ABSTRACT
Sequential combination of immunogenic cell death (ICD)-induced interventions with subsequent immunotherapy has shown efficacy in preclinical models and clinical evaluation. Recently, a clinical trial enrolling small cell lung cancer patients treated with amrubicin together with PD-1 blockade confirmed the notion that ICD sensitizes tumors to immune checkpoint inhibitors.
KEYWORDS:
The long-term efficacy of anticancer therapies depends on the reinstatement of immunosurveillance by adaptive immune circuitries and the subsequent generation of immunological memory against tumor-associated antigens (TAAs). Anticancer immunity in cancer patients can be reinstated by chemotherapy- or radiotherapy-elicited immunogenic cell death (ICD). Therapeutic exposure to antineoplastic agents, such as anthracyclines, oxaliplatin, crizotinib, or lubinectidin, all are clinically approved for the treatment of a variety of cancer, can stimulate the tumor to emit danger-associated molecular patterns (DAMPs).Citation1–3 Via the ligation of pattern recognition receptors (PRR) expressed on antigen presenting cells, DAMPs act as immunological adjuvant signals and ultimately stimulate TAA-specific anticancer immune responses. Thus, the lysosomal secretion of ATP by tumor cells induces the chemotactic attraction of dendritic cells that express purinergic receptor P2X7 (P2RX7). Annexin A1 (ANXA1) released from dying cancer cells can ligate formyl peptide receptor 1 (FPR1) on such DCs, guiding them toward apoptotic malignant cells. Exposure of calreticulin serves as an eat-me signal and stimulates TAA uptake via LDL-receptor-related protein 1 (LRP1, best known as CD91) expressed by DC. Exodus of high mobility group box 1 (HMGB1) stimulates TAA processing via the toll like receptor 4 (TLR4) axis and facilitates MHC class I-restricted cross-presentation of TAA by DC. Together with a type-1 interferon (IFN) response and C-X-C motif chemokine ligand 10 (CXCL10)-mediated stimulation, this ultimately leads to the priming of T cells and the clonal expansion of cancer-specific cytotoxic T lymphocytes.Citation4,Citation5 Further mechanism that are implicated in the regulation of cancer immunogenicity are complex and include the induction of a DNA damage response, the onset of ER stress and the release of mitochondrial DNA.Citation6–8
There is ample preclinical evidence that the induction of ICD can sensitize models of established lung cancer to subsequent immunotherapy with immune check-point blockade in mice. Thus, the tyrosine kinase inhibitor crizotinib, which induces ICD through off-target effects, yields a 90% cure rate in a model of orthotopic non-small cell lung cancers (NSCLC), when combined with standard-of-care chemotherapy and subsequent PD-1-based immune checkpoint blockade.Citation9 Consistently, in mice bearing genetically induced KRAS+/TP53− NSCLC, the ICD-induced agent oxaliplatin enhances the effect of subsequent CTLA-4 and PD-1-checkpoint inhibition.Citation10 In both studies, the frequency of tumor-infiltrating immune effectors increased over that of immunosuppressive cells, indicating that the induction of ICD converted ‘cold’ into ‘hot’ tumors, rendering them susceptible to subsequent immune checkpoint blockade.
