ABSTRACT
Substantial evidence indicates that brain-derived neurotrophic factor (BDNF) plays an important role in tumorigenesis, in addition to its primary role in neuronal activity. Gastrointestinal stromal tumors (GISTs), which are the most common mesenchymal neoplasms of the gastrointestinal tract, contain multiple types of tumor-infiltrating lymphocytes (TILs) that express relevant immune checkpoint proteins. However, no data have been reported on the role of BDNF in GISTs. This study aimed to investigate the expression pattern and prognostic value of BDNF in GIST patients with different degrees of risk, as well as the relationship between BDNF expression and immune checkpoints. Immunohistochemistry (IHC) demonstrated that higher BDNF expression was more likely to be present in high-risk patients and suggested a poor prognosis. A similar phenomenon was demonstrated in plasma. Even more interesting was that a positive correlation was present between BDNF and PD-L1+ expression on TILs. Moreover, high BDNF expression levels in combination with a high PD-L1+ TIL count predict extremely poor survival. The combination of BDNF expression and TIL PD-L1+ expression as a single biomarker was a powerful significant independent predictor of prognosis. Taken together, BDNF expression may serve as a significant prognostic factor, as the combination of BDNF expression and the PD-L1+ TIL subset led to superior prediction of GIST prognosis. Furthermore, our research coupled a neurotrophin with immunity, which provides novel evidence of neural and immune regulation in a clinical study of GIST.
Introduction
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophic factor family, is the most studied and well characterized in the central nervous system. In mammals, several lines of evidence have suggested that BDNF exerts its role through tropomyosin receptor kinase B (TrkB) and the low affinity p75 neurotrophin receptor (p75NTR) in terms of neuronal activity,Citation1 including proper growth, development, synaptic plasticity,Citation2,Citation3 neuronal differentiation,Citation4,Citation5 and neurotransmission.Citation6 In addition, BDNF protein is detectable in several non-neural tissues, such as endothelial cells,Citation7,Citation8 cardiomyocytes,Citation9 and vascular smooth muscle cells.Citation7 Therefore, BDNF may be involved in cancer via angiogenesis,Citation10 which suggests a potential role as a therapeutic target in tumors. Furthermore, numerous studies have reported that BDNF overexpression frequently indicates an aggressive phenotype and poor prognosis in cancers,Citation11–13 which implies that BDNF has oncogenic characteristics. However, the role of BDNF in gastrointestinal stromal tumors (GISTs) remains unclear.
Infiltration and expansion of nerve fibers can be observed in various tumors, including gastric cancer,Citation14 pancreatic cancerCitation15 and breast cancer.Citation16 Molecules of the neurotrophin family signal through Trk receptors to support neuronal survival and axonal growth. Moreover, upregulation of neurotrophins or Trk receptors is frequently observed in cancers.Citation17,Citation18 Recent research has shown that cancer cell-derived BDNF promotes axonogenesis, which in turn facilitates tumor cell invasion.Citation19 In addition, the immune and nervous systems are commonly coopted by tumors to favor cancer progression,Citation20 which contributs to proliferative, invasion and poor prognosis. Sympathetic signaling induces the infiltration of FoxP3+ Tregs, myeloid-derived suppressor cells and M2-type macrophages and decreases the numbers of CD8+ cells and natural killer cells, which potentially contributes to immune suppression. In addition, sympathetic nerves also express programmed cell death-1 (PD-1) in some cancer types.Citation19 In pancreatic ductal adenocarcinoma, the tumor-associated neurotransmitter GABA (γ-aminobutyric acid), facilitates macrophage infiltration by mediating Ca2+ signaling.Citation21 In melanoma, NGFR-positive cells coupled with high expression of BDNF, contributes to T cell resistance. Moreover, a patient-intrinsic NGFR signature predicts anti-PD-1 therapy resistance, and tumor fractions with high NGFR expression are associated with immune exclusion.Citation22
Immunotherapeutic strategies harnessing the different components of the immune system to eliminate viable tumor cells are a promising therapeutic strategy for GIST. Investigators have shown that abundant TILs are located in the microenvironment of GISTs where they play an important role in tumor surveillance and progression.Citation23 PD-1 and its ligands (programmed death-ligand 1/programmed death-ligand 2 (PD-L1/PD-L2)), as well as T cell immunoglobulin-3 (TIM3) and its ligand Galectin 9, are considered important immune checkpoint proteins involved in immune escape.Citation23 Inspired by the above, we wondered whether tumor-intrinsic BDNF expression predicts immunotherapy resistance, which would cause a worse prognosis.
