ABSTRACT
Various reports have pointed out the potential of cytokines as diagnostic and prognostic biomarkers for pancreatic ductal adenocarcinoma (PDA). Nonetheless, the evidence is contradictory and the role of chronic inflammation and relationship between circulatory and corresponding tumoral cytokine levels remain elusive. Utilizing a broad array of cytokines, we identified two opposing parameters: serum levels of interleukin 2 (IL2) and macrophage migration inhibitory factor (MIF) are diagnostic and prognostic factors. While low IL2 levels are associated with PDA, they also relate to a favorable prognosis of patients. In contrast, high MIF levels are associated with PDA and simultaneously related to an unfavorable outcome. MIF levels are associated with the intratumoral density of M2 macrophages (CD163+). Focusing on the tumor-to-serum gradient, we unveiled a different pattern of compartmental cytokine expression between IL2 and MIF. Our findings indicate that an extra-tumoral source of IL2 exists in PDA patients leading to increased detectability in the circulatory system. In case of MIF, the tumor microenvironment is presumably the main site of production and thereby reflected by serum measurements. Taken together, our study describes IL2 and MIF levels as biomarker candidates for diagnosis and prognosis of PDA, highlighting the need for compartmental cytokine analyses. From the perspective of tumor immunobiology, we identify multiple inflammatory states (proposed as types I–III) and see that systemic chronic dysregulation, independent of tumor microenvironment, can be measured and is a possible tool for stratification. Thus, direct correlation of local cytokine levels to peripheral blood levels needs to be regarded with caution.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDA) is a devastating disease and usually detected at a late stage, which contributes to the dismal 5-year survival rate of only 10%.Citation1 The sole potentially curative treatment for PDA is surgical resection, but at the time of diagnosis only 15–20% % qualify for surgery.Citation2,Citation3 To date, the most common diagnostic biomarker in clinical practice is carbohydrate 19–9 (CA 19–9). However, CA 19–9 has its detection value in symptomatic patients (often unresectable), but little clinical usefulness in screening asymptomatic patients.Citation4,Citation5
The identification of reliable parameters for early diagnosis as well as prognosis of PDA is an unmet need and pivotal to improve clinical decision-making and patient outcomes. Cytokines as inflammatory molecules play an important role in the development and progression of PDA, and tumor cell-intrinsic cytokines have the potential to shape local immunity.Citation6,Citation7 Originating from the tumor microenvironment, cytokines orchestrate various pro- and antitumoral functions and thereby potentially qualify as biomarkers for PDA.Citation8 Existing evidence demonstrates that a broad range of cytokines have a diagnostic or prognostic potential.Citation9 But previous data is heterogenous, for example, elevated as well as decreased circulatory interleukin 2 (IL2) levels have both been described as diagnostic markers for disease.Citation9–11 Another cytokine of interest is macrophage migration inhibitory factor (MIF), which is widely secreted by immune and nonimmune cells. MIF exists as a multimeric protein and has multiple functions, including inhibition of T cell activation and alteration of macrophage functionality and differentiation.Citation12,Citation13 Also, tumoral overexpression of MIF has been described in various malignancies (including PDA) being associated with poor prognosis, higher metastatic potential, and higher tumor burden.Citation14–18 However, uncertainty exists regarding the role of circulatory MIF levels and their diagnostic and prognostic value in PDA patients.
The relationship between circulatory cytokine levels and the corresponding tumoral immune landscape is another unresolved issue. Do serum levels of cytokines mirror cytokine expression in the tumoral microenvironment? This is an important question, as insights into the tumor-to-serum gradient of cytokines could improve the understanding of tumor-immune interactions in PDA patients. This also potentially can have predictive potential for circulating biomarkers. Also, chemotherapy or immunotherapeutic strategies commonly alter local cytokine networks. Thus, it is crucial to provide evidence regarding the interconnection of systemic and tumoral cytokine expression to allow proper interpretation of serum cytokine levels.
In this study, we systematically investigated the circulatory and corresponding tumoral levels of 50 cytokines on protein level as well as the local immune cell infiltration patterns of PDA patients. Our aim was to identify diagnostic and prognostic serum markers for PDA and further elucidate their relation to the tumoral immune landscape.
