ABSTRACT
Although previous studies suggest that cancer cachexia is a poor prognostic factor for immune checkpoint inhibitor monotherapy, the impact of cancer cachexia on chemoimmunotherapy is unclear. We investigated the impact of cancer cachexia on the therapeutic outcomes of chemoimmunotherapy for non-small cell lung cancer (NSCLC). We retrospectively analyzed patients’ medical records with NSCLC who received chemoimmunotherapy in 12 institutions in Japan between January and November 2019. We defined cancer cachexia as weight loss exceeding 5% of the total body weight or a body mass index of < 20 kg/m2 and weight loss of more than 2% of the total body weight within 6 months before chemoimmunotherapy initiation, with laboratory results exceeding reference values. This study enrolled 235 patients with NSCLC, among whom 196 were eligible for analysis, and 50 (25.5%) met the criteria for cachexia diagnosis. Patients with cancer cachexia had a significantly higher frequency of a programmed death-ligand 1 (PD-L1) expression of ≥ 50% (48%, p = .01) and shorter progression-free survival (PFS; log-rank test: p = .04) than patients without cachexia. There was no significant difference in overall survival (OS) between the cachexia and no-cachexia groups (log-rank test: p = .14). In the PD-L1 ≥ 50% population, there was no significant difference in PFS and OS (log-rank test: p = .19 and p = .79, respectively) between patients with NSCLC in the cachexia or no-cachexia groups. Cancer cachexia might be a poor prognostic factor in patients with NSCLC receiving chemoimmunotherapy.
INTRODUCTION
Immune checkpoint inhibitors (ICIs) are therapeutic drugs that modulate the immune response to cancer cells. ICIs target regulatory molecules such as programmed death-ligand 1 (PD-L1) and are used alone (monotherapy) or in combination with cytotoxic agents to treat tumors with a high mutational burden.Citation1–3 The benefits of ICIs in several carcinomas have been shown.Citation4–8 In previous clinical trials involving previously treated patients with non-small cell lung cancer (NSCLC), nivolumab, pembrolizumab, and atezolizumab administered alone, each had a longer overall survival (OS) than docetaxel.Citation1,Citation9–11 Combined chemoimmunotherapy has also been shown to be superior to cytocidal anticancer agents and has become one of the standard treatments for NSCLC.Citation12–15 Moreover, combined chemoimmunotherapy leads to a lower risk of disease progression than ICI monotherapy.
It has been reported that cancer cachexia may be a poor prognostic factor for disease control and survival after ICI monotherapy.Citation16–18 However, there are few reports investigating whether cancer cachexia is a prognostic factor for chemoimmunotherapy in patients with NSCLC. This study investigated the impact of cancer cachexia on the therapeutic outcome of combined chemoimmunotherapy in patients with NSCLC.
MATERIALS AND METHODS
Patients
A total of 235 patients from 12 institutions in Japan were enrolled in this study between January and November 2019. The median follow-up duration was 13.8 months. The patients’ pre-treatment height and weight were extracted from electronic medical records. The body mass index (BMI) was calculated by dividing each patient’s weight (kg) by his/her height squared (m2). The World Health Organization has classified BMI into four categories: underweight, BMI < 18.5 kg/㎡; normal weight, 18.5 kg/㎡ ≤ BMI ≤ 24.9 kg/㎡; overweight, 25 kg/㎡ ≤ BMI ≤ 29.9 kg/㎡; and obesity, BMI ≥ 30 kg/㎡. Based on previous reports, cancer cachexia was defined as weight loss of more than 5% of the body weight within the 6 months before chemoimmunotherapy initiation, or weight loss of more than 2% of the body weight when the BMI was less than 20 kg/m2, along with laboratory values above the expected reference values (C-reactive protein [CRP] > 0.5 mg/dL, serum albumin [Alb] < 3.2 g/dL, or hemoglobin [Hb] < 12 g/dL).Citation19,Citation20 Patients who had received steroids within two weeks before chemoimmunotherapy initiation were excluded from the study. Patients with epidermal growth factor (EGFR) or anaplastic lymphoma kinase (ALK) driver mutations were eligible if they had received treatment with at least one approved tyrosine kinase inhibitor.