The purpose of the present study was to examine the clinical relevance of BDNF expression and the combination of BDNF expression and TIL PD-L1+ expression, as well as the combined prognostic value for GIST patients. Here, we found increased BDNF expression in high-risk GIST patients, which indicates a poor outcome. Furthermore, high expression levels of BDNF in combination with a high number of PD-L1+ TILs predict extremely poor survival. Similar to BDNF expression and PD-L1+ TILs, the combination of BDNF expression and PD-L1+ TILs as a single biomarker was a powerful independent predictor of prognosis.
Materials and Methods
Patients and clinical specimens
A tissue microarray (TMA) was constructed and contained 249 pathologist-certified and clinically annotated GIST specimens obtained between September 2004 and September 2013 from patients who were enrolled in the Department of General Surgery, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University. Informed consent was provided by all patients. Clinical information was retrospectively collected from the hospital archives, as shown in . Again, plasma samples from 88 patients with different risk levels were randomly selected to detect changes in the BDNF concentration in the blood. This study was approved by the Research Ethics Committee of Ren Ji Hospital and was performed in accordance with ethical standards as outlined in the Declaration of Helsinki (Ethical approval number: 2012031).
Table 1. Patient and tumor characteristics
ELISA
The concentration of BDNF in patient plasma was examined by ELISA according to the manufacturer’s protocol. A kit (Human BDNF ELISA kit, PCDBH0042) was designed to test human tissue samples.
Immunohistochemistry (IHC)
The IHC protocol used has been previously described.Citation24 BDNF expression and immune checkpoints located on tumor-infiltrating immune cells were classified semiquantitatively and were independently scored by two pathologists blinded to the clinical outcomes; any differences were resolved by mutual agreement, as previously described.Citation25,Citation26 Briefly, the BDNF expression score and the intensity of immune infiltrates that were positively stained for immune checkpoints were assigned a semiquantitative designation, as follows: ‘-’, ‘+’, ‘++’ and ‘++++’, where ‘-’ = “none” (no staining), ‘+’ = “weak staining or few scattered infiltrating immune cells”, ‘++’ = “moderate staining or moderate infiltration of immune cells”, or ‘+++’ = “strong staining or dense infiltration of immune cells” (), Figure S1a and Figure S2). ‘++’ and ‘+++’ were considered to represent higher expression, while the others were considered to represent lower expression () and Figure S1b). The primary antibodies used were as follows: BDNF (GeneTex, GTX132621, 1: 200), PD-L1 (CST, # 13684, 1: 200), PD-1 (CST, 43248, 1: 100), TIM3 (Abcam, ab185703, 1: 200), Galectin 9 (Abcam, ab69630, 1: 200), CD3 (Servicebio, GB13440, 1: 100), and CD8 (CST, 85336, 1: 100).
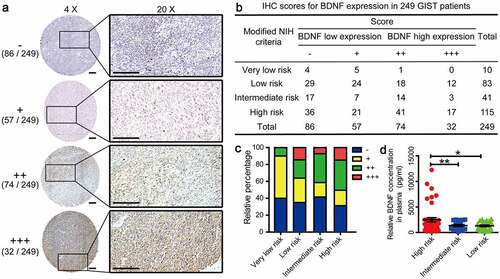
Figure 1. IHC scores for BDNF expression in tumor tissues from 249 GIST patient
Multiplexed immunohistochemistry
The protocol for multiplexed immunohistochemistry was performed as previously described using TSA (Tyramide Signal Amplification) technology.Citation27,Citation28 Formalin-fixed and paraffin-embedded GIST tissues were deparaffinized and rehydrated for multiple fluorescence detection. The primary antibody and HRP-labeled secondary antibody were added successively, and then a TSA reaction was performed to covalently bind the fluorescence signal to the antibody binding protein. Next, the antibody was removed by microwave treatment, while the fluorescent signal that was bound to the antigen remained stable. This procedure was performed several times for fluorescence staining using different antibodies. FITC-TSA, CY3-TSA, CY5-TSA and DAPI was used to label BDNF, CD3, PD-L1 and nucleus, respectively. A section that received no primary antibody served as a negative control.
Statistical analyses
Data are shown as means ± SD. GraphPad Prism 5 software was used to calculate cumulative survival time by the Kaplan–Meier method via the log-rank test or Cox regression analysis, as shown by the Kaplan–Meier (KM) curve. Fisher’s exact test and the chi-square test were used for comparisons between groups using SPSS 20.0 (Chicago, IL, USA). Correlations between markers were obtained using the Spearman correlation method. All tests were two-sided except as indicated, and P < .05 was considered statistically significant.