MATERIAL AND METHODS
Study design and patient cohort
The study was approved by the local ethics committee (301/2001) and written informed consent from all patients was obtained. Analysis and data collection follows the STROBE recommendations for observational studies.Citation19 The electronic pancreas database of the Department for General, Visceral and Transplantation Surgery at the University Hospital Heidelberg, which is maintained prospectively, was searched for patients undergoing a surgical procedure for suspected PDA between 03/2007 and 07/2011. Only previously untreated patients with available frozen tumor tissue samples and preoperatively obtained serum samples (according to the European Pancreas Center of the University Hospital Heidelberg) as well as available clinical baseline and outcome parameters were included. Blood samples from healthy controls were provided by the Blood bank of the University Hospital Heidelberg and used as reference.
Data collection and outcome parameters
The following baseline data were obtained from the prospectively maintained database: Gender, age, weight, body mass index, comorbidities (cardiovascular, pulmonary, renal, hepatic, autoimmune, diabetes), intake of glucocorticoids or immunosuppressive drugs. Preoperative serum values of white blood cells (WBC) and c-reactive protein (CRP) were extracted from the laboratory information system. Pathological reports included tumor grading, resection margin status and pTNM tumor stage according to the TNM Staging Manual, American Joint Committee on Cancer (AJCC).Citation20 Furthermore, overall survival in days was assessed.
Multiplex cytokine quantification
Multiplex cytokine quantification was performed on tissue lysates from cryopreserved pancreatic tumor tissues. Frozen pancreatic tumor tissues were collected and lysed (Bio-Plex Cell Lysis Kit, BioRad, USA; 171304011), afterward lysates were repeatedly vortexed, frozen (−80°C for 10 minutes), thawed (on ice) and ultrasonically bathed (for 10 minutes). This procedure was followed by centrifugation of the supernatants (13.000 rpm, for 20 minutes at 4°C). When blood samples were analyzed, no lysis was performed. Protein concentrations of all samples were determined (Pierce BCA Protein Assay, Thermo Fisher Scientific, Germany; 23227) and adjusted to 200 µg/ml. The concentrations were quantified by multiplex protein arrays, according to the instructions of the manufacturer (BioRad, USA). A two-laser array reader simultaneously quantifies all proteins of interest, allowing precise measurement of different soluble factors. The concentrations were calculated with Bio-Plex Manager 4.1.1 based on a 5-parameter logistic plot regression formula. Bio-Plex Pro Human Cytokine Screening Panel 48-plex (BioRad, USA; 12007283), Bio-Plex Pro Human Cytokine ICAM-1 (BioRad, USA; 171B6009M) and Bio-Plex Pro Human Cytokine VCAM-1 (BioRad, USA; 171B6022M) were used for cytokine quantification. This method of cytokine-based immune phenotyping has frequently been reported in the past with confirmed validity.Citation21,Citation22
Measurement of CRP and WBC
Blood samples of the PDA patients were analyzed in the Core Laboratory Facilities of the University Hospital Heidelberg for the count of WBC and CRP (normal range <5 mg/dl), according to accredited standards (DIN EN ISO 15189, D-ML-13060-01-00).
Laser capture microdissection
This procedure allows to isolate selected cell populations from a section of complex tissue under direct microscopic observation. Using PDA sections, tumoral and stromal compartment was isolated from each other by laser capture microdissection. This was necessary to assess the exclusive local cytokine concentration in the tumoral compartment. The procedure was performed according to the standard protocol of the inventers. Briefly, after focusing the tissue section (20-fold magnification), the tumoral and stromal area was manually separated using the Leica Laser Microdissection Software (Leica, Germany). The final dissection was performed with a carbon dioxide laser pulse.
