ABSTRACT
Immunogenic cell death (ICD) has initially been discovered in the context of chemotherapy. High-dose crizotinib also stimulates ICD, as we described for non-small cell lung cancer lacking activating chromosomal aberrations of ALK or ROS1, the usual targets of crizotinib, indicating that crizotinib may act through off-target effects. However, we found that low-dose of ALK inhibitors, crizotinib and ceritinib, may stimulate ICD in anaplastic large cell lymphoma, in which ALK is activated due to a chromosomal translocation, suggesting on target ICD-promoting effects.
Cancer does not develop without the failure of immunosurveillance.Citation1 Accordingly, the clinical efficacy of cancer therapies heavily relies on the (re)establishment of anticancer immune responses. Immunogenic cell death (ICD) is an immunologically “noisy” modality of cell death accompanied by the exposure or release of danger-associated molecular patterns (DAMPs) that alert innate immune effectors (mostly dendritic cells) to finally launch a cognate immune response (mostly mediated by cytotoxic T lymphocytes) against dead-cell antigens.Citation2,Citation3 Prominent ICD-linked DAMPs include adenosine triphosphate (ATP, which is released during cell death), calreticulin (CALR, which is exposed on the cell surface at a premortem stage), high mobility group box 1 (HMGB1, which exits the nucleus and is released from cells as they succumb) and type-I interferons (which are actively synthesized and activate other downstream genes including genes coding for chemokines to favor an immune response).Citation2 ICD was initially discovered in the context of cytotoxic anticancer chemotherapies.Citation4 However, it is important to note that only a fraction of cytotoxicants is able to induce ICD, correlating with their clinical long-term efficacy against cancer as well as with their capacity to inhibit DNA-to-RNA transcription.Citation5
Several years ago, we found that crizotinib, a tyrosine kinase inhibitor used for the treatment of cancers with oncogenic activating inversions or translocations of anaplastic lymphoma kinase (ALK, which is activated in anaplastic large cell lymphoma, ALCL, as well as in a fraction of lung adenocarcinomas) and ROS proto-oncogene 1 (ROS1, which is activated in another small fraction of non-small cell lung cancers) can be employed at relatively high doses (≥10 µM) to induced ICD in lung adenocarcinoma cells as well as in unrelated malignant cell lines (such as skin fibrosarcomas) that lack activating mutations of ALK and ROS1, meaning that these effects must be considered as “off-target”.Citation6 Indeed, high-dose crizotinib turned out to inhibit DNA-to-RNA transcription.Citation5 Thus, in preclinical models, high-dose crizotinib can be advantageously combined with chemotherapy and immunotherapy to eradicate established lung cancers.Citation6
Recently, we examined the possibility that crizotinib might exert “on-target” effects as well, by treating ALCL cell lines, which, by definition, are addicted to the oncogenic action of ALK. We found that low-dose crizotinib (≤5 µM) as well as comparably low doses of an alternative ALK inhibitor, ceritinib, both induced the whole spectrum of ICD hallmarks including ATP secretion, CALR exposure, HMGB1 release and the activation of a type-I interferon response in human and mouse cell lines.Citation7 In vivo, in preclinical models, established ALCL responded to ceritinib. The duration of the response depended on the immune system, meaning that ALCL evolving in immunocompetent mice responded much better to ceritinib than ALCL implanted into immunodeficient strains. Importantly, mouse ALCL cells treated with ceritinib in vitro were capable of inducing a protective anti-ALCL immune response when they were injected subcutaneously into immunocompetent mice, thus slowing down the growth of live ALCL cells injected two weeks later.Citation7
The fact that low-dose ALK inhibitors can induce ICD in ALK-dependent cell lines pleads in favor of an “on-target” effect ()). However, to ascertain that this interpretation is correct, we performed a series of additional experiments that confirm the “on-target” effects of the two ALK inhibitors crizotinib and ceritinib (). Thus, we used small hairpin RNAs (shRNAs) to reduce the expression of ALK, showing that this genetic (as opposed to pharmacological) manipulation was able to induce the stigmata of ICD in ALK-dependent ALCL cells ()). In addition, we found that pharmacological inhibition of downstream effectors of ALK such as phosphoinositide 3-kinases induced the hallmarks of ICD in ALCL cells ()). Finally, we demonstrated that ALCL cells that had been selected for crizotinib resistance showed reduced signs of ICD-associated DAMP release or exposure ()). Altogether, these results plead in favor of an “on-target” ICD-inducing effect of ALK inhibitors.Citation7