Several clinical reports support the hypothesis that ICD-induced treatments sensitize malignant neoplasms to subsequent immunotherapy with immune checkpoint blockade.Citation11 NSCLC patients that received a combination of standard of care chemotherapy with immunostimulatory irradiation together with subsequent PD-L1 blockade exhibited a significantly higher overall survival than the placebo group. Thus, the combination of standard-of-care chemotherapy with immunogenic irradiation and immune checkpoint blockade was more efficient than chemoradiotherapy alone.Citation12 Furthermore, induction therapy followed by PD-1 blockade in metastatic triple-negative breast cancer patients yielded the highest objective response rate (35% as compared to 20% in the overall cohort) when the women were initially treated with the ICD-induced anthracycline doxorubicin.Citation13 Recently, the notion that ICD stimulates tumors to immune checkpoint blockade was corroborated by another study that showed the efficacy of the anthracycline amrubicin (AMR) in combination with anti-PD-1 targeting immunotherapy in patients with relapsed small cell lung cancer (SCLC).Citation14 Here, the overall response rate (ORR) was 52% with a median progression-free survival (PFS) of 4 months and a PFS rate at 1 year of 14.4%.Citation14 This warrants further investigation and supports the rationale of other studies that combine immunogenic chemotherapy with immune checkpoint blockade such as the ongoing clinical trial NCT04253145 employing the immunogenic agent lurbinectedin with anti-PD-L1 in SCLC (). Similar to chemotherapy, induction of ICD by radiotherapy has been linked to increased sensitivity to immune checkpoint blockade in various preclinical settingsCitation15–17 as well as in some clinical trials targeting lung cancer (notably the PACIFIC studyCitation18 and a recent study by Altorki and colleagues).Citation19
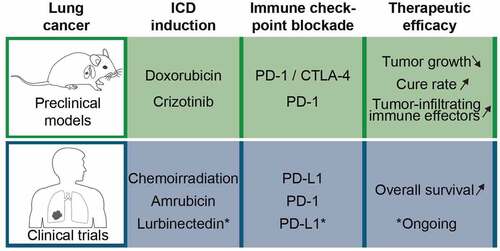
Figure 1. Pre-clinical and clinical evidence for the efficacy of immunogenic chemotherapy plus immune checkpoint blockade in lung cancer. In mouse studies, the induction of immunogenic cell death (ICD) by crizotinib or doxorubicin sensitizes established orthotopic lung cancers to subsequent immunotherapy with immune checkpoint blockade. Consistently, non-small cell lung cancer patients responded to the combination of ICD-inducing chemoradiotherapy or amrubicin with immune checkpoint blockade by an improved overall survival. An ongoing trial evaluates the combination of lurbinectedin with PD-L1 targeting immunotherapy against small cell lung cancers
A growing literature now points to the critical importance of treatment sequencing for immuno-oncology regimens, especially when it comes to ICD inducers and immune checkpoint inhibitors.Citation20–24 In sum, ample clinical evidence supports the concept of an ICD-mediated sensitization to immune checkpoint blockade in treatment-resistant diseases such as lung cancer. We anticipate that future clinical trials will establish optimal combination regimens that will further increase efficacy while reducing chemotherapy-associated side effects.
Disclosure statement
GK and OK are cofounders of Samsara Therapeutics. GK is a cofounder of Therafast Bio.
Additional information
Funding
References
- Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–3. doi:10.1038/nri.2016.107.
- Kepp O, Zitvogel L, Kroemer G. Lurbinectedin: an FDA-approved inducer of immunogenic cell death for the treatment of small-cell lung cancer. Oncoimmunology. 2020;9:1795995. doi:10.1080/2162402X.2020.1795995.
- Liu P, Zhao L, Kepp O, Kroemer G. Crizotinib - a tyrosine kinase inhibitor that stimulates immunogenic cell death. Oncoimmunology. 2019;8:1596652. doi:10.1080/2162402X.2019.1596652.
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi:10.1146/annurev-immunol-032712-100008.
- Yamazaki T, Galluzzi L. Mitochondrial control of innate immune signaling by irradiated cancer cells. Oncoimmunology. 2020;9:1797292. doi:10.1080/2162402X.2020.1797292.
- Sprooten J, Agostinis P, Garg AD. Type I interferons and dendritic cells in cancer immunotherapy. Int Rev Cell Mol Biol. 2019;348:217–262. doi:10.1016/bs.ircmb.2019.06.001.
- Wu Chuang A, Kepp O, Kroemer G, Bezu L. Endoplasmic reticulum stress in the cellular release of damage-associated molecular patterns. Int Rev Cell Mol Biol. 2020;350:1–28. doi:10.1016/bs.ircmb.2019.11.006.
- Chabanon RM, Rouanne M, Lord CJ, Soria JC, Pasero P, Postel-Vinay S. Targeting the DNA damage response in immuno-oncology: developments and opportunities. Nat Rev Cancer. 2021. doi:10.1038/s41568-021-00386-6.
- Liu P, Zhao L, Pol J, Levesque S, Petrazzuolo A, Pfirschke C, Engblom C, Rickelt S, Yamazaki T, Iribarren K, et al. Crizotinib-induced immunogenic cell death in non-small cell lung cancer. Nat Commun. 2019;10:1486. doi:10.1038/s41467-019-09415-3.
- Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, Yamazaki T, Poirier-Colame V, Newton A, Redouane Y, et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity. 2016;44:343–354. doi:10.1016/j.immuni.2015.11.024.
- Kepp O, Zitvogel L, Kroemer G. Clinical evidence that immunogenic cell death sensitizes to PD-1/PD-L1 blockade. Oncoimmunology. 2019;8:e1637188. doi:10.1080/2162402X.2019.1637188.
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, De Wit M, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–2350. doi:10.1056/NEJMoa1809697.
- Voorwerk L, Slagter M, Horlings HM, Sikorska K, van de Vijver KK, de Maaker M, Nederlof I, Kluin RJC, Warren S, Ong S, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25:920–928. doi:10.1038/s41591-019-0432-4.
- Akamatsu H, Teraoka S, Hayashi H, Fujimoto D, Hayata A, Haratani K, Ozawa Y, Yoshida T, Iwasa T, Shimokawa T, et al. Pembrolizumab plus amrubicin in patients with relapsed SCLC: multi-institutional, single-arm phase 2 study. JTO Clin Res Rep. 2021;2:100184. doi:10.1016/j.jtocrr.2021.100184.
- Yamazaki T, Kirchmair A, Sato A, Buqué A, Rybstein M, Petroni G, Bloy N, Finotello F, Stafford L, Navarro Manzano E, et al. Mitochondrial DNA drives abscopal responses to radiation that are inhibited by autophagy. Nat Immunol. 2020;21:1160–1171. doi:10.1038/s41590-020-0751-0.
- Pilones KA, Hensler M, Daviaud C, Kraynak J, Fucikova J, Galluzzi L, Demaria S, Formenti SC. Converging focal radiation and immunotherapy in a preclinical model of triple negative breast cancer: contribution of Vista blockade. Oncoimmunology. 2020;9:1830524. doi:10.1080/2162402X.2020.1830524.
- Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. doi:10.1158/1078-0432.CCR-09-0265.
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, De Wit M, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–1929. doi:10.1056/NEJMoa1709937.
- Altorki NK, McGraw TE, Borczuk AC, Saxena A, Port JL, Stiles BM, Lee BE, Sanfilippo NJ, Scheff RJ, Pua BB, et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial. Lancet Oncol. 2021;22:824–835. doi:10.1016/S1470-2045(21)00149-2.
- Hashizume R, Zhang A, Mueller S, Prados MD, Lulla RR, Goldman S, Saratsis AM, Mazar AP, Stegh AH, Cheng SY, et al. Inhibition of DNA damage repair by the CDK4/6 inhibitor palbociclib delays irradiated intracranial atypical teratoid rhabdoid tumor and glioblastoma xenograft regrowth. Neuro Oncol. 2016;18:1519–1528. doi:10.1093/neuonc/now106.
- Petroni G, Buqué A, Yamazaki T, Bloy N, Di Liberto M, Chen-Kiang S, Formenti SC, Galluzzi L. Radiotherapy delivered before CDK4/6 inhibitors mediates superior therapeutic effects in ER(+) breast cancer. Clin Cancer Res. 2021;27:1855–1863. doi:10.1158/1078-0432.CCR-20-3871.
- Wang Y, Liu S, Yang Z, Algazi AP, Lomeli SH, Wang Y, Othus M, Hong A, Wang X, Randolph CE, et al. Anti-PD-1/L1 lead-in before MAPK inhibitor combination maximizes antitumor immunity and efficacy. Cancer Cell. 2021;39:1375–1387 e1376. doi:10.1016/j.ccell.2021.07.023.
- Ma J, Benitez JA, Li J, Miki S, Ponte de Albuquerque C, Galatro T, Orellana L, Zanca C, Reed R, Boyer A, et al. Inhibition of nuclear PTEN tyrosine phosphorylation enhances glioma radiation sensitivity through attenuated DNA repair. Cancer Cell. 2019;35:504–518 e507. doi:10.1016/j.ccell.2019.01.020.
- Truman JP, García-Barros M, Kaag M, Hambardzumyan D, Stancevic B, Chan M, Fuks Z, Kolesnick R, Haimovitz-Friedman A. Endothelial membrane remodeling is obligate for anti-angiogenic radiosensitization during tumor radiosurgery. PLoS One. 2010;5. doi:10.1371/annotation/6e222ad5-b175-4a00-9d04-4d120568a897.