Immunohistochemistry and whole-slide immune cell quantification
PDA tissue specimen were fixed in 4% phosphate buffered formaldehyde (ROTI Histofix, Roth, Germany; P087) and embedded in paraffin after placement in ethanol. Afterward, the samples were sectioned in 3 µm thick slices. The staining procedure itself was performed on a BOND-MAX (Leica, Germany). After deparaffinization, tissues were rehydrated (BOND Dewax Solution, Leica, Germany; AR9222). Then, heat-induced epitope retrieval (HIER) at 100°C (BOND Epitope Retrieval Solution 1 or 2, Leica, Germany; HIER 1 AR9961, HIER 2 AR9640) was applied and blocking of endogenous peroxidase activity was performed by incubation with 3% peroxide block for 20 minutes (BOND Polymer Refine Detection System, Leica, Germany; DS9800). Finally, the samples were blocked with 10% normal goat serum (Vector, USA; S-1000-20). PDA tissue specimens were analyzed by application of primary antibodies at room temperature for 30 minutes: CD3 (1:100, HIER 1, rabbit monoclonal, clone SP7, Abcam, UK; ab16669), CD4 (1:100, HIER 1, mouse monoclonal, clone 4B12, Leica, Germany; CD4-368-L-CE-H), CD8 (1:50, HIER 1, mouse monoclonal, clone 4B11, Leica, Germany, CD8-4B11-L-CE), CD20 (1:100, HIER 1, mouse monoclonal, clone L26, Leica, Germany; CD20-L26-L-CE), CD163 (1:500, HIER 2, rabbit monoclonal, clone EPR19518, Abcam, UK; ab182422), NKp46 (1:175, HIER 1, monoclonal mouse, clone 195314, R&D Systems, USA; MAB1850-500), FoxP3 (1:100, HIER 2, mouse monoclonal, clone 236A/E7, Thermo Fisher Scientific, Germany; 14–4777). The whole procedure was performed on a BOND-MAX (Leica, Germany) detecting the color reaction for 3,3-di-amino-benzidine with a BOND Polymer Refine Detection System (Leica, Germany) and counterstaining was performed with hematoxylin on a BOND Polymer Refine Detection System (Leica, Germany)
To quantify immune cells, whole-slide images were acquired with a Leica Aperio AT2 scanner (Leica, Germany). After scanning the slides at 40-fold magnification, they were examined using an image analysis software (VIS software suite, Visiopharm, Denmark). The tumoral tissue was manually annotated and quantification of immune cells was performed semi-automatically, as reported previously.Citation23–25
List of reagents
Statistical analyses
For non-paired samples, Mann Whitney or unpaired t tests were used. Paired samples were compared using Wilcoxon matched-pairs signed rank tests. The overall survival time was defined using the latest information. For survival analysis, the patients were dichotomized based on cytokine concentration. The patients were stratified into two groups (high and low) by using an unsupervised methodology and determining the median cutpoints (threshold for serum IL2: 11.0 pg/ml, for MIF: 3000 pg/ml). Survival curves were visualized using the Kaplan Meier estimators. The log-rank test was used to compare overall survival between patient groups. Differences were considered significant in case of a p-value ≤ and represented as follows: *: p ≤ 0.05, **p ≤ 0.005 and ***p ≤ 0.0001. All analyses were performed using the statistical software GraphPad Prism 8.4.1 software.
RESULTS
Patients and baseline characteristics
We received serum and tumor tissue samples from 48 PDA patients who underwent pancreatic surgery between March 2007 and July 2011. As provided in , the cohort consisted of 25 men (52%) and 23 women (48%), with a mean age of 66.3 (± 1.4). In all patients, the histopathological diagnosis was PDA, with 47 patients (98%) having the tumor status pT3 and 1 patient (2%) with pT4. None of the patients received prior neoadjuvant chemotherapy. The control cohort consisted of 30 healthy individuals with a mean age of 61 (± 1.5) and no known medical condition.
Table 1. Patient characteristics
IL2, MIF and other cytokines are potential diagnostic markers for patients with PDA
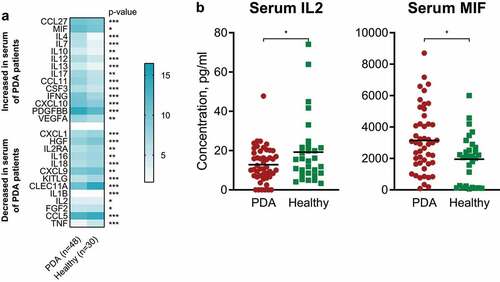
To investigate the differences of serum cytokine levels between PDA patients and healthy individuals, we evaluated the concentration of circulatory cytokines in blood samples of untreated PDA patients (n = 48) () and a control cohort of healthy individuals (n = 30).
