ABSTRACT
The standard of care for stage III non-small cell lung cancer (NSCLC) is chemoradiotherapy (CRT) followed by durvalumab. Although doses higher than 66 Gy are standard in our center, they were used in only 6.9% of patients in the PACIFIC trial. We report our experience with durvalumab after high-dose radiotherapy. The database of a tertiary hospital for patients with stage III NSCLC who were treated with CRT and adjuvant durvalumab was evaluated. Progression-free survival (PFS), overall survival (OS), and local-regional failure (LRF) were measured from the administration of durvalumab. Thirty-nine patients were included. All were treated with intensity-modulated radiation (mean dose 69.9 Gy); Median follow-up time was 20.4 months (range 1–35.4). At 12 months, PFS was 49%, OS 79%, and LRF 14%. Intrathoracic failure at first progression was demonstrated in 8 (21%) patients. Adverse events requiring corticosteroids occurred in 10(25.6%) patients: pneumonitis – 6 (15.4%), hepatitis – 2 (5.1%), and arthralgia and pericarditis – 1 (2.6%). One patient (2.6%) died of pneumonitis. The occurrence of pneumonitis was significantly associated with lung V5 (55% vs. 42%, p = .04) and V20 (28% vs. 19%, p = .01) and mean lung dose (14.8 Gy vs.11.6 Gy, p = .05). The similar 12-month PFS and OS rates of our cohort and the PACIFIC trial support the use of high-dose radiotherapy in patients with stage III NSCLC. Treatment-related mortality was similar to the PACIFIC results. The intrathoracic failure rate in our cohort was lower than that reported from the PACIFIC trial, suggesting that radiation dose escalation may improve local control.
Introduction
The treatment of locally advanced non-small cell lung cancer (NSCLC) has been rigorously debated. Early multiple phase II and one small randomized phase III trial suggested that chemotherapy concurrent with dose-escalated radiotherapy, made possible with the introduction of advanced technologies such as intensity-modulated radiotherapy (IMRT) and volumetric modulated arc therapy (VMAT), yielded an improved outcome compared to combined chemotherapy and standard-dose radiotherapy.Citation1–4 However, these claims were reversed in the RTOG 0617 trial of Bradley et al.Citation5 wherein 60 Gy of concurrent chemoradiotherapy not only provided improved local control over the higher 74 Gy dose but also led to better overall survival. As a consequence of these disappointing results, the standard of care for stage III NSCLC remained unchanged for 15 y. PD and PD-L1 blockers were shown in a number of trials to be effective in metastatic NSCLC.Citation6,Citation7 In 2017, this data led to a breakthrough, when the PACIFIC trial demonstrated an absolute 17% improvement in 18-months PFS with the administration of durvalumab as adjuvant treatment to standard concurrent chemoradiotherapy.Citation8 Later updates have shown similar improvements in OS which was durable up to 4 y after randomization.Citation9 Nevertheless, the intrathoracic response rate was not significantly increased over historical controls and intrathoracic progression was up to 38.5% of the durvalumab arm,Citation10 indicating that the majority of the benefit of durvalumab was in the prevention of distant disease.Citation9
Thus, it remains unclear if there is an interaction or an improvement in outcome with dose-escalated radiotherapy in the setting of adjuvant durvalumab. The aim of the present institutional study was to evaluate the effect of radiotherapy of >66 Gy followed by durvalumab on the outcome of patients with stage III NSCLC.
Patients and methods
Study cohort and setting
The pharmacy registry of a tertiary university-affiliated medical center was retrospectively searched for all patients treated with durvalumab following definitive radiotherapy for biopsy-proven AJCC stage III NSCLC, with or without concurrent chemotherapy, from January 2018 to June 2020. Patient data were collected from the complete electronic medical records. Each case had been reviewed by a multidisciplinary tumor board prior to treatment. Patients underwent standard fluorodeoxyglucose (FDG)-positron emission tomography (PET), magnetic resonance imaging (MRI) of the brain, and mediastinal staging with endobronchial ultrasound (EBUS) when appropriate. Patients with recurrent disease or malignant pleural effusion were excluded from the study as were patients who had prior antineoplastic systemic therapy or were scheduled for surgical resection.
The study was approved by the institutional regulatory board.