The primary endpoint was progression-free survival (PFS) from the start of chemoimmunotherapy. The secondary endpoints were overall survival (OS) and objective response rate (ORR).
Patients’ characteristics such as age, sex, histology type, PD-L1 expression, EGFR gene mutation status, ALK rearrangement status, laboratory test results (Hb, CRP, and Alb levels), height, weight, Eastern Cooperative Oncology Group (ECOG) performance status (PS), smoking history, PFS, OS, best overall response, ORR, and disease control rate (DCR) were retrieved from medical records. The eighth edition of the American Joint Commission on Cancer staging system was used for tumor, node, and metastasis staging. Patient response was assessed using the new guideline for solid cancer response assessment (RECIST guidelines: revised version 1. 1). To measure PD-L1 expression, a 22C3 antibody (Agilent Technologies, Santa Clara, CA, USA) was used. The PD-L1 tumor proportion score was calculated as a percentage of at least 100 viable tumor cells with complete or partial membrane staining and was analyzed by SRL, inc. The body weight of patients during the 6 months that preceded chemoimmunotherapy was determined by interviewing the patients or their family members or by weight measurement in the hospitals. This study was approved by the Ethics Review Board of the Kyoto Prefectural University of Medicine and was conducted with consent from the Ethics Review Board of each hospital (approval no. ERB-C-1803).
Statistical analysis
P-values < 0.05 were considered statistically significant. Patients were classified into two groups according to their cachexia status or PD-L1 expression. Fisher’s exact test or the chi-square test was used to compare the factors between groups.
PFS was defined as the period between chemoimmunotherapy initiation and disease progression, treatment discontinuation, or death. OS was defined as the period between chemoimmunotherapy initiation and death. PFS and OS were censored on final survival confirmation in those patients whose disease did not progress or who survived. PFS and OS were calculated using the Kaplan–Meier method, and the differences were verified using the log-rank test. Student’s t-test was used to compare age and BMI. Multivariate analysis for cancer cachexia was performed by logistic regression analysis. In univariate and multivariate analyses, a Cox proportional hazard model was used to estimate hazard ratios (HR) and 95% confidence intervals (CI). Based on previous reports, ECOG-PS (PS ≥2), sex, age (≥ 75 years), smoking status, PD-L1 (≥ 50%), postoperative recurrence, and driver mutation were selected as covariates.Citation12,Citation21,Citation22 Schoenfeld residual tests were performed to assess the Cox proportional hazards assumptions. Tumor response was evaluated using RECIST, version 1.1. EZR statistical software, version 1.54, was used for all statistical analyses.Citation23
RESULTS
Patients’ characteristics
Of the 235 enrolled patients, 39 were excluded for the following reasons: 11 patients had been treated with steroids, 4 patients had incomplete body weight assessment findings during the study period, the CRP, Hb, and Alb levels of 17 patients were missing, the EGFR and ALK mutation status was not assessed in 5 patients, and 2 patients received chemoimmunotherapy before tyrosine kinase inhibitors; therefore, 196 patients were examined ().
The median overall age was 69 years (). Among these patients, 72.4% were men, 96.9% had ECOG-PS 0/1, 81.1% were in clinical stage III/IV, 6.1% had EGFR gene mutations, 76.5% were smokers, and 32.1% had a PD-L1 tumor proportion score (TPS) ≥ 50%. The median BMI was 21.5 kg/m2: 13.3% were underweight, 70.4% had a normal weight, 15.3% were overweight, and 1.0% were obese. Fifty patients met the criteria for cachexia diagnosis. Their median age was 70 years, 78.0% were men, 86.0% were smokers, 10.0% had EGFR gene mutations, and 92.0% were in clinical stage III/IV. The median BMI of the group with cachexia was 19.6 kg/m2: 26.0% were underweight, 64.0% had normal weight, and 10.0% were overweight.