In serum, differential cytokine patterns between PDA patients and healthy controls were observed. Decreased levels of IL2 were significantly associated with diagnosis of PDA ( and ). The same observation was made for CXCL1, HGF, IL2RA, IL16, IL18, CXCL9, KITLG, CLEC11A, IL1B, FGF2, CCL5 and TNF. On the other hand, increased levels of MIF, CCL27, IL4, IL7, IL10, IL12, IL13, IL17, CCL11, CSF3, IFNG, CXCL10, PDGFBB and VEGFA were significantly associated with PDA ( and ).
Figure 1. IL2, MIF and other cytokines are potential diagnostic markers for patients with PDA
Circulatory IL2 and MIF are associated with poor overall survival
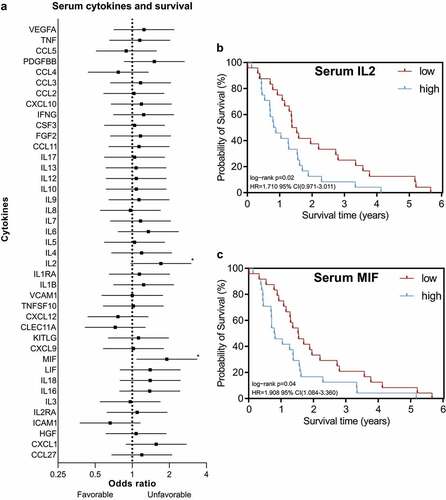
Next, the prognostic value of circulatory cytokine levels in the same cohort was investigated and parameters were analyzed in relation to the clinical outcome of patients.
Serum IL2 and MIF were significantly associated with poor overall survival ( and ). No other cytokine was associated with survival ().
Figure 2. Circulatory IL2 and MIF are associated with poor overall survival
Tumor-to-serum gradient of IL2 and MIF and their relationship to the tumoral immune landscape
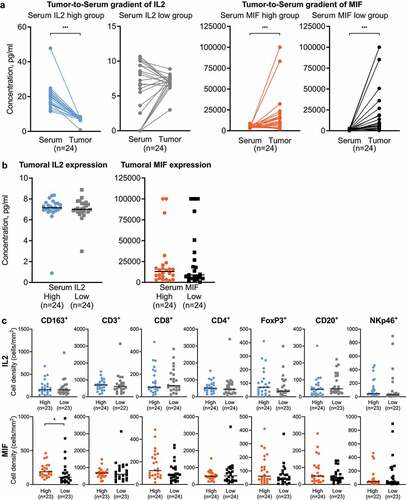
The identification of circulatory IL2 and MIF as a prognostic unfavorable parameter in PDA patients prompted us to study the corresponding tumoral IL2 and MIF expression, in order to investigate whether local cytokine expression in PDA consequently relates to systemic levels of IL2 and MIF. Also, we intended to assess whether circulatory IL2 and MIF levels relate to the local immune microenvironment. We analyzed the corresponding PDA tissues of our cohort, after performing a laser capture microdissection to separate tumor tissue from stroma.
In patients with high serum IL2 levels (unfavorable prognosis), we detected a significantly higher IL2 concentration in the serum than in the tumoral tissue, whereas in patients with low serum IL2 (favorable prognosis) no such gradient was detected (). In contrast, a significantly higher concentration of MIF in the tumoral tissue was detected in both groups (high and low serum MIF levels) (). Also, we found that the tumoral expression of IL2 and MIF did not differ between the corresponding low and high serum concentration groups (). Focusing on the quantities of intratumoral immune cells (CD3+, CD8+, CD4+, FoxP3+, CD20+, CD163+, NKp46+), we found that the cell count did not significantly vary in the low and high serum IL2 groups (). However, comparing the low and high serum MIF groups, we observed that significantly higher intratumoral CD163+ M2 macrophages were noted in patients with high serum MIF levels (unfavorable prognosis) ().