Results
Baseline clinicopathologic characteristics of GIST patients
In all, 249 tissue samples that were pathologically diagnosed as GIST in patients treated between September 2004 and September 2013 were enrolled from the pathology archives. Of these, 42 patients were treated with imatinib in addition to surgery. shows their clinical parameters. The median age was 59 years, and 52.61% of patients were male. The mean tumor size was 7.2 cm, and the median was 6.0 cm, with a range of 0.5–30 cm. Regarding the modified National Institute of Health (NIH) consensus, 37.35% of samples were predicted to be low risk, 16.47% were predicted to be intermediate risk, and 46.18% were predicted to be high risk, of which 43 (17.27%) cases were predicted to have serosal invasion. More than half of the cases (69.48%) had a mitotic count of less than 5 per 50 high-power fields (HPFs). Tumor bleeding was present in 45 of 249 samples (18.07%). In this cohort, complete follow-up data for GIST patients were available. Relapse occurred in 50 patients (20.08%) by the end of follow-up. The median overall survival (OS) and median progression-free survival (PFS) were 56 months and 53 months, respectively.
BDNF expression and its relationship with clinicopathological features
To evaluate BDNF expression levels in GIST tissues, IHC was performed in a set of TMAs containing 249 cases. According to the IHC scores, 106 cases had high BDNF expression and 143 cases had low BDNF expression (). Moreover, the proportion of patients with higher BDNF expression (scores of ‘++ ’ and ‘+++’) gradually increased along with the risk degree of the NIH classification (). As a member of the nerve growth factor family, BDNF is secreted into the extracellular space. Hence, we examined the changes in the blood concentration of BDNF in 88 randomly selected patients with different degrees of risk. The results showed that the concentration of BDNF in plasma from high-risk patients was significantly increased compared with that in plasma from intermediate-risk and low-risk patients, as shown in ). Then, the chi-square test was used to assess the correlations between BDNF expression and clinicopathological features in tissue samples. The results demonstrated that BDNF expression was closely correlated with NIH risk degree (p = .033), presence of mitotic figures (p = .005), Ki67 index (p = .020), and recurrence (p < .001). No significant associations were observed between BDNF expression and age, sex, tumor size, tumor bleeding or serosal invasion ().
Table 2. Correlations between BDNF expression and clinicopathologic features in GIST patients
Higher BDNF expression predicted poor prognosis of GIST patients
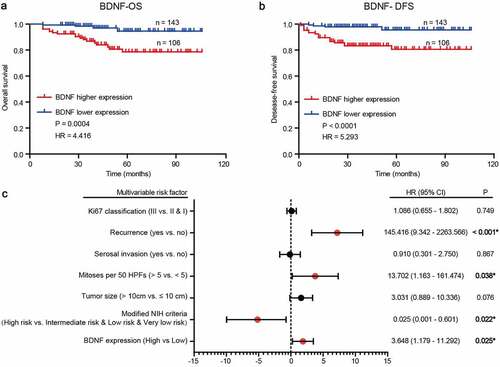
Next, the survival rate in terms of BDNF expression in GISTs was analyzed using the Kaplan–Meier method and log-rank test. The results revealed significantly superior survival in patients with lower BDNF expression than in those with higher BDNF expression (). Moreover, univariate and multivariate analyses using a Cox proportional hazards model were applied to analyze the relationship between BDNF expression and patient outcomes. The results showed that BDNF expression, NIH risk degree, tumor size, mitotic figures, Ki67 index, recurrence, and serosal invasion were significantly correlated with overall survival (). A multivariate Cox regression analysis demonstrated that BDNF expression, NIH risk degree, mitotic figures, and recurrence were independent predictors of poor prognosis ()).
Table 3. Univariate analyses of prognostic parameters for survival in patients with gastrointestinal stromal tumor (GIST)
Figure 2. Prognostic value of BDNF in GIST patients
The sensitivity and specificity of BDNF for GIST prognosis
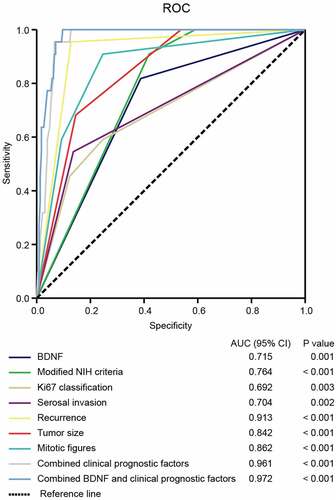
To further confirm the prognostic accuracy of BDNF in GIST, logistic regression was conducted to compare the sensitivity and specificity of BDNF in GIST prognosis. Multiple models were constructed, including BDNF as a single clinicopathological feature, combinations of clinicopathological features, and BDNF combined with clinicopathological features. A receiver operating characteristic (ROC) curve comparison of the prognostic accuracy of BDNF with clinicopathological features was then performed. As shown in , statistical analyses demonstrated that the area under the curve (AUC) for BDNF combined with other clinicopathological features was higher than that for any other single or combined factor (AUC = 0.972, p < .001). These results indicated that BDNF combined with other clinicopathological features had greater sensitivity and specificity and was a stronger prognostic predictor than any single risk factor or their combination.