Table 1. Characteristics of patients
Patients with cancer cachexia had significantly fewer postoperative recurrences and a significantly higher frequency of PD-L1 ≥ 50% than those in the no-cachexia group (p = .02, p = .01, respectively). Multivariate logistic regression analyses revealed that PD-L1 ≥ 50% (Odds ratio: 2.48, 95% CI: 1.21–5.12), postoperative recurrence (Odds ratio: 0.16, 95% CI: 0.03–0.70), and age ≥ 75 (Odds ratio: 2.63, 95%CI: 1.08–6.44) were associated with cancer cachexia independent of other patient characteristics ().
Table 2. Multivariate logistic regression analysis for factors associated with cancer cachexia
To assess whether PD-L1 ≥ 50% is common in patients with NSCLC having cachexia, we examined the PD-L1 expression data of patients with NSCLC who started chemotherapy between February 2017 and June 2020 at the Kyoto Prefectural University of Medicine (Supplementary Figure 1). We excluded 156 postoperative patients and 46 EGFR/ALK mutation-positive patients in this analysis to avoid the influence of other clinical factors. The frequency of PD-L1 ≥ 50% was significantly higher in the cachexia group than in the no cachexia group (p = .02) (Supplementary Figure 2).
There was no significant difference in the rate of discontinuation of all treatment components among the patients with NSCLC having cachexia or not (p = .66) (Supplementary Table 1).
Treatment efficacy of chemoimmunotherapy in patients with NSCLC and cancer cachexia
The ORR of patients with cachexia was 62.0% (95% CI: 47.2%–74.3%), whereas that of patients without cachexia was 56.8% (95% CI: 48.4–65.0%) (p = .64). The DCR was 94.0% (95% CI: 83.5–98.7%) in patients with cachexia and 87.7% (95% CI: 81.2%–92.5%) in patients without cachexia (p = .29).
Prognostic association of chemoimmunotherapy in patients with NSCLC and cancer cachexia
Among the 196 patients with NSCLC, 124 patients had disease progression, and 63 patients had died by the cutoff date. The cachexia group (n = 50) had a significantly shorter PFS (log-rank test p = .04) and tended to have a shorter OS (log-rank test p = .14) than the group without cachexia (n = 146) (). In the univariate analysis, the cachexia group had a significantly shorter PFS than the no-cachexia group (HR: 1.49, 95% CI: 1.01–2.19, p = .04). This result was confirmed in the multivariate analysis (HR: 1.64, 95% CI: 1.06–2.55, p = .03) (). Schoenfeld residual tests in the sex section of the multivariate analysis indicated a potential violation of the proportional hazard assumption (p < .05). Visual inspection of the log-log and Schoenfeld residual plots showed no serious violations, and the analysis was carried out as planned. A similar trend was observed when dividing the group with cancer cachexia into two sub-groups: those with weight loss > 5% with laboratory results exceeding reference values and those with BMI < 20 kg/m2 and weight loss > 2% with laboratory results exceeding reference values (Supplementary Figures 3 and 4). We have investigated the relationship between cachexia and disease progression in various subgroups. There was an interaction between cachexia and smoking status (p for interaction = 0.02) (Supplementary Table 3). In this study, in the univariate analysis, there was no significant difference in the OS between the cachexia and no-cachexia groups. Similarly, there was no significant difference in OS between the cachexia and no-cachexia groups in the multivariate analysis. The multivariate analysis that was focused on only the essential items ([ECOG-PS ≥2], sex, age [≥ 75 years], smoking status, PD-L1 [≥ 50%] showed similar results (Supplementary Table 2).