Figure 3. Tumor-to-Serum gradient of IL2 and MIF and their relationship to the tumoral immune landscape
Increased IL2 levels are not associated with systemic inflammation markers
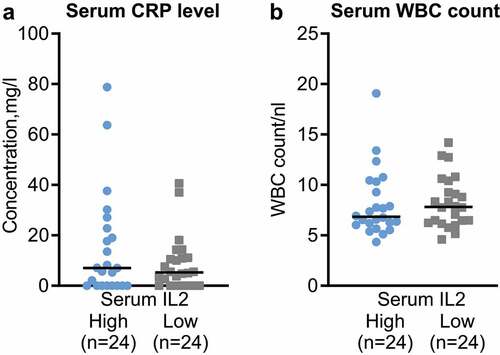
To investigate the link to systemic inflammation in PDA patients, we analyzed the c-reactive protein (CRP) and white blood cell (WBC) levels of the patients and compared the high and low serum IL2 groups.
This analysis demonstrated that the CRP and WBC levels did not significantly differ in the high and low serum IL2 cohort ( and ).
Figure 4. Increased IL2 levels are not associated with systemic inflammation markers
DISCUSSION
Cytokines are produced by a variety of different cell types, including immune and tumor cells. They have context-dependent pleiotropic functions and strongly mediate inflammation. Another characteristic is the redundancy of cytokines enabling them to exhibit overlapping functions in close proximity to the site of their production.Citation26,Citation27 Further, the production of high quantities of local cytokines can consequently lead to their entry into the circulatory system and detectability in the serum of patients.Citation28 However, while many cytokines have been described as diagnostic or prognostic biomarkers for PDA, the evidence is contradictory. Previous work highlighted that depending on the sample type (blood or tissue) inconsistent results regarding the diagnostic and prognostic value of cytokines were found.Citation9 To our knowledge, no systematic evaluation of cytokine levels and their corresponding tumoral expression has been reported so far. In this regard, different aspects need to be considered: A high production of a cytokine in the tumor microenvironment could lead to a “spillover” in the periphery. Also, a fundamental defect or aberration in systemic cytokine regulation could favor cancer growth or dissemination and therefore be present mainly in the circulatory system. The absence of a specific cytokine in the serum could serve as a functional or prognostic parameter. As cytokine level changes in the periphery can lead to subsequent further cytokine alterations, a complex regulatory pattern can emerge. Alternatively, a preexisting dysregulation of the immune system could augment tumor growth and local immunological immune dysregulation. This also would lead to a specific cytokine signature in the periphery but not inside the tumor microenvironment. We hereby propose a state model of immune dysregulation that encompasses a) tumor-driven direct dysregulation (type I) b) tumor-driven indirect dysregulation (via modulation of other organ sites, type II) and c) non-tumor-driven dysregulation (inflammatory or genetic driver for dysbalanced immunological setup, type III). Assessing these differential states holds the key to an improved understanding and translational possibilities. Detecting fundamental defects in immunobiology (see type III) beyond the direct/indirect tumor effects could help to stratify patients more effectively. Consequently, the knowledge of relationships between serum cytokine levels and local expression patterns will allow to adequately interpret serum measurements and utilize them as clinically useful parameters. Moreover, a deeper insight could pave the way for evaluating easily accessible serum cytokines as markers for e.g. treatment response in PDA, another major clinical need.
In our study, a large panel of cytokines was analyzed in the serum of PDA patients, which led to the identification of IL2 and MIF as significant diagnostic and prognostic markers in PDA. Protein measurements allow for robust quantification and excellent reproducibility, corroborating previous findings and allowing new insights. Both cytokines have different expression patterns regarding their tumor-to-serum gradient. While low serum levels of IL2 are related to the diagnosis of PDA, they also predict a better overall survival of patients. In PDA patients with high serum IL2 levels, a strong IL2 gradient toward the serum was detected indicating another source of IL2 upregulation than the pancreatic tumor tissue. Several immune cells have been shown to produce IL2 when activated, therefore lymphatic organs such as lymph nodes could be a potential alternative source of IL2 production in PDA patients.Citation29 IL2 is also commonly upregulated in systemic inflammation as driven by infections, autoimmune states or microbiome modulation.Citation30,Citation31 And CRP is associated with a worse outcome in PDA patients.Citation32,Citation33 In our cohort, CRP and WBC levels were not linked to high versus low levels of circulatory IL2. This indicates an independent mechanism for IL2 regulation. Another aspect is the multifaceted antitumoral function of IL2, e. g. historically used in metastatic melanoma and metastatic renal cancer.Citation34,Citation35 Still, we observed an unfavorable prognostic role of increased circulatory IL2 levels. In the past, low as well as high circulatory IL2 levels have been attributed with a diagnostic potential in PDA patients. However, the prognostic role of serum IL2 levels is unclear.Citation9 Given the generally reported anti-tumoral properties of IL2, the question arises why low circulatory levels are associated with better patient survival in our cohort. The key to this seems to lie in differential IL2 gradients, emphasizing the need to look beyond peripheral blood alone. Unfavorable prognosis is associated not only with high serum IL2 levels, but also with a significant tumor-to-serum IL2 gradient toward the serum. Coordination of immune responses is also orchestrated by compartmental differences in cytokine concentrations. Our results indicate that high serum IL2 levels could potentially “distract” local immune responses. Interestingly, corresponding tissue analysis revealed that neither local IL2 expression nor tumoral composition of immune cells did relate to serum IL2 levels in PDA patients. This finding suggests that alteration of circulatory IL2 levels is either due to indirectly tumor-driven (type II) or non-tumor-driven (type III) dysregulation. Further investigation is needed to unravel functional effects and to understand the relationship of extra-tumoral IL2 expression and outcome in PDA patients.