Figure 3. The sensitivity and specificity of BDNF for GIST prognosis
The positive correlation between BDNF expression, PD-L1+ TILs and CD3 cell density
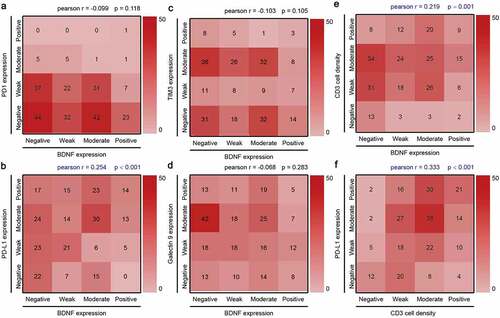
In addition to their well-characterized nerve growth promotion effects, neurotrophins can also recruit leukocytes. However, no data have been reported in GISTs regarding neurotrophins, let alone the relationship between neurotrophins and immune checkpoints. First, IHC was performed to assess the expression of immune checkpoints, including PD-L1, PD-1, TIM3 and Galectin 9. Representative IHC scores for PD-L1, CD3, PD-1, TIM3 and Galectin 9 are shown in Figure S1a and Figure S2. The results showed an increased likelihood of higher PD-L1 expression (scores of ‘++’ and ‘+++’) in high-risk patients (Figure S1b and c). Intriguingly, a Pearson correlation analysis demonstrated a weak but statistically significant correlation between BDNF expression and PD-L1+ TILs ()). However, no correlation was observed between BDNF expression and other immune checkpoint protein-positive cells, including PD-1 ()), TIM3 ()) and Galectin 9 ()). Moreover, immunofluorescence staining showed that BDNF and PD-L1 could be expressed on the same immune cells (Figure S3), which suggests that an interaction maybe present between them. Furthermore, Pearson correlation analysis demonstrated that CD3 cell density was positively correlated with BDNF expression (), r = 0.219, p = .001) and PD-L1+ TILs (), r = 0.333, p < .001), with statistical significance.
Figure 4. The relationship between BDNF expression and immune checkpoint proteins
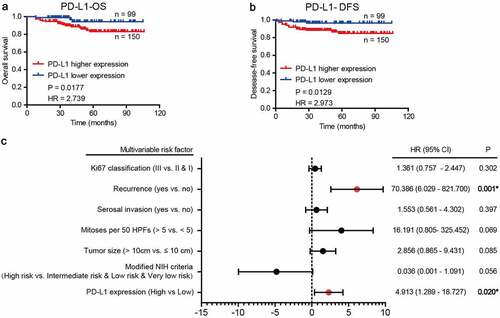
TIL PD-L1+ expression and patients’ survival
Next, we investigated the relationship between PD-L1+ TILs and survival in the collected cohort, as well as the relationship between CD3 cell density and survival. Optimal cutoffs for negative versus positive staining were determined as described above. In the study of CD3, no statistical significance was observed between the CD3 cell density and patients’ survival (Figure S4). Next, we found that high expression of PD-L1 was associated with poor GIST-specific survival (), p = .0177). The relationship between GIST relapse-free survival and the expression of these molecules followed the same trends (), p = .0129). The chi-square test demonstrated that the distribution of PD-L1+ TILs was closely correlated with NIH risk degree (p = .040) and tumor size (p = .028) (). However, no statistical significance was observed between PD-L1+ TILs and other clinicopathological features, including age, sex, mitotic figures, Ki67 index, tumor bleeding, recurrence or serosal invasion (). Moreover, univariate analyses using a Cox proportional hazards model showed that PD-L1 expression on TILs was significantly correlated with patient outcome (Supplementary ). Additionally, multivariate Cox regression analysis demonstrated that PD-L1 expression and recurrence were independent predictors of poor prognosis ()).