Figure 2. Kaplan–Meier curves for (a) PFS and (b) OS of patients with NSCLC, according to the presence of cachexia. PFS: progression-free survival, OS: overall survival, NSCLC: non-small cell lung cancer, HR: hazard ratio, CI: confidence interval
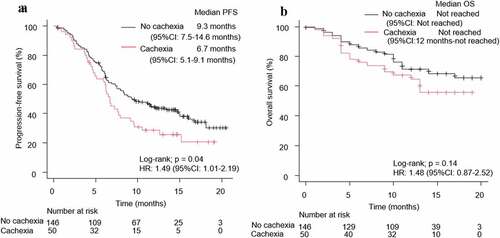
Table 3. Cox proportional-hazards models for time to progression-free survival and overall survival in patients with non-small cell lung cancer regardless of their PD-L1 status
When cachexia was defined only by weight loss (more than 5% of the body weight within the 6 months preceding chemoimmunotherapy initiation or weight loss of more than 2% when the BMI was less than 20 kg/m2), the PFS tended to be worse in the cachexia group than in the no-cachexia group (HR: 1.36, 95% CI: 0.96–1.93, log-rank test p = .08), but there was no difference in OS (HR: 1.13, 95% CI: 0.69–1.84, log-rank test p = .63) (Supplementary Figure 5).
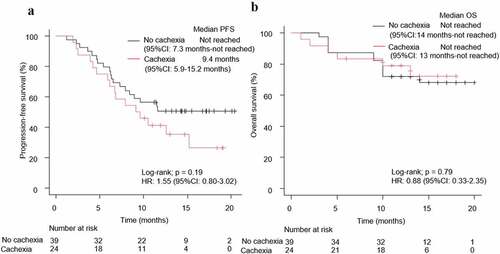
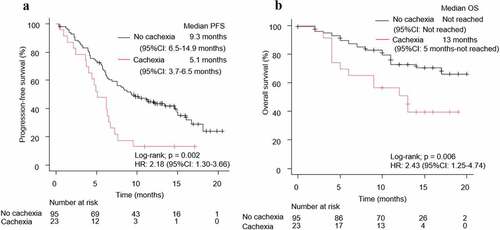
Patients were also classified according to their PD-L1 expression score (PD-L1 ≥ 50% and PD-L1 < 50%). In the PD-L1 < 50% group, patients with NSCLC having cachexia (n = 23) had a significantly shorter PFS (HR: 2.18, 95% CI: 1.30–3.66, log-rank test p = .002) and OS (HR: 2.43, 95% CI: 1.25–4.74, log-rank test p = .006) than those without cachexia (n = 95) (). In contrast, in the PD-L1 ≥ 50% group, there were no significant differences in the PFS (HR: 1.55, 95% CI: 0.80–3.02, log-rank test p = .19) and OS (HR: 0.88, 95% CI: 0.33–2.35, log-rank test p = .79) among the cachexia (n = 24) and no-cachexia (n = 39) groups (). In the cachexia group (n = 47), the ORRs were 75% (95% CI: 53.3–90.2%) and 52.2% (95% CI: 30.6–73.2%) while that in the no-cachexia group (n = 134) were 69.2% (95% CI: 52.4–83.4%) and 52.6% (95% CI: 42.1–63.0%) when divided into two groups based on PD-L1 expression rate of 50% (Supplementary Figure 6A).
Figure 3. Kaplan–Meier curves for (a) PFS and (b) OS of patients with NSCLC patients and a PD-L1 < 50%, according to the presence of cachexia. PFS: progression-free survival, OS: overall survival, NSCLC: non-small cell lung cancer, HR: hazard ratio, CI: confidence interval, PD-L1: programmed death-ligand 1
DISCUSSION
Cancer cachexia is a multifactorial syndrome characterized by a persistent loss of skeletal muscle mass that cannot be recovered with conventional dietary supplements, leading to progressive dysfunction.Citation20
Inflammation is considered the main cause of cancer cachexia. Inflammatory cytokines such as interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α adversely influence systemic disorders such as metabolic disorders, skeletal muscle loss, and fat breakdown.Citation20,Citation24 We used laboratory test results (serum CRP, Hb, and Alb) to detect systemic inflammation.Citation19 In our study, patients with cancer cachexia had a significantly shorter PFS than those without cachexia. Since chemotherapy is the standard treatment for NSCLC, cachexia can be a poor prognostic factor for chemoimmunotherapy and ICI monotherapy. In this study, weight loss alone was not a poor prognostic factor, and systemic inflammation had to be included as a criterion. It has been reported that the definition by Fearon et al. overestimates the diagnosis of cachexia and may not contribute to the prognosis.Citation25 It is important to evaluate not only weight loss but also other factors such as inflammation, anemia, and anorexia.