In case of MIF, we found that increased serum levels are related to a worse patient outcome. Contrary to IL2, a significant MIF gradient toward the tumor was detected in high and low serum MIF groups. However, in our cohort, tumoral MIF expression was not related to serum MIF levels. But still, circulatory MIF levels were associated with an increased density of intratumoral CD163+ M2 macrophages. These findings indicate that serum MIF levels -independently from their local expression- potentially allow conclusions to be drawn about the tumor microenvironment. In line with previous data, MIF is known to support the polarization of M2 macrophages contributing to an immunosuppressive and tumor-supporting microenvironment.Citation36–38 Notably, MIF exists in two conformational isoforms: oxidized MIF and reduced MIF. While reduced MIF is mainly found in the plasma of healthy individuals, oxidized MIF is primarily detected and upregulated in acute and chronic inflammatory states and solid tumor patients.Citation12,Citation39 However, in a clinical trial with an anti-oxidized-MIF antibody, no clinical benefit was seen in tumor patients. Therefore, we detected total MIF concentrations (= both isoforms) as an overall marker for MIF in the PDA tissue of patients.
Some limitations should be considered when interpreting our conclusions. We investigated a homogenous cohort of representative patients with resectable PDA, but our findings have to be interpreted with caution in unresectable disease or after neoadjuvant chemotherapy. Due to the limited number of patients (but with rather homogeneous clinical features) and only very small resulting subgroups the multivariate testing could not be performed reliably. Further, it also needs to be considered that the monocentric retrospective study design is exploratory.
All in all, circulatory IL2 and MIF levels are systematically altered in PDA patients and thereby are biomarker candidates for diagnosis and prognosis of disease. Our data further underlines the different tumor-to-serum gradients of IL2 and MIF. For IL2, apart from the local tumor tissue and systemic inflammation, another extra-pancreatic source seems to be the reason of increased serum expression in PDA patients. On the other hand, serum MIF levels possibly reflect the tumor microenvironment regarding the infiltration with tumor-supporting M2 macrophages. Our observations indicate that chronic inflammatory states have differential roles and varying consequences: either beneficial or detrimental for PDA patients. We propose the above mentioned three state model (type I–III) for classification of the inflammatory parameters. Prospective clinical trials are needed to validate our findings and further investigate regulatory mechanisms of IL2 and MIF in PDA patients. Hence, circulatory IL2 and MIF levels have to be interpreted with caution, especially regarding their ability to mirror their corresponding local expression. This possibly also applies to other cytokines, especially for clinical decision-making or biomarker studies. Our work highlights that the immunological context is not only governed by the composition of cytokines, but also their compartmentalization.
CONTRIBUTORS
Conceptualization, A.A. and N.H.; Methodology, A.A., S.K., F.L. and N.H.; Formal Analysis, A.A. and N.H.; Investigation, A.A. and R.K.; Resources, R.K., T.H., N.G., C.S., I.Z., D.J. and N.H.; Data Curation, A.A. and N.H.; Writing - Original Draft, A.A.; Writing - Review & Editing, A.A, R.K., S.K., N.G., C.S., I.Z., D.J. and N.H.; Visualization, A.A. and N.H.; Supervision, I.Z., D.J. and N.H.; Project Administration, A.A. and N.H.; Funding Acquisition, I.Z., D.J. and N.H.
