol. 2017;3(1):58–66. doi:10.1001/jamaoncol.2016.3049
- Yang TS, Lu SN, Chao Y, Sheen IS, Lin CC, Wang TE, Chen SC, Wang JH, Liao LY, Thomson JA, et al. A randomised phase II study of pegylated arginine deiminase (ADI-PEG 20) in Asian advanced hepatocellular carcinoma patients. Br J Cancer. 2010;103(7):954–960. doi:10.1038/sj.bjc.6605856
- Beddowes E, Spicer J, Chan PY, Khadeir R, Corbacho JG, Repana D, Steele JP, Schmid P, Szyszko T, Cook G, et al. Phase 1 dose-escalation study of pegylated arginine deiminase, cisplatin, and pemetrexed in patients with argininosuccinate synthetase 1-deficient thoracic cancers. J Clin Oncol. 2017;35(16):1778–1785. doi:10.1200/JCO.2016.71.3230
- Harding JJ, Do RK, Dika IE, Hollywood E, Uhlitskykh K, Valentino E, Wan P, Hamilton C, Feng X, Johnston A, et al. A phase 1 study of ADI-PEG 20 and modified FOLFOX6 in patients with advanced hepatocellular carcinoma and other gastrointestinal malignancies. Cancer Chemother Pharmacol. 2018;82(3):429–440. doi:10.1007/s00280-018-3635-3
- Lowery MA, Yu KH, Kelsen DP, Harding JJ, Bomalaski JS, Glassman DC, Covington CM, Brenner R, Hollywood E, Barba A, et al. A phase 1/1B trial of ADI-PEG 20 plus nab-paclitaxel and gemcitabine in patients with advanced pancreatic adenocarcinoma. Cancer. 2017;123(23):4556–4565. doi:10.1002/cncr.30897
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–1086. doi:10.1158/2159-8290.CD-18-0367
- Teng F, Meng X, Kong L, Yu J. Progress and challenges of predictive biomarkers of anti PD-1/PD-L1 immunotherapy: a systematic review. Cancer Lett. 2018;414:166–173. doi:10.1016/j.canlet.2017.11.014
- Kim SH, Roszik J, Grimm EA, Ekmekcioglu S. Impact of l-arginine metabolism on immune response and anticancer immunotherapy. Front Oncol. 2018;8:67. doi:10.3389/fonc.2018.00067
- Lowe MM, Boothby I, Clancy S, Ahn RS, Liao W, Nguyen DN, Schumann K, Marson A, Mahuron KM, Kingsbury GA, et al. Regulatory T cells use arginase 2 to enhance their metabolic fitness in tissues. JCI Insight. 2019;4(24):e129756. doi:10.1172/jci.insight.129756
- Brin E, Wu K, Lu HT, He Y, Dai Z, He W. PEGylated arginine deiminase can modulate tumor immune microenvironment by affecting immune checkpoint expression, decreasing regulatory T cell accumulation and inducing tumor T cell infiltration. Oncotarget. 2017;8(35):58948–58963. doi:10.18632/oncotarget.19564
- Pavlyk I, Foster J, Dexter K, Sobasowski J, Bomalarski J, Berlato C, Balkwill F, Szlosarek PW.Abstract 2217: Pegylated arginine deiminase sensitizes ASS1-negative and KRAS mutant non-small cell lung cancer to PD-1 blockade immunotherapy. Cancer Res 2020;80(16Suppl):2217. doi:10.1158/1538-7445.am2020-2217
- Szlosarek PW, Phillips MM, Pavlyk I, Steele J, Shamash J, Spicer J, Kumar S, Pacey S, Feng X, Johnston A, et al. expansion phase 1 study of pegargiminase plus pemetrexed and cisplatin in patients with argininosuccinate synthetase 1–deficient mesothelioma: safety, efficacy, and resistance mechanisms. JTO Clin Res Rep. 2020;1(4):100093. doi: 10.1016/j.jtocrr.2020.100093