Systemic chemotherapy
Concurrent chemotherapy was given at the discretion of the treating oncologist. The predominate regimen used was a combination of cisplatin and etoposide (SWOG), as previously published.Citation11
Radiotherapy
All patients underwent computed tomography (CT) simulation with intravenous contrast when appropriate. T-bar and Vac-Lok bags were used for immobilization. Planning was done on EclipseTM, v. 13.5 (Varian, Palo Alto, CA, USA). The gross tumor volume (GTV) included all gross disease identified on FDG-PET. As the dose was escalated in these patients, a very minimal clinical target volume (CTV) of 2–3 mm was used. Expansion of the planning treatment volume (PTV) was based on four-dimensional CT or other respiratory excursion assessment. IMRT treatment plans were generated in all cases utilizing standard thoracic dose volume. PTV was optimized such that 95% of the planned dose covered 95% of the planned volume.
IGRT
All patients were treated with daily image-guided radiation therapy (IGRT) using daily kilo-voltage (KV) imaging and, with time, daily cone beam CT (CBCT).
Restaging and durvalumab therapy
In view of the results of the PACIFIC trial which encouraged a minimal time lag between completion of radiotherapy and initiation of durvalumab, patients underwent CT scanning 2–4 weeks after radiotherapy was completed to ensure lack of progressive disease (PD) and were then started on durvalumab therapy. Durvalumab was delivered intravenously per protocol at a dose of 10 mg/kg every 2 weeks for up to 12 months or to disease progression or unacceptable toxicity.
Patient follow-up and toxicity reporting
All patients were followed for local control and survival outcome until death or end of follow-up. Toxicity, both acute and late, was recorded and graded based on the Common Terminology Criteria for Adverse Events (CTCAE), v.5.0.
Statistical analysis
Oncological outcomes of progression-free survival (PFS), overall survival (OS), and locoregional failure (LRF) were analyzed using the Kaplan–Meier method and competing risks analysis, as needed. Cox regression analysis was used to assess associations between oncological outcomes and clinical characteristics. For associations between dosimetric parameters and treatment-related adverse events, the Mann–Whitney U-test was used.
Results
According to pharmacy registry, a total of 67 patients received durvalumab at our center during the study period. We identified 39 patients that were treated following definitive radiotherapy for stage III. The clinical characteristics of the patients and the treatment parameters are presented in , respectively. The chemotherapy regimen consisted of platinum doublets, with 25 (64%) and 13 (33%) with cisplatin and carboplatin doublets, respectively. Only one patient was treated without concurrent chemotherapy due to comorbidities, and two switched or stopped their chemotherapy due to toxicity. Mean radiation dose was 69.9 Gy. Prior stereotactic body radiotherapy (SBRT) for suspected lung lesions was given to two patients. Only one patient stopped the radiotherapy after a 56 Gy dose was given due to esophagitis. Median time to initiation of durvalumab was 2.2 months (range 0.6–5.3), and median number of cycles and duration of treatment were 21 (range 1–26) cycles and 10.2 (0.5–15.8) months. At the end of follow-up, 15 (38%) patients finished 1 y of adjuvant durvalumab, and 2 (5%) were still ongoing.
Table 1. Baseline characteristics of 39 patients with stage III NSCLC
Table 2. Treatment of 39 patients with stage III NSCLC
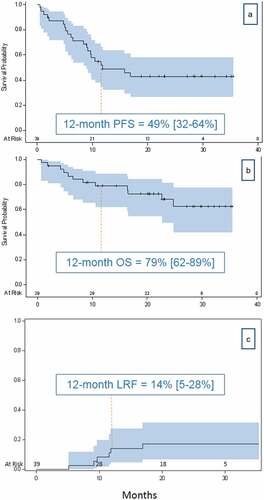
shows the oncological outcomes of the cohort. Median follow-up time was 20.4 months (range 1–35.4). The respective 1- and 2-y survival rates were as follows: PFS, 49% and 43%; OS, 79% and 68%; and LRF, 14% and 17%. Median PFS was 11.8 months; median OS was not reached. The complete metabolic response rate as defined by no viable tumor on PET CT was 18%. Univariate Cox regression analysis did not show a significant association of survival outcomes with any of the patient or treatment characteristics examined. The only exception was number of durvalumab cycles received (HR of 0.86 and 0.83 for PFS and OS, respectively, p < .001). Details are provided in .