Disclosure Statement
None declared.
DATA AVAILABILITY STATEMENT
All data are available upon request from the authors. All data relevant to the study are included in the article.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–9.
- Kardosh A, Lichtensztajn DY, Gubens MA, Kunz PL, Fisher GA, Clarke CA. Long-term survivors of pancreatic cancer: a California population-based study. Pancreas. 2018;47:958.
- Cress RD, Yin D, Clarke L, Bold R, Holly EA. Survival among patients with adenocarcinoma of the pancreas: a population-based study (United States). Cancer Causes & Control. 2006;17:403–409.
- Lee KIMJE. KT, Lee JK, Paik SW, Rhee JC, Choi KW. Clinical Usefulness of Carbohydrate Antigen 19-9 as a Screening Test for Pancreatic Cancer in an Asymptomatic Population Journal of Gastroenterology and Hepatology. 2004;19:182–186.
- Tong Y, Song Z, Zhu W. Study of an elevated carbohydrate antigen 19-9 concentration in a large health check-up cohort in China. Clinical Chemistry and Laboratory Medicine (CCLM). 2013;51:1459–1466.
- Greer JB, Whitcomb DC. Inflammation and pancreatic cancer: an evidence-based review. Curr Opin Pharmacol. 2009;9:411–418.
- Li J, Byrne KT, Yan F, Yamazoe T, Chen Z, Baslan T, Richman L, Lin J, Sun Y, Rech A, et al. Tumor cell-intrinsic factors underlie heterogeneity of immune cell infiltration and response to immunotherapy. Immunity. 2018;49(1):93–178. e7.
- Luo D, Kuang F, Du J, Zhou M, Peng F, Gan Y, Fang C, Yang X, Li B, Su S, et al. Characterization of the Immune Cell Infiltration Profile in Pancreatic Carcinoma to Aid in Immunotherapy. Front Oncol. 2021;11:1614.
- Yako YY, Kruger D, Smith M, Brand M. Cytokines as biomarkers of pancreatic ductal adenocarcinoma: a systematic review. PloS One. 2016;11:e0154016.
- Zhang P, Zou M, Wen X, Gu F, Li J, Liu G, Dong J, Deng X, Gao J, Li X, et al. Development of serum parameters panels for the early detection of pancreatic cancer. Int J of Cancer. 2014;134(11):2646–2655.
- Mroczko B, Groblewska M, Gryko M, Kędra B, Szmitkowski M. Diagnostic usefulness of serum interleukin 6 (IL‐6) and C‐reactive protein (CRP) in the differentiation between pancreatic cancer and chronic pancreatitis. J Clin Lab Anal. 2010;24:256–261.
- Thiele M, Kerschbaumer RJ, Tam FW, Völkel D, Douillard P, Schinagl A, Kuehnel H, Smith J, McDaid JP, Bhangal G, et al. Selective targeting of a disease-related conformational isoform of macrophage migration inhibitory factor ameliorates inflammatory conditions. The J of Immunology. 2015;195(5):2343–2352.
- Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800.
- Takano K, Sakata J, Hirose Y, Miura K, Ichikawa H, Nagahashi M, Shimada Y, Kameyama H, Kobayashi T, Wakai T, et al. Macrophage migration inhibitory factor expression predicts clinical outcomes in patients with resected pancreatic ductal adenocarcinoma. HPB. 2019;21:S424–S5.
- Wang D, Wang R, Huang A, Fang Z, Wang K, He M, Yia J, Li W. Upregulation of macrophage migration inhibitory factor promotes tumor metastasis and correlates with poor prognosis of pancreatic ductal adenocarcinoma. Oncol Rep. 2018;40(5):2628–2636.
- Meyer-Siegler KL, Iczkowski KA, Vera PL. Further evidence for increased macrophage migration inhibitory factor expression in prostate cancer. BMC Cancer. 2005;5:1–12.