- Zheng X, Fernando V, Sharma V, Walia Y, Letson J, Furuta S. Correction of arginine metabolism with sepiapterin-the precursor of nitric oxide synthase cofactor BH 4-induces immunostimulatory-shift of breast cancer. Biochem Pharmacol. 2020;176:113887. doi:10.1016/j.bcp.2020.113887
- Wolchok Jd, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi:10.1158/1078-0432.CCR-09-1624
- Chuk MK, Chang JT, Theoret MR, Sampene E, He K, Weis SL, Helms WS, Jin R, Li H, Yu J, et al. FDA approval summary: accelerated approval of pembrolizumab for second-line treatment of metastatic melanoma. Clin Cancer Res. 2017;23(19):5666–5670. doi:10.1158/1078-0432.CCR-16-0663
- Huang HY, Wu WR, Wang YH, Wang JW, Fang FM, Tsai JW, Li SH, Hung HC, Yu SC, Lan J, et al. ASS1 as a novel tumor suppressor gene in myxofibrosarcomas: aberrant loss via epigenetic dna methylation confers aggressive phenotypes, negative prognostic impact, and therapeutic relevance. Clin Cancer Res. 2013;19(11):2861–2872. doi:10.1158/1078-0432.CCR-12-2641
- Blando J, Sharma A, Higa MG, Zhao H, Vence L, Yadav SS, Kim J, Sepulveda AM, Sharp M, Maitra A, et al. Comparison of immune infiltrates in melanoma and pancreatic cancer highlights VISTA as a potential target in pancreatic cancer. Proc Natl Acad Sci U S A. 2019;116(5):1692–1697. doi:10.1073/pnas.1811067116
- Trujillo JA, Sweis RF, Bao R, Luke JJ. T cell-inflamed versus non-T cell-inflamed tumors: a conceptual framework for cancer immunotherapy drug development and combination therapy selection. Cancer Immunol Res. 2018;6(9):990–1000. doi:10.1158/2326-6066.CIR-18-0277
- Delanoy N, Michot JM, Comont T, Kramkimel N, Lazarovici J, Dupont R, Champiat S, Chahine C, Robert C, Herbaux C, et al. Haematological immune-related adverse events induced by anti-PD-1 or anti-PD-L1 immunotherapy: a descriptive observational study. Lancet Haematol. 2019;6(1):e48–e57. doi:10.1016/S2352-3026(18)30175-3
- Tozuka T, Sugano T, Noro R, Takano N, Hisakane K, Takahashi S, Tanaka T, Kashiwada T, Takeuchi S, Kunugi S, et al. Pembrolizumab-induced agranulocytosis in a pulmonary pleomorphic carcinoma patient who developed interstitial lung disease and ocular myasthenia gravis. Oxf Med Case Rreports. 2018;2018:omy094. doi: 10.1093/omcr/omy094
- Kapp K, Prufer S, Michel CS, Habermeier A, Luckner-Minden C, Giese T, Bomalaski J, Langhans CD, Kropf P, Muller I, et al. Granulocyte functions are independent of arginine availability. J Leukoc Biol. 2014;96(6):1047–1053. doi:10.1189/jlb.3AB0214-082R
- Cho JH, Lee R, Kim E, Choi YE, Choi EJ. PRMT1 negatively regulates activation-induced cell death in macrophages by arginine methylation of GAPDH. Exp Cell Res. 2018;368(1):50–58. doi:10.1016/j.yexcr.2018.04.012
- Bankey PE, Banerjee S, Zucchiatti A, De M, Sleem RW, Lin CL, Miller-Graziano CL, De AK. Cytokine induced expression of programmed death ligands in human neutrophils. Immunol Lett. 2010;129(2):100–107. doi:10.1016/j.imlet.2010.01.006
- Abou-Alfa GK, Qin S, Ryoo BY, Lu SN, Yen CJ, Feng YH, Lim HY, Izzo F, Colombo M, Sarker D, et al. Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann Oncol. 2018;29(6):1402–1408. doi:10.1093/annonc/mdy101
- Nunes SC. Exploiting cancer cells metabolic adaptability to enhance therapy response in cancer. Adv Exp Med Biol. 2020;1219:297–310.