The cachexia group before chemoimmunotherapy initiation had significantly fewer cases of postoperative recurrence and more elderly patients (age ≥ 75) than the no-cachexia groups. In cancer cachexia, the tumor burden increases the production of cytokines and catabolic factors.Citation26 Compared to patients with stage III/IV disease, the lower tumor burden may explain the lower prevalence of cancer cachexia among patients with postoperative recurrence.Citation21 However, it should be noted that in patients with NSCLC, PD-L1 expression may vary between primary and recurrent disease, and postoperative recurrence cases may not be an accurate representation of the current PD-L1 expression.Citation27 It has been reported that aging and tumor stage are positively correlated with pre-treatment weight loss at the time of diagnosis of NSCLC, which was consistent with our results.Citation28 Surprisingly, the group with cancer cachexia had a significantly higher proportion of patients with PD-L1 ≥ 50% than the no-cachexia group. The frequency of PD-L1 ≥ 50% was 26.0–34.0% in previous pivotal studies and 29.0% in a single-center study in Japan.Citation12,Citation14,Citation29 In our study, the proportion of PD-L1 ≥ 50% in the overall analysis set was 32.1%, whereas the proportion of PD-L1 ≥ 50% in the cachexia group was as high as 48.0%. Considering the possibility of selection bias caused by assessing the PD-L1 TPS only in patients with NSCLC who received chemoimmunotherapy, we assessed the PD-L1 TPS in patients with NSCLC who received chemotherapy in a single center. We excluded postoperative cases and EGFR/ALK mutation-positive cases because of their potential impact on the PD-L1 tumor proportion score.Citation27,Citation30 Since we obtained similar results in patients with NSCLC who received chemotherapy, the higher proportion of PD-L1 ≥ 50% in the cachexia group than in the no-cachexia group in patients with NSCLC may be common.
PD-L1 is upregulated by multiple inflammatory signals (IL-6, TNF-α, interferon-γ, etc.), functioning in a negative feedback loop during inflammation. Our results may then be related to the pathology of inflammation in patients with cachexia.Citation31,Citation32
Cachexia has been reported to exacerbate toxicity and complications of cancer treatment.Citation33 Although detailed information on adverse events was not available in this study, there were no significant differences in the treatment discontinuation rate or treatment-related deaths between the cachexia and no-cachexia groups.
When the association between cachexia and disease progression was examined in various subgroups without adjustment, there was an interaction between smoking status and cachexia (Supplementary Table 3). This may be due to the limited number of patients with NSCLC who were never smokers and had cachexia (n = 7); five of these patients had EGFR/ALK mutations, which had a poor prognosis in this analysis.
Our analyses indicated that in the PD-L1 ≥ 50% population, there was no significant difference in PFS and OS between the cachexia group and no-cachexia groups at the time of analysis. A previous single-center retrospective study reported that cancer cachexia might desensitize the therapeutic effect of ICIs in patients with NSCLC and a high PD-L1 expression.Citation17 This may be because cachexia-related mediators such as IL-6, IL-1β, and TNF-α suppress CD8+ tumor infiltrating lymphocytes (TILs) and reduce anti-tumor immunity in patients with NSCLC having cancer cachexia. Conversely, a high PD-L1 expression strongly influenced the therapeutic effect of chemoimmunotherapy in patients with NSCLC having cachexia. Chemotherapeutic agents promote an antitumor immune response by inducing immunogenic cell death and promoting CD8+ TILs Citation34,Citation35. The combination of ICIs and chemotherapeutic agents may attenuate the negative impact of cachexia on anti-tumor immunity.