Table 4. Correlations between PD-L1+ TIL density and clinicopathologic features in GIST patients
Figure 5. Prognostic value of PD-L1+ TIL in GIST patients
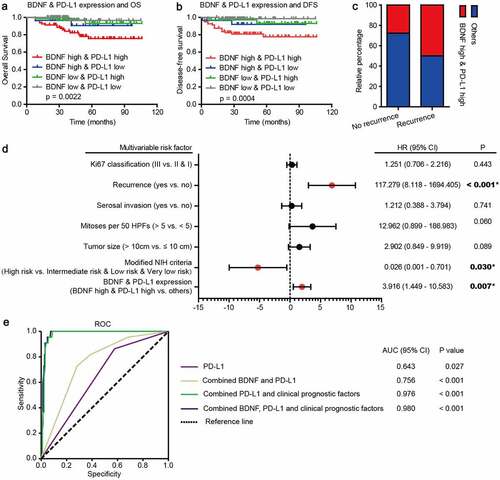
The relationship between the co-distribution of BDNF expression and PD-L1+ TILs and patient survival
Due to the positive correlation between BDNF expression and PD-L1+ TILs, we wondered whether a combination of these would improve prognostic prediction. The analysis showed that both high BDNF expression and distribution of PD-L1+ TILs predicted a worse prognosis than other combinations, as shown in . Interestingly, both high BDNF expression and PD-L1+ expression on TILs were associated with a higher probability of recurrence ()). Concordantly, similar to NIH risk criteria and recurrence, the combination of BDNF expression and PD-L1+ TILs as a single biomarker was a powerful independent predictor of prognosis according to univariate (Table S1) and multivariate analyses (), p = .007, HR = 3.916, 95% CI 1.449–10.583). In summary, BDNF expression was highly synergistic with PD-L1+ TIL distribution, and these factors together predicted unsatisfactory outcomes. Moreover, statistical analyses showed that, among multiple models, the AUCs were highest for BDNF and PD-L1 combined with clinicopathological features (), AUC = 0.980, p < .001). These results indicated that BDNF and PD-L1 combined with other clinicopathological features had the greatest sensitivity and specificity and was a stronger prognostic model than the others.
Figure 6. Relationship of BDNF expression and PD-L1+ expression on TILs with patient survival
Discussion
As a member of the neurotrophin family, BDNF plays an important role in the development and repair of the mammalian nervous system.Citation29 In addition, several lines of evidence have indicated that BDNF is elevated in non-nervous system solid tumors and acts as an oncogenic factor. In HCC, higher BDNF expression was significantly correlated with multiple and advanced stages.Citation30 In colorectal cancer, elevated BDNF expression in tumor tissues was closely related to poor prognosis and metastasis,Citation31 and thus, increased BDNF expression in serum specimens has the potential to become a new laboratory marker for early clinical diagnosis.Citation32 In addition, similar phenomena have been observed in other tumors, including breast, lung, pancreas and prostate cancers as well as myelomas and lymphoid tumors.Citation33 However, the clinical relevance of BDNF expression and its prognostic value for GIST patients remain unclear. Here, we found that increased BDNF expression was closely related to tumor malignancy indicators and poor prognosis. Moreover, blood samples from high-risk patients had significantly higher concentrations of BDNF than samples from medium- and low-risk patients, which suggests that BDNF concentration has the potential to be a diagnostic indicator in these patients. These findings support the role of BDNF in oncogenesis. However, its detailed function and underlying mechanism in GIST remain unclear and warrant further studies.
In previous studies, BDNF was usually investigated together with TrkB, which is a carcinogenic factor that exerts its role via activation of the Trk receptor to elicit a series of downstream signaling pathways, including PI3K/Akt, Ras-Raf-MEK-ERK, and PLCγ and the transactivation of EGFR, among others.Citation34 In addition, a high level of TrkB expression predicts metastases and poor prognosis.Citation35,Citation36 In one study, neutralizing BDNF or inhibiting TrkB kinase activity by K252a both disrupted apoptosis and invasion in HCC cell lines.Citation30 In colon cancer, K252a, a Trk tyrosine receptor kinase inhibitor, could reverse the facilitation effect of BDNF on tumor cell viability, migration, and invasion.Citation31 In addition, preclinical trials of TrkB-targeted therapies showed promising results in several cancers including breast cancer, renal cell carcinoma (RCC), ovarian cancer, NSCLC, primary brain cancer, and sarcoma.Citation37 Moreover, in addition to more aggressive clinical features, previous studies have shown that TrkB is also associated with resistance to anticancer drugs.Citation38 Hence, we wondered whether the BDNF/TrkB axis is associated with imatinib resistance in GISTs. A subsequent analysis showed that even among patients treated with imatinib, those with higher BDNF expression had a higher probability of recurrence (Supplementary Figure 5a), which was similar to the probability of recurrence in patients treated without imatinib (Figure S5b). Furthermore, among imatinib treated patients, no significant difference was observed in overall survival (Figure S5c, log-rank test, p = .1377) or disease-free survival (Figure S5d, log-rank test, p = .0620) between the BDNF higher and lower expression groups, but a slightly worse prognosis was seen in the BDNF higher expression groups. Among patients who did not receive imatinib, patients with high BDNF expression exhibited significantly worse outcomes (Figure S5e and f). These phenomena suggest that BDNF expression is related to imatinib resistance. In summary, we hypothesize that the BDNF/TrkB axis is likely an effective target to prevent GIST advancement. More studies should be designed to clarify this conjecture and should include the collection of tissue samples from relapsed patients treated with imatinib to assess the change in BDNF expression.