- Funamizu N, Hu C, Lacy C, Schetter A, Zhang G, He P, Gaedcke J, Ghadimi MB, Ried T, Yfantis HG, et al. Macrophage migration inhibitory factor induces epithelial to mesenchymal transition, enhances tumor aggressiveness and predicts clinical outcome in resected pancreatic ductal adenocarcinoma. Int J of Cancer. 2013;132(4):785–794.
- Tomiyasu M, Yoshino I, Suemitsu R, Okamoto T, Sugimachi K. Quantification of macrophage migration inhibitory factor mRNA expression in non-small cell lung cancer tissues and its clinical significance. Clinical Cancer Research. 2002;8:3755–3760.
- Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577.
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474.
- Halama N, Spille A, Lerchl T, Brand K, Herpel E, Welte S, Keim S, Lahrmann B, Klupp F, Kahlert C, et al. Hepatic metastases of colorectal cancer are rather homogeneous but differ from primary lesions in terms of immune cell infiltration. Oncoimmunology. 2013;2(4):e24116.
- Halama N, Zoernig I, Berthel A, Kahlert C, Klupp F, Suarez-Carmona M, Suetterlin T, Brand K, Krauss J, Lasitschka F, et al. Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by anti-CCR5 therapy in cancer patients. Cancer Cell. 2016;29(4):587–601.
- Halama N, Zoernig I, Spille A, Westphal K, Schirmacher P, Jaeger D, Grabe N. Estimation of immune cell densities in immune cell conglomerates: an approach for high-throughput quantification.PloS One. 2009;4(11):e7847.
- Halama N, Zoernig I, Spille A, Michel S, Kloor M, Grauling-Halama S, Westphal K, Schirmacher P, Jaeger D, Grabe N. Quantification of prognostic immune cell markers in colorectal cancer using whole slide imaging tumor maps. Anal Quant Cytol Histol. 2010;32(6):333–340.
- Halama N, Braun M, Kahlert C, Spille A, Quack C, Rahbari N, Koch M, Weitz J, Kloor M, Zoernig, et al. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin Cancer Res. 2011;17(4):678–689.
- Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. New England J of Medicine. 2006;354:610–621.
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820.
- Mack CL. Serum cytokines as biomarkers of disease and clues to pathogenesis. Hepatology. 2007;46(1):6-8.
- Owen DL, Mahmud SA, Vang KB, Kelly RM, Blazar BR, Smith KA, Farrar MA. Identification of cellular sources of IL-2 needed for regulatory T cell development and homeostasis. The J of Immunology. 2018;200(12):3926–3933.
- Blander JM, Longman RS, Iliev ID, Sonnenberg GF, Artis D. Regulation of inflammation by microbiota interactions with the host. Nat Immunol. 2017;18:851–860.
- Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, Ter Horst R, Jansen T, Jacobs L, Bonder MJ, et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. 2016;167(4):36–1125. e8.
- Szkandera J, Stotz M, Absenger G, Stojakovic T, Samonigg H, Kornprat P, Schaberl-Moser R, Alzoughbi W, Lackner C, Ress AL, et al. Validation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. Br J Cancer. 2014;110(1):183–188.
- Arenas-Ramirez N, Woytschak J, Boyman O. Interleukin-2: biology, design and application. Trends Immunol. 2015;36:763–777.
- Jiang T, Zhou C, Ren S. Role of IL-2 in cancer immunotherapy. Oncoimmunology. 2016;5:e1163462.
- Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Pérez-Gracia JL, Rodríguez-Ruiz ME, Ponz-Sarvise M, Castanón E, Melero I, et al. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2019;120(1):6–15.
- Yaddanapudi K, Putty K, Rendon BE, Lamont GJ, Faughn JD, Satoskar A, Lasnik A, Eaton JW, Mitchell RA. Control of tumor-associated macrophage alternative activation by macrophage migration inhibitory factor. The J of Immunology. 2013;190(6):2984–2993.
- Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol. 2017;10:1–12.
- Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108(4):914–923.
- Mahalingam D, Patel MR, Sachdev JC, Hart LL, Halama N, Ramanathan RK, Sarantopoulos J, Voelkel D, Youssef A, de Jong FA, et al. Phase I study of imalumab (BAX69), a fully human recombinant antioxidized macrophage migration inhibitory factor antibody in advanced solid tumours. Br J Clin Pharmacol. 2020;86(9):1836–1848.