In patients with cancer cachexia who have not reached the refractory stage, the cachectic state has been reported to be reversible.Citation20,Citation36 Patients who transition from a state of cancer cachexia to no cachexia have a better prognosis than those who remain in a state of cancer cachexia.Citation37
In our study, the patients with cachexia responded better to chemoimmunotherapy in the group with PD-L1 ≥ 50% than in the group with PD-L1 < 50% (Supplementary Figure 4a). Thus, in patients with NSCLC having cachexia and a PD-L1 ≥ 50%, the high antitumor efficacy of chemoimmunotherapy may reduce the tumor burden and lead to a shift from cachexia to no cachexia.
Even in pre-treatment cachexia, patients with NSCLC and a PD-L1 ≥ 50% may be considered for chemoimmunotherapy. However, the lack of a significant difference in survival in the presence or absence of cachexia in the PD-L1 ≥ 50% group could be due to the greatly reduced statistical power (n = 63) of this comparison. Furthermore, there was no adjustment for background factors in the subgroup analysis according to the PD-L1 expression score; this may have contributed to the survival, such as postoperative recurrence, in the multivariate analysis. In our study, the median follow-up period was slightly short to evaluate OS. It is necessary to increase the number of cases and follow-up the patients for a longer period to test the hypothesis.
Weight loss due to cachexia involves the abnormal metabolism of skeletal muscle and fat.Citation38 Previous studies summarizing data from the time when ICIs were not commercially available reported that weight loss during treatment is associated with shortening of PFS and OS.Citation39,Citation40 In patients with NSCLC and PD-L1 ≥ 50%, BMI variations have been reported to be associated with clinical outcomes after pembrolizumab monotherapy.Citation41 The development of a treatment method to improve cancer cachexia is desired.
This study has some limitations. First, it did not evaluate skeletal muscle mass, which is used to define cachexia. However, approximately 90% of the patients with cachexia can be diagnosed using weight loss > 5% or BMI < 20 kg/m2 and weight loss > 2% alone.Citation42 Second, there may have been bias in obtaining information on the body weight within 6 months preceding the chemoimmunotherapy initiation. Third, only Japanese patients were included in this study. Racial differences are unclear. Further research, including other races, could strengthen our findings.
In conclusion, cancer cachexia might be associated with a shorter PFS in patients with NSCLC who received chemoimmunotherapy. Further studies, including patients of other races, are needed to assess the effects of cancer cachexia in this patient population.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of each hospital, including the Kyoto Prefectural University of Medicine (Approval no. ERB-C-1803). Since this was a retrospective study, informed consent was waived, and an official website was used as an opt-out method, which was approved by the Ethics Committee of each hospital.
Availability of data and material
The datasets generated during the current study are not publicly available due to ethical constraints but are available from the corresponding author on reasonable request.
Supplemental Material
Download ()Acknowledgments
The authors sincerely appreciate all the physicians and patients who participated in this study. Additionally, we thank Editage (www.editage.jp) for helping with the English-language editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–9. doi:10.1056/NEJMoa1606774.
- Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers S, Salman P, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378:2093–2104. doi:10.1056/NEJMoa1801946.
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi:10.1056/NEJMoa1501824.
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob -J-J, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–1356. doi:10.1056/NEJMoa1709684.
- Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P, Porta C, George S, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi:10.1056/NEJMoa1712126.
- Kang YK, Boku N, Satoh T, Ryu M-H, Chao Y, Kato K, Chung HC, Chen J-S, Muro K, Kang WK, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi:10.1016/S0140-6736(17)31827-5.
- Ferris RL, Blumenschein BG, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi:10.1056/NEJMoa1602252.
- Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Hegg R, Im S-A, Shaw Wright G, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121. doi:10.1056/NEJMoa1809615.
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi:10.1056/NEJMoa1507643.
- Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E,et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi:10.1056/NEJMoa1504627.
- Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi:10.1016/S0140-6736(16)32517-X.
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi:10.1056/NEJMoa1801005.
- Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi:10.1056/NEJMoa1716948.
- Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi:10.1056/NEJMoa1810865.
- Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, Richardet E, Bennouna J, Felip E, Juan-Vidal O, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:198–211. doi:10.1016/S1470-2045(20)30641-0.
- Roch B, Coffy A, Jean-Baptiste S, Palaysi E, Daures J-P, Pujol J-L, Bommart S. Cachexia - sarcopenia as a determinant of disease control rate and survival in non-small lung cancer patients receiving immune-checkpoint inhibitors. Lung Cancer. 2020;143:19–26. doi:10.1016/j.lungcan.2020.03.003.
- Miyawaki T, Naito T, Kodama A, Nishioka N, Miyawaki E, Mamesaya N, Kawamura T, Kobayashi H, Omori S, Wakuda K, et al. Desensitizing effect of cancer cachexia on immune checkpoint inhibitors in patients with advanced NSCLC. JTO Clin Res Rep. 2020;1:100020. doi:10.1016/j.jtocrr.2020.100020.
- Turcott JG, Martinez-Samano JE, Cardona AF, Bassarmal SS, Ramírez-Tirado LA, Zatarain-Barrón ZL, Barrón F, Corrales L, Martín C, Barragán-Castillo PA, et al. The role of Cachexia Grading System in Patients with Non-Small Cell Lung Cancer Treated with Immunotherapy: implications for Survival. Nutr Cancer. 2021;73:794–801. doi:10.1080/01635581.2020.1769691.
- Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, et al. Cachexia: a new definition. Clin Nutr. 2008;27(6):793–799. doi:10.1016/j.clnu.2008.06.013.
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–495. doi:10.1016/S1470-2045(10)70218-7.
- Yoshioka H, Shimokawa M, Seto T, Morita S, Yatabe Y, Okamoto I, Tsurutani J, Satouchi M, Hirashima T, Atagi S,et al. Final overall survival results of WJTOG3405, a randomized phase III trial comparing gefitinib versus cisplatin with docetaxel as the first-line treatment for patients with stage IIIB/IV or postoperative recurrent EGFR mutation-positive non-small-cell lung cancer. Ann Oncol. 2019;30:1978–1984. doi:10.1093/annonc/mdz399.
- Fujimoto D, Miura S, Yoshimura K, Wakuda K, Oya Y, Yokoyama T, Yokoi T, Asao T, Tamiya M, Nakamura A, et al. Pembrolizumab plus chemotherapy-induced pneumonitis in chemo-naïve patients with non-squamous non-small cell lung cancer: a multicentre, retrospective cohort study. Eur J Cancer. 2021;150:63–72. doi:10.1016/j.ejca.2021.03.016.
- Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi:10.1038/bmt.2012.244.
- Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Fearon K, Hütterer E, Isenring E, Kaasa S,et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11–48. doi:10.1016/j.clnu.2016.07.015.
- Vanhoutte G, van de Wiel M, Wouters K, Sels M, Bartolomeeussen L, De Keersmaecker S, Verschueren C, De Vroey V, De Wilde A, Smits E, et al. Cachexia in cancer: what is in the definition? BMJ Open Gastroenterol. 2016;3:e000097. doi:10.1136/bmjgast-2016-000097.
- Anderson LJ, Albrecht ED, Garcia JM. Update on management of cancer-related cachexia. Curr Oncol Rep. 2017;19(1):3. doi:10.1007/s11912-017-0562-0.
- Lacour M, Hiltbrunner S, Lee SY, Soltermann A, Rushing EJ, Soldini D, Weder W, Curioni-Fontecedro A. Adjuvant Chemotherapy Increases Programmed Death-Ligand 1 (PD-L1) Expression in Non-small Cell Lung Cancer Recurrence. Clin Lung Cancer. 2019;20:391–396. doi:10.1016/j.cllc.2019.05.013.
- Lau SKM, Gannavarapu BS, Carter K, Gao A, Ahn C, Meyer JJ, Sher DJ, Jatoi A, Infante R, Iyengar P, et al. Impact of Socioeconomic Status on Pretreatment Weight Loss and Survival in Non-Small-Cell Lung Cancer. J Oncol Pract. 2018;14:e211–20. doi:10.1200/JOP.2017.025239.