Table 3. Univariate analysis of patient and treatment parameters and survival outcomes
Figure 1. Kaplan–Meier graphs of PFS (a) and OS (b) and a competing risks graph of LRF (c) with 95% confidence intervals. The competing risk for LRF is death
Imaging demonstrated first disease progression in 17 patients (43%): in the lung in 8 patients (47%), the brain in 5 (29%), and other sites in 4 (24%). Local therapy was administered in 9/17 patients (53%) and consisted of brain resection in 1, stereotactic radiosurgery in 4, and SBRT in 4. Median time to progression after local therapy was 8.6 months. A full explanation of patterns of failure is included in .
Table 4. Reported adverse events in 39 patients with stage III NSCLC during durvalumab therapy
The adverse events are detailed in . The most common reported toxicities (any grade) during durvalumab therapy were fatigue in 30 patients (77%), dyspnea in 28 (72%), and endocrine abnormalities in 18 (46%). Treatment-related adverse events requiring discontinuation of durvalumab and corticosteroids occurred in 10/39 (27%) patients after a median of 8 (1–21) cycles. Only 3/10 (30%) were able to successfully resume durvalumab and complete the full year of therapy. Patients without treatment-related toxicities received a significantly higher number of cycles of treatment (19.2 vs 14, p = .05). Occurrence of treatment-related toxicity was found to be non-significantly associated with poorer PFS and OS (HR 1.4, p = .45 and HR 2.4, p = .18) respectively.
Table 5. Patterns of failure
One patient (3%) patient died of pneumonitis. The occurrence of pneumonitis during durvalumab maintenance after chemoradiotherapy was significantly associated with a higher percentage of the lung volume receiving at least 5 Gy (V5) and 20 Gy (V20) relative to no pneumonitis (55% vs. 42%, p = .04 and 28% vs. 19%, p = .01, respectively), and higher mean lung dose (MLD) values (14.8 Gy vs.11.6 Gy, p = .05). The full dosimetry data are found in .
Discussion
Recent advances in active systemic treatments have led to an increasing importance of local therapy in breast, prostate, and other cancers, including NSCLC,Citation12–14 not only for local control but also for survival. The present study sought to answer the question raised by the results of the PACFIC trial: Does the decrease in distant failure rates with adjuvant immunotherapy serve as a rationale for aggressive local therapy?
A previous study in our institution showed that a radiation dose of 72 Gy with concurrent chemotherapy was safe and was associated with a remarkable 64% complete pathologic response rate.Citation15 Therefore, in the present trial, we chose to continue our in-house regimen followed by durvalumab, based on the consolidation of our own data with the data from the PACIFIC trial. Certainly, the question of high-dose radiotherapy in stage III NSCLC is controversial. The data from RTOG 0617 showed not only decreased overall survival with higher dose radiotherapy but also decreased local control.Citation5 This study is very important; however, it is not the only randomized trial asking this question. A previous smaller study by Yuan et al.Citation16 was positive for higher dose radiotherapy. In addition, other works have shown potential for improved outcome with higher doses as long as cardiac toxicity is minimized.Citation17,Citation18 Combining these data with our own results regarding metabolic CR as mentioned above, we felt it was highly important to examine the PACIFIC regimen with higher radiation doses.
Our findings demonstrated that the toxicity with this combination is acceptable. Treatment-related pneumonitis occurred in only 15% of cases with only one grade 5 event. These data are consistent with both the results of the PACIFIC trial as well as recently published real-world data from Memorial Sloan Kettering Cancer Center.Citation19 In addition, as expected, the standard dose volume metrics such as V20 and MLD correlated with the occurrence of pneumonitis in our cohort, suggesting that the classic relationship between dose volume metrics and toxicity was preserved and that durvalumab was not an additive factor.
The PACIFIC trial demonstrated 55.9% 1-y progression-free survival an 81.6% overall survivalCitation8,Citation9 in the experimental arm. Since the publication of those results, a number of groups have reported real-world data on the same patient population. Diselets et al.Citation20 recently reported a multicenter cohort study that demonstrated an impressive 92% 1-y overall survival. They specifically emphasized the importance of PDL >50%, which may be confirmed in future studies. Other groups have shown similar results in outcome with future concerns mainly for learning to manage toxicity associated with this regimen.Citation21–23
The 12-month outcome results in this study, namely, PFS 49%, OS 79%, and LRF 14%, are similar to other published studies using chemoradiotherapy and adjuvant durvalumab. What is noteworthy in our study is the 18% complete metabolic response rate compared to 1.5% in the PACIFIC trial.Citation10 This finding could suggest that in selected patients, high-dose radiotherapy combined with the PACIFIC regimen may lead to improved outcome. Further studies are needed to confirm this hypothesis.