In addition, recent studies have suggested that hypothalamic BDNF may exert oncolytic effects by downregulating leptin production in adipocytes via sympathetic neural β-adrenergic signaling to augment T-cell cytotoxicity in enriched environments.Citation39 However, cancer cell-derived BDNF induced by NGFR contributes to T cell resistance, which predicts anti-PD-1 therapy resistance.Citation22 Immune resistance to targeted immunological checkpoints will also emerge in GIST patients with high BDNF expression. In recent years, immunotherapy, especially targeted immune checkpoint therapy, such as that against cytotoxic T-lymphocyte associated protein 4 (CTLA-4), PD-1 and PD-L1, has shown promising prospects in multiple tumors.Citation40,Citation41 GISTs, a class of mesenchymal neoplasms, contain multiple types of TILs and relevant immune checkpoint proteins, which provides an opportunity and rationale for developing effective immunotherapies.Citation42 PD-1 and its ligands (PD-L1/PD-L2), as well as TIM3 and its ligand Galectin 9, are considered the primary immune checkpoint proteins involved in immune escape.Citation23 Therefore, we studied their expression on TILs in GISTs and analyzed their correlation with BDNF. The results revealed a statistically significant positive correlation only between BDNF expression and PD-L1+ TILs. Moreover, we stratified the tumors based on BDNF expression and PD-L1+ TILs. Immune tolerance may occur in patients with high BDNF expression and PD-L1+ TIL infiltration, which is accompanied by the worst prognosis. In addition, a positive correlation was present between BDNF expression and CD3 cell density and PD-L1 expression and CD3 cell density. And immunofluorescence staining showed that BDNF and PD-L1 could co-locate on CD3 cells. Hence, we speculate that BDNF maybe promote PD-L1 expression and its localization in immune cells, which promotes the formation of an immunosuppressive microenvironment.
Recently, increasing concern has been focused on immunotargeted combination therapy. The combination reported here has strong scientific merit, but clinical trials are still needed.Citation43 In principle, combining treatment could enhance immunotherapy efficacy through increased neoantigens released as a result of rapid tumor death induced by targeted therapy.Citation44 For example, the receptor tyrosine kinase inhibitors sunitinib and pazopanib in combination with anti-PD-1 showed promising overall response rates of 40–50% in patients with metastatic RCC.Citation45 Vemurafenib, an FDA-approved BRAF inhibitor, can increase the expression of tumor antigens and MHC molecules, which increases the sensitivity of tumor cells to immune attack during melanoma treatment.Citation46 Therefore, we speculate that anti-PD-L1 combined with a neutralizing antibody to BDNF or inhibitors of TrkB kinase activity (K252a) would be superior to the effect of each alone. We also speculate as well as whether BDNF neutralizing antibodies or inhibitors of TrkB kinase activity can restore the sensitivity of tumor cells to T cell attack. Further studies should be conducted to understand the function of BDNF in the neuroimmune tumor microenvironment and its interaction with PD-L1, which will lead to new therapeutic strategies for GIST patients.
In conclusion, we first demonstrated that in GIST patients, a higher BDNF and PD-L1+ TIL subset distribution and concurrent BDNF and PD-L1+ TIL subset distribution were significantly associated with poor prognosis. The positive relationship between BDNF expression and PD-L1+ TIL subset distribution might indicate better efficacy when targeted BDNF therapy is combined with immune checkpoint inhibitors. However, the effect on GIST requires further investigation.
Supplemental Material
Download ()Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Colucci-D’Amato L, Speranza L, and Volpicelli F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression. Neurodegeneration and Brain Cancer. 2020;21(7777).
- Itami C, Kimura, F, Kohno, T, Matsuoka, M, Ichikawa, M, Tsumoto, T, and Nakamura, S. Brain-derived neurotrophic factor-dependent unmasking of “silent” synapses in the developing mouse barrel cortex Proc Natl Acad Sci U S A. 2003 Oct 28;100(2):13069–10.
- Edelmann E, Lessmann V, and Brigadski T. Pre- and postsynaptic twists in BDNF secretion and action in synaptic plasticity. Neuropharmacology. 2014; 76 Pt C;610–627.
- Bartkowska K, Paquin A, Gauthier AS, Kaplan DR, and Miller FD. Trk signaling regulates neural precursor cell proliferation and differentiation during cortical development. Development (Cambridge, England). 2007;134:4369–4380.
- Vilar M, and Mira H. Regulation of Neurogenesis by Neurotrophins during Adulthood: expected and Unexpected Roles. Front Neurosci. 2016;10:26.
- Lin PY, Kavalali ET, and Monteggia LM. Genetic Dissection of Presynaptic and Postsynaptic BDNF-TrkB Signaling in Synaptic Efficacy of CA3-CA1 Synapses. Cell Rep. 2018;24:1550–1561.