- Takeda M, Kasai T, Naito M, Tamiya A, Taniguchi Y, Saijo N, Naoki Y, Okishio K, Shimizu S, Kojima K, et al. Programmed death-ligand 1 expression with clone 22C3 in non-small cell lung cancer: a single institution experience. Clin Med Insights Oncol. 2019;13:1179554918821314. doi:10.1177/1179554918821314.
- Li C, Liu J, Xie Z, Zhu F, Cheng B, Liang H, Li J, Xiong S, Chen Z, Liu Z, et al. PD-L1 expression with respect to driver mutations in non-small cell lung cancer in an Asian population: a large study of 1370 cases in China. Ther Adv Med Oncol. 2020;12:1758835920965840. doi:10.1177/1758835920965840.
- Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B, Wu X, Ma J, Zhou M, Li X, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18(1):10. doi:10.1186/s12943-018-0928-4.
- Yi M, Niu M, Xu L, Luo S, Wu K. Regulation of PD-L1 expression in the tumor microenvironment. J Hematol Oncol. 2021;14(1):10. doi:10.1186/s13045-020-01027-5.
- Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4(1):17105. doi:10.1038/nrdp.2017.105.
- Heinhuis KM, Ros W, Kok M, Steeghs N, Beijnen JH, Schellens JHM. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol. 2019;30(2):219–235. doi:10.1093/annonc/mdy551.
- Li JY, Chen YP, Li YQ, Liu N, Ma J. Chemotherapeutic and targeted agents can modulate the tumor microenvironment and increase the efficacy of immune checkpoint blockades. Mol Cancer. 2021;20:27. doi:10.1186/s12943-021-01317-7.
- Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2012;10:90–99. doi:10.1038/nrclinonc.2012.209.
- Kimura M, Naito T, Kenmotsu H, Taira T, Wakuda K, Oyakawa T, Hisamatsu Y, Tokito T, Imai H, Akamatsu H, et al. Prognostic impact of cancer cachexia in patients with advanced non-small cell lung cancer. Support Care Cancer. 2015;23(6):1699–1708. doi:10.1007/s00520-014-2534-3.
- Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014;14(11):754–762. doi:10.1038/nrc3829.
- Le-Rademacher J, Lopez C, Wolfe E, Foster NR, Mandrekar SJ, Wang X, Kumar R, Adjei A, Jatoi A. Weight loss over time and survival: a landmark analysis of 1000+ prospectively treated and monitored lung cancer patients. J Cachexia Sarcopenia Muscle. 2020;11:1501–1508. doi:10.1002/jcsm.12625.
- Takayama K, Atagi S, Imamura F, Tanaka H, Minato K, Harada T, Katakami N, Yokoyama T, Yoshimori K, Takiguchi Y, et al. Quality of life and survival survey of cancer cachexia in advanced non-small cell lung cancer patients-Japan nutrition and QOL survey in patients with advanced non-small cell lung cancer study. Support Care Cancer. 2016;24:3473–3480. doi:10.1007/s00520-016-3156-8.
- Cortellini A, Ricciuti B, Tiseo M, Bria E, Banna GL, Aerts JG, Barbieri F, Giusti R, Cortinovis DL, Migliorino MR, et al. Baseline BMI and BMI variation during first line pembrolizumab in NSCLC patients with a PD-L1 expression ≥ 50%: a multicenter study with external validation. J Immunother Cancer. 2020;8(2):e001403. doi:10.1136/jitc-2020-001403.
- Antoun S, Morel H, Souquet P-J, Surmont V, Planchard D, Bonnetain F, Foucher P, Egenod T, Krakowski I, Gaudin H, et al. Staging of nutrition disorders in non-small-cell lung cancer patients: utility of skeletal muscle mass assessment. J Cachexia Sarcopenia Muscle. 2019;10(4):782–793. doi:10.1002/jcsm.12418.