Interestingly, thoracic failures in our cohort (both intrathoracic only and combined intra- and extra-thoracic) occurred in 8/39 (21%) patients and 8/17 (47%) of patients with evaluable progression. These are much lower than the pacific thoracic failure rates, which were reported by Raben et al.,Citation10 to be 38.5% in the entire intention-to-treat duravalumab arm, and 84% of patients with evaluable progression. While the in-field vs. out-of-field data are currently unpublished, this difference might suggest that better local control can be achieved with higher radiation doses as demonstrated in our cohort.
The present study was limited by the retrospective design and its inherent biases and the small size of the cohort which restricts the generalizability of the results. Nevertheless, our real-life results based on consecutive patients attending a large tertiary cancer center are hypothesis-generating.
In conclusion, the combination of high-dose chemoradiotherapy and adjuvant durvalumab is tolerable and leads to good results, warranting further investigation.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Schild SE, Hillman SL, Tan AD, Ross HJ, McGinnis WL, Garces YA, Graham DL, Adjei AA, Jett JR; Mayo Clinic. North central cancer treatment group. Long-term results of a trial of concurrent chemotherapy and escalating doses of radiation for unresectable non-small cell lung cancer: NCCTG N0028 (Alliance). J Thorac Oncol. 2017;12(4):697–7. doi:10.1016/j.jtho.2016.12.021.
- Rengan R, Rosenzweig KE, Venkatraman E, Koutcher LA, Fox JL, Nayak R, Amols H, Yorke E, Jackson A, Ling CC, et al. Improved local control with higher doses of radiation in large-volume stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;60(3):741–747. doi:10.1016/j.ijrobp.2004.04.013.
- Guckenberger M, Wilbert J, Richter A, Baier K, Flentje M. Potential of adaptive radiotherapy to escalate the radiation dose in combined radiochemotherapy for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;79(3):901–908. doi:10.1016/j.ijrobp.2004.04.013.
- Yuan S, Sun X, Li M, Yu J, Ren R, Yu Y, Li J, Liu X, Wang R, Li B, et al. A randomized study of involved-field irradiation versus elective nodal irradiation in combination with concurrent chemotherapy for inoperable stage III nonsmall cell lung cancer. Am J Clin Oncol. 2007;30(3):239–244. doi:10.1097/01.coc.0000256691.27796.24.
- Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, Bogart J, Hu C, Forster K, Magliocco A, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187–199. doi:10.1016/S1470-2045(14)71207-0.
- OAK Study Group; Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi:10.1016/S0140-6736(16)32517-X.
- Vokes EE, Ready N, Felip E, Horn L, Burgio MA, Antonia SJ, Arén Frontera O, Gettinger S, Holgado E, Spigel D, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol. 2018;29(4):959–965. doi:10.1093/annonc/mdy041.
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, De Wit M, et al. PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi:10.1056/NEJMoa1709937.
- Faivre-Finn C, Vicente D, Kurata T, Planchard D, Paz-Ares L, Vansteenkiste JF, Spigel DR, Garassino MC, Reck M, Senan S, et al. Four-year survival with durvalumab after chemoradiotherapy in stage III NSCLC-an update from the PACIFIC Trial. J Thorac Oncol. 2021;16(5):860–867. doi:10.1016/j.jtho.2020.12.015.
- Raben D, Rimner A, Senan S, Broadhurst H, Pellas T, Dennis PA, Faivre-Finn C. Patterns of disease progression with durvalumab in stage III non-small cell lung cancer (PACIFIC). Int J Radiat Oncol Biol Phys. 2019;105(3):683. doi:10.1016/j.ijrobp.2019.08.034.
- Gandara DR, Chansky K, Albain KS, Leigh BR, Gaspar LE, Lara PN Jr, Burris H, Gumerlock P, Kuebler JP, Bearden JD 3rd,, Southwest Oncology Group. Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non-small-cell lung cancer: phase II Southwest Oncology Group Study S9504. J Clin Oncol. 2003; 21(10): 2004–2010. doi: 10.1200/JCO.2003.04.197
- Punglia RS, Morrow M, Winer EP, Harris JR. Local therapy and survival in breast cancer. N Engl J Med. 2007;356(23):2399–2405. doi:10.1056/NEJMra065241.
- Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360(9327):103–106. doi:10.1016/s0140-6736(02)09408-4.
- Mitchell KG, Farooqi A, Ludmir EB, Corsini EM, Zhang J, Sepesi B, Vaporciyan AA, Swisher SG, Heymach JV, Zhang J, et al. Improved overall survival with comprehensive local consolidative therapy in synchronous oligometastatic non-small-cell lung cancer. Clin Lung Cancer. 2020;21(1):37–46 e37. doi:10.1016/j.cllc.2019.07.007.
- Allen AM, Shochat T, Flex D, Kramer E, Zer MR, Peled A, Dudnik N, Fenig E, Saute E. High-dose radiotherapy as neoadjuvant treatment in non-small-cell lung cancer. Oncology. 2018;95(1):13–19. doi:10.1159/000487928.
- Yuan S, Sun X, Li M, Yu J, Ren R, Yu Y, Li J, Liu X, Wang R, Li B, et al. A randomized study of involved-field irradiation versus elective nodal irradiation in combination with concurrent chemotherapy for inoperable stage III nonsmall cell lung cancer. Am J Clin Oncol. 2007;30(3):239–244. doi:10.1097/01.coc.0000256691.27796.24.
- Johnson MD, Sura K, Mangona VS, Glick A, Wallace M, Ye H, Grills IS. Matched-pair analysis of high dose versus standard dose definitive chemoradiation for locally advanced non-small-cell lung cancer. Clin Lung Cancer. 2017;18(2):149–155. doi:10.1016/j.cllc.2016.06.004.
- Zhao Q, Liu M, Wang Z, Huang W, Allen LX, Zhou T, Zhang J, Zhang Z, Wang Q, Yu S, et al. High dose radiation therapy based on normal tissue constraints with concurrent chemotherapy achieves promising survival of patients with unresectable stage III non-small cell lung cancer. Radiother Oncol. 2020;145:7–12. doi:10.1016/j.radonc.2019.11.024.
- Offin M, Shaverdian N, Rimner A, Lobaugh S, Shepherd AF, Simone CB 2nd, Gelblum DY, Wu AJ, Lee N, Kris MG, et al. Clinical outcomes, local-regional control and the role for metastasis-directed therapies in stage III non-small cell lung cancers treated with chemoradiation and durvalumab. Radiother Oncol. 2020;149:205–211. doi:10.1016/j.radonc.2020.04.047.
- Desilets A, Blanc-Durand F, Lau S, Hakozaki T, Kitadai R, Malo J, Belkaid W, Richard C, Messaoudene M, Cvetkovic, et al. Durvalumab therapy following chemoradiation compared with a historical cohort treated with chemoradiation alone in patients with stage III non-small cell lung cancer: a real-world multicentre study. Eur J Cancer. 2021;142:83–91. doi:10.1016/j.ejca.2020.10.008.
- Chu CH, Chiu TH, Wang CC, Chang WC, Huang AC, Liu CY, Wang CL, Ko HW, Chung FT, Hsu PC, et al. Consolidation treatment of durvalumab after chemoradiation in real-world patients with stage III unresectable non-small cell lung cancer. Thorac Cancer. 2020;11(6):1541–1549. doi:10.1111/1759-7714.13426.
- Faehling M, Schumann C, Christopoulos P, Hoffknecht P, Alt J, Horn M, Eisenmann S, Schlenska-Lange A, Schütt P, Steger F, et al. Durvalumab after definitive chemoradiotherapy in locally advanced unresectable non-small cell lung cancer (NSCLC): real-world data on survival and safety from the German expanded-access program (EAP). Lung Cancer. 2020;150:114–122. doi:10.1016/j.lungcan.2020.10.006.
- Jung HA, Noh JM, Sun JM, Lee SH, Ahn JS, Ahn MJ, Pyo H, Ahn YC, Park K. Real world data of durvalumab consolidation after chemoradiotherapy in stage III non-small-cell lung cancer. Lung Cancer. 2020;146:23–29. doi:10.1016/j.lungcan.2020.05.035.