- Nakahashi T, Fujimura, H, Altar, CA, Li, J, Kambayashi, J, Tandon, NN, and Sun, B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000;470:113–117.
- Wang H, Ward N, Boswell M, and Katz DM. Secretion of brain-derived neurotrophic factor from brain microvascular endothelial cells. Eur J Neurosci. 2006;23:1665–1670.
- Pius-Sadowska E, and Machaliński B. BDNF - A key player in cardiovascular system. J Mol Cell Cardiol. 2017;110: 54–60.
- Lam CT, Yang ZF, Lau CK, Tam KH, Fan ST, Poon RT. Brain-derived neurotrophic factor promotes tumorigenesis via induction of neovascularization: implication in hepatocellular carcinoma. Clinical Cancer Research: An Official J American Association for Cancer Res. 2011;17(3123–3133):3123–3133. doi:10.1158/1078-0432.CCR-10-2802.
- Yang ZF, Ho, DW, Lam, CT, Yu, WC, Poon, RT, and Fan, ST. Identification of brain-derived neurotrophic factor as a novel functional protein in hepatocellular carcinoma. Cancer Res. 2005;65:219–225.
- Hu Y, Wang, YD, Guo, T, Wei, WN, Sun, CY, Zhang, L, and Huang, J. Identification of brain-derived neurotrophic factor as a novel angiogenic protein in multiple myeloma. Cancer Genet Cytogenet. 2007;178:1–10. doi:10.1016/j.cancergencyto.2007.05.028.
- Adriaenssens E, Vanhecke E, Saule P, Mougel A, Page A, Romon R, Nurcombe V, Le Bourhis X, Hondermarck H. Nerve growth factor is a potential therapeutic target in breast cancer. Cancer Res. 2008;68(346–351):346–351. doi:10.1158/0008-5472.CAN-07-1183.
- Zhao C-M, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, Andersen GT, Flatberg A, Johannessen H, Friedman RA, Renz BW, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6(250). doi:10.1126/scitranslmed.3009569.
- Stopczynski RE, Normolle DP, Hartman DJ, Ying H, DeBerry JJ, Bielefeldt K, Rhim AD, DePinho RA, Albers KM, Davis BM, et al. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 2014;74(1718–1727):1718–1727. doi:10.1158/0008-5472.CAN-13-2050.
- Pundavela J, Roselli, S, Faulkner, S, Attia, J, Scott, RJ, Thorne, RF, Forbes, JF, Bradshaw, RA, Walker, MM, and Jobling, P et al. Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer. Mol Oncol. 2015;9:1626–1635. doi:10.1016/j.molonc.2015.05.001.
- Dollé L, Adriaenssens E, El Yazidi-Belkoura I, Le Bourhis X, Nurcombe V, Hondermarck H. Nerve growth factor receptors and signaling in breast cancer. <![CDATA[Current Cancer Drug Targets]]>. 2004;4(6):463–470. doi:10.2174/1568009043332853.
- Hayakawa Y, Sakitani K, Konishi M, Asfaha S, Niikura R, Tomita H, Renz BW, Tailor Y, Macchini M, and Middelhoff M, et al. Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell. 2017;31:21–34 . doi:10.1016/j.ccell.2016.11.005.
- Silverman DA, Martinez VK, Dougherty PM, and Myers JN. Cancer-Associated Neurogenesis and Nerve-Cancer Cross-talk. Cancer Res. 2021;81:1431–1440.
- Cervantes-Villagrana RD, Albores-García D, Cervantes-Villagrana AR, and García-Acevez SJ. Tumor-induced neurogenesis and immune evasion as targets of innovative anti-cancer therapies. Signal Transduction and Targeted Therapy. 2020;5:99.
- Jiang SH, Zhu LL, Zhang M, Li RK, Yang Q, Yan JY, Zhang C, Yang JY, Dong FY, Dai M, et al. GABRP regulates chemokine signalling, macrophage recruitment and tumour progression in pancreatic cancer through tuning KCNN4-mediated Ca(2+) signalling in a GABA-independent manner. Gut. 2019;68:1994–2006.
- Boshuizen J, and Vredevoogd DW. Reversal of pre-existing NGFR-driven tumor and immune therapy resistance. Nat Commun. 11(3946): 2020.
- Tan Y, Trent JC, Wilky BA, Kerr DA, and Rosenberg AE. Current status of immunotherapy for gastrointestinal stromal tumor. Cancer Gene Ther. 2017;24:130–133.
- Xu C, Tian G, Jiang C, Xue H, Kuerbanjiang M, Sun L, Gu L, Zhou H, Liu Y, Zhang Z. NPTX2 promotes colorectal cancer growth and liver metastasis by the activation of the canonical Wnt/β-catenin pathway via FZD6. Cell Death Dis. 2019;10:217.