Figure 1. On-target effects of ALK inhibitors inducing ICD. The scheme recapitulates the major arguments suggesting that crizotinib and ceritinib indeed stimulate ICD via on-target effects, namely (a) low-dose effects that are expected to inhibit ALK but not any other tyrosine kinase; (b) mimicry by genetic inhibition of ALK, (c) mimicry by pharmacological inhibition of down-stream targets and (d) abolition of ICD-inducing effects after selection for resistance
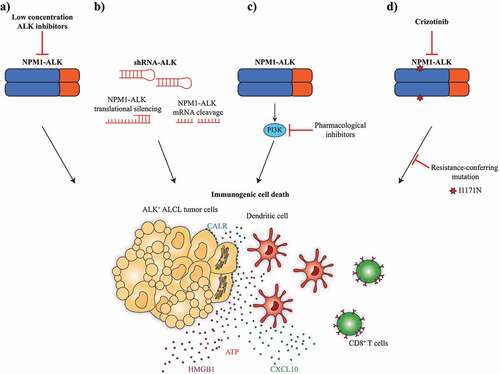
Altogether, these results suggest that inhibition of a trophic receptor tyrosine kinases such as ALK can induce ICD. In a way, these results are reminiscent of the observation that the inhibition of insulin growth factor-1 receptor (IGF1R), which is another receptor tyrosine kinase,Citation8,Citation9 or that of its downstream effector protein kinase B (PKB, best known as AKT)Citation10 can favor anticancer immune responses as well. However, in this latter case the inhibition of IGF1R or PKB/AKT is not sufficient to achieve anticancer immune responses. Indeed, pharmacological inhibitors of IGF1R (such as picropodophyllin and linsitinib) or PKB/AKT (such as isobacachalcone) were unable to reduce tumor growth in preclinical models unless they were combined with ICD-inducing chemotherapeutic agents such as oxaliplatin, alone or in combination with PD-1 blockade.Citation8–10 These examples suggest that tyrosine kinase inhibitors may trigger ICD either as standalone agents or in combination with other antineoplastic agents.
In summary, depending on the cellular and molecular context, tyrosine kinase inhibitors can induce ICD via “on-target” or “off-target” effects, alone or in combination with other therapeutic agents. It will be important to understand the detailed molecular rules that dictate this dual dichotomy. Irrespective of these uncertainties, it appears clear that future research on this class of “targeted” antineoplastic agents cannot neglect the immunological facets of their mode of action.
Disclosure Statement
GK is a co-founder of everImmune, Samsara Therapeutics and Therafast Bio. All remaining authors indicate that there is no potential conflict of interest.
Additional information
Funding
References
- López-Otín C, Kroemer G. Hallmarks of Health. Cell. 2021;184(1):33–3. doi:10.1016/j.cell.2020.11.034.
- Fucikova J, Kepp O, Kasikova L, Petroni G, Yamazaki T, Liu P, Zhao L, Spisek R, Kroemer G, Galluzzi L. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020;11(11):1013. doi:10.1038/s41419-020-03221-2.
- Zitvogel L, Perreault C, Finn OJ, Kroemer G. Beneficial autoimmunity improves cancer prognosis. Nat. Rev. Clin. Oncol. 2021 Sept;18(9):591–602. doi:10.1038/s41571-021-00508-x.
- Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007;13(1):54–61. doi:10.1038/nm1523.
- Humeau J, Sauvat A, Cerrato G, Xie W, Loos F, Iannantuoni F, Bezu L, Lévesque S, Paillet J, Pol J, et al. Inhibition of transcription by dactinomycin reveals a new characteristic of immunogenic cell stress. EMBO Mol. Med. 2020;12(5):e11622. doi:10.15252/emmm.201911622.
- Liu P, Zhao L, Pol J, Levesque S, Petrazzuolo A, Pfirschke C, Engblom C, Rickelt S, Yamazaki T, Iribarren K, et al. Crizotinib-induced immunogenic cell death in non-small cell lung cancer. Nat. Commun. 2019. doi:10.1038/s41467-019-09415-3.
- Petrazzuolo A, Perez-Lanzon M, Martins I, Liu P, Kepp O, Minard-Colin V, Maiuri MC, Kroemer G. Pharmacological inhibitors of anaplastic lymphoma kinase (ALK) induce immunogenic cell death through on-target effects. Cell Death Dis. 2021;12(8):713. doi:10.1038/s41419-021-03997-x.
- Wu Q, Tian A-L, Li B, Leduc M, Forveille S, Hamley P, Galloway W, Xie W, Liu P, Zhao L, et al. IGF1 receptor inhibition amplifies the effects of cancer drugs by autophagy and immune-dependent mechanisms. J. Immunother. Cancer 2021;9(6):6. doi:10.1136/jitc-2021-002722.
- Wu Q, Tian A-L, Kroemer G, Kepp O. Autophagy indu ction by IGF1R inhibition with picropo dophyllin and linsit inib. Autophagy. 2021:Jun:1–2. doi:10.1080/15548627.2021.1936934.
- Wu Q, Tian A-L, Durand S, Aprahamian F, Nirmalathasan N, Xie W, Liu P, Zhao L, Zhang S, Pan H, et al. Isobacachalcone induces autophagy and improves the outcome of immunogenic chemotherapy. Cell Death Dis. 2020;11(11):1015. doi:10.1038/s41419-020-03226-x.