- Doi T, Piha-Paul SA, Jalal SI, Saraf S, Lunceford J, Koshiji M, Bennouna J. Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J Clin Oncol. 2018;36(1):61–67. doi:10.1200/JCO.2017.74.9846
- Frenel JS, Le Tourneau C, O’Neil B, Ott PA, Piha-Paul SA, Gomez-Roca C, van Brummelen EMJ, Rugo HS, Thomas S, Saraf S, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J Clin Oncol. 2017;35(36):4035–4041. doi:10.1200/JCO.2017.74.5471
- Giaccone G, Kim C, Thompson J, McGuire C, Kallakury B, Chahine JJ, Manning M, Mogg R, Blumenschein WM, Tan MT, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol. 2018;19(3):347–355. doi:10.1016/S1470-2045(18)30062-7
- Hamid O, Robert C, Ribas A, Hodi FS, Walpole E, Daud A, Arance AS, Brown E, Hoeller C, Mortier L, et al. Antitumour activity of pembrolizumab in advanced mucosal melanoma: a post-hoc analysis of KEYNOTE-001, 002, 006. Br J Cancer. 2018;119(6):670–674. doi:10.1038/s41416-018-0207-6
- Hsu C, Lee SH, Ejadi S, Even C, Cohen RB, Le Tourneau C, Mehnert JM, Algazi A, van Brummelen EMJ, Saraf S, et al. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: results of the KEYNOTE-028 Study. J Clin Oncol. 2017;35(36):4050–4056. doi:10.1200/JCO.2017.73.3675
- Kang J, Jeong JH, Hwang HS, Lee SS, Park DH, Oh DW, Song TJ, Kim KH, Hwang S, Hwang DW, et al. Efficacy and safety of pembrolizumab in patients with refractory advanced biliary tract cancer: tumor proportion score as a potential biomarker for response. Cancer Res Treat. 2020;52(2):594–603. doi:10.4143/crt.2019.493
- Lin R, Mo Y, Zha H, Qu Z, Xie P, Zhu ZJ, Xu Y, Xiong Y, Guan KL. CLOCK acetylates ASS1 to drive circadian rhythm of ureagenesis. Mol Cell. 2017;68(1):198–209 e6. doi:10.1016/j.molcel.2017.09.008
- Heinhuis KM, Ros W, Kok M, Steeghs N, Beijnen JH, Schellens JHM. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol. 2019;30(2):219–235. doi:10.1093/annonc/mdy551
- Ock CY, Kim S, Keam B, Kim S, Ahn YO, Chung EJ, Kim JH, Kim TM, Kwon SK, Jeon YK, et al. Changes in programmed death-ligand 1 expression during cisplatin treatment in patients with head and neck squamous cell carcinoma. Oncotarget. 2017;8(58):97920–97927. doi:10.18632/oncotarget.18542
- Tran L, Allen CT, Xiao R, Moore E, Davis R, Park SJ, Spielbauer K, Van Waes C, Schmitt NC. Cisplatin alters antitumor immunity and synergizes with PD-1/PD-L1 inhibition in head and neck squamous cell carcinoma. Cancer Immunol Res. 2017;5(12):1141–1151. doi:10.1158/2326-6066.CIR-17-0235
- Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, Kogadeeva M, Picotti P, Meissner F, Mann M, et al. L-Arginine Modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. 2016;167(3):829–842. doi:10.1016/j.cell.2016.09.031
- Mussai F, Wheat R, Sarrou E, Booth S, Stavrou V, Fultang L, Perry T, Kearns P, Cheng P, Keeshan K, et al. Targeting the arginine metabolic brake enhances immunotherapy for leukaemia. Int J Cancer. 2019;145(8):2201–2208. doi:10.1002/ijc.32028
- Werner A, Koschke M, Leuchtner N, Luckner-Minden C, Habermeier A, Rupp J, Heinrich C, Conradi R, Closs EI, Munder M. Reconstitution of T cell proliferation under arginine limitation: activated human T cells take up citrulline via L-type amino acid transporter 1 and use it to regenerate arginine after induction of argininosuccinate synthase expression. Front Immunol. 2017;8:864. doi:10.3389/fimmu.2017.00864
- Lee JS, Adler L, Karathia H, Carmel N, Rabinovich S, Auslander N, Keshet R, Stettner N, Silberman A, Agemy L, et al. Urea cycle dysregulation generates clinically relevant genomic and biochemical signatures. Cell. 2018;174(6):1–12. doi:10.1016/j.cell.2018.07.019
- Barnes TA, Amir E. HYPE or HOPE: the prognostic value of infiltrating immune cells in cancer. Br J Cancer. 2017;117(4):451–460. doi:10.1038/bjc.2017.220