- Tian GA, Zhu CC, Zhang XX, Zhu L, Yang XM, Jiang SH, Li RK, Tu L, Wang Y, Zhuang C, et al. CCBE1 promotes GIST development through enhancing angiogenesis and mediating resistance to imatinib. Sci Rep. 2016;6:31071.
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra137.
- Sun Z, Nyberg R, Wu Y, Bernard B, and Redmond WL. Developing an enhanced 7-color multiplex IHC protocol to dissect immune infiltration in human cancers. PloS One. 2021;16:e0247238.
- Nguyen T, Kocovski N, Macdonald S, Yeang HXA, Wang M, and Neeson PJ. Multiplex Immunohistochemistry Analysis of Melanoma Tumor-Infiltrating Lymphocytes. Methods in Molecular Biology (Clifton, NJ). 2021;2265:557–572.
- Arévalo JC, Wu SH. Neurotrophin signaling: many exciting surprises! Cellular and Molecular Life Sciences: CMLS. 2006;63:1523–1537.
- Guo D, Hou X, Zhang H, Sun W, Zhu L, Liang J, Jiang X. More expressions of BDNF and TrkB in multiple hepatocellular carcinoma and anti-BDNF or K252a induced apoptosis, supressed invasion of HepG2 and HCCLM3 cells. J Experimental & Clinical Cancer Research: CR. 2011;30:97.
- Tanaka K, Okugawa Y, Toiyama Y, Inoue Y, Saigusa S, Kawamura M, Araki T, Uchida K, Mohri Y, Kusunoki, M. Brain-derived neurotrophic factor (BDNF)-induced tropomyosin-related kinase B (Trk B) signaling is a potential therapeutic target for peritoneal carcinomatosis arising from colorectal cancer. PloS One. 2014;9:e96410.
- Brierley GV, Priebe IK, Purins L, Fung KY, Tabor B, Lockett T, Nice E, Gibbs P, Tie J, McMurrick P et al. Serum concentrations of brain-derived neurotrophic factor (BDNF) are decreased in colorectal cancer patients. Cancer Biomarkers: Section A of Disease Markers. 2013;13:67–73.
- Neurotrophin Trk MJ. Receptors: new Targets for Cancer Therapy. Rev Physiol Biochem Pharmacol. 2018;174:67–79.
- Meng L, Liu B, Ji R, Jiang X, Yan X, and Xin Y. Targeting the BDNF/TrkB pathway for the treatment of tumors. Oncol Lett. 2019;17:2031–2039.
- Zhang Y, Fujiwara Y, Doki Y, Takiguchi S, Yasuda T, Miyata H, Yamazaki M, Ngan CY, Yamamoto H, Ma Q, et al. Overexpression of tyrosine kinase B protein as a predictor for distant metastases and prognosis in gastric carcinoma. Oncology. 2008;75:17–26.
- Sclabas GM, Fujioka S, Schmidt C, Li Z, Frederick WA, Yang W, Yokoi K, Evans DB, Abbruzzese JL, Hess KR, et al. Overexpression of tropomysin-related kinase B in metastatic human pancreatic cancer cells. Clinical Cancer Research: An Official J American Association for Cancer Res. 2005;11:440–449.
- Lange AM, and Lo HW. Inhibiting TRK Proteins in Clinical Cancer Therapy. Cancers. 2018;10: 105.
- Lucarelli E, Kaplan D, Thiele CJ. Activation of trk-A but not trk-B signal transduction pathway inhibits growth of neuroblastoma cells. European Journal of Cancer (Oxford, England: 1990). 1997;33:2068–2070.
- Cao L, Liu X, Lin EJ, Wang C, Choi EY, Riban V, Lin B, During MJ. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell. 2010;142:52–64.
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133.
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723.
- Watson GA, Kelly D, Melland-Smith M, Gleeson J, McEntee G, Kelly CM, McCaffrey JA. Get the GIST? An overview of gastrointestinal stromal tumours. Ir J Med Sci. 2016;185:319–326.
- Sharma P, and Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214.
- Vanneman M, and Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–251.
- Amin A, Ernstoff MS, Infante JR, Heng Dyc, Rini BI, Plimack ER, Mcdermott DF, Kollmannsberger CK, Reaume MN, Spratlin JL. A phase I study of nivolumab (anti-PD-1; BMS-936558; ONO-4538) in combination with sunitinib, pazopanib, or ipilimumab in patients (pts) with metastatic renal cell carcinoma (mRCC). J Clinical Oncology. 2013;31:1904–1911.
- Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, Mitra D, Boni A, Newton LP, Liu C, Peng W, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clinical Cancer Research: An Official J American Association for Cancer Res. 2013;19:1225–1231.
