ABSTRACT
Background: Passive standing tests are a first-line, practical means of assessing individuals with chronic orthostatic symptoms.
Purpose: To identify the proportion reaching heart rate (HR) criteria for postural tachycardia syndrome (POTS) during a 10-minute passive standing test (PST) if measurement of the lowest supine HR incorporated a 2-minute period of post-test monitoring, rather than being restricted to the 5-minute pre-test values only, and to determine the proportion whose POTS would be missed by shorter periods upright.
Methods: Consecutive individuals ≥ 12 years from 2008 to 2017 who presented with chronic fatigue or lightheadedness and whose PST met criteria for POTS.
Results: Of the 93 enrolled (70% female, median age 17 years), the mean (SD) HR was higher in the 5 min supine before the 10 min upright than in the 2 min supine afterwards (67.6 [10.0] vs. 65.7 [10.9]; P = 0.01). Thirteen (14%; 95% CI, 7–21%) satisfied HR criteria for POTS using the supine HR from only the post-test period. The median time to reaching the HR criteria for POTS was 3 min. Of those reaching HR criteria, 53% (95% CI, 43–63%) would be missed by a 2-minute and 27% (95% CI, 19–37%) by a 5-minute test.
Interpretation: More adolescents and young adults are diagnosed with POTS during a 10-minute PST when the definition of their lowest supine HR includes a 2-minute post-test measurement along with the conventional pre-test measure. A full 10 min of standing is required to avoid underdiagnosing POTS in both clinical and epidemiologic studies.
Introduction
Postural tachycardia syndrome (POTS) is a relatively common circulatory condition, estimated to affect 0.1–1% of the population [Citation1], disproportionately affecting women in the adolescent and young adult age range [Citation1–3]. POTS can be associated with a variety of clinical syndromes including myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) [Citation4] and Ehlers-Danlos syndrome [Citation5,Citation6]. The diagnosis of POTS requires an exaggerated increase in heart rate (HR) from supine to standing of at least 30 beats per minute for adults (20 years of age or older), or at least 40 bpm for adolescents over the course of 10 min upright, in the absence of orthostatic hypotension during the first 3 min [Citation7]. The diagnosis also requires the presence of chronic orthostatic symptoms such as fatigue, lightheadedness, blurry vision, weakness, cognitive difficulties, and nausea [Citation1–3,Citation8–10]. Although tilt table testing was the initial method of orthostatic testing in POTS studies [Citation8], in-office passive standing tests (PST), during which the individual leans against a wall [Citation5,Citation11], or active standing tests (without any support) [Citation12–15] have also been used for several decades. Limitations to tilt testing can include a lack of availability, lack of universal insurance coverage for the testing, and the higher cost of the equipment. This has led to an increased interest in standing tests as the first-line investigation for chronic orthostatic symptoms [Citation16,Citation17], including among those with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) [Citation4].
The specific methods for performing a standing test, however, have not received much attention in the published literature. Two methodological issues deserve further exploration. First, the measurement of the HR increase is assumed to be the difference between the peak HR standing and the lowest pre-test supine measurement. However, in the clinical assessment of adolescents and young adults referred for evaluation of orthostatic intolerance symptoms, we have observed that some HRs in the 5-minute pre-test period can be higher than those measured in the 2-minute supine period immediately following a 10-minute standing test. In some instances, the post-test HR values accord more closely with the individual’s expected age-specific supine HR. An accurate measurement of the usual, representative supine HR is needed to avoid errors in the diagnosis of POTS.
Second, some groups have used shorter periods of time for orthostatic testing in those with POTS. In studies published before the 2011 Consensus criteria for POTS, Schondorf and colleagues employed a 5-minute period of head-up tilt [Citation8], and Braune and colleagues felt that an upright position of 2 min would be sufficient for the diagnosis in most patients [Citation18]. Using a 2-minute period of standing, Hoad and colleagues reported a 27% prevalence of POTS among those with ME/CFS [Citation19]. In a recent review of pediatric orthostatic intolerance, Stewart and colleagues suggested a 5-minute tilt test might suffice for diagnosing POTS [Citation3].
Abbreviated testing would be more time-efficient in a busy clinic, and might be tolerated better by patients with more severe orthostatic symptoms. However, it is unclear what proportion of those with POTS would be missed by a shorter period upright. Any reduction in the diagnostic yield of the test would create a potential for under-treatment of POTS. For accurate estimates of prevalence in epidemiologic studies, it would be important to know the proportion of patients who would be missed if the test period was less than 10 min.
Our clinic has employed the same 10-minute PST protocol in the evaluation of those with orthostatic symptoms for over 10 years, providing an opportunity to address these two methodological issues. The objective of this retrospective study was two-fold:
To identify the proportion of individuals who would meet the HR criteria for POTS during a PST if measurement of the lowest supine HR incorporated a 2-minute period of post-test supine monitoring, rather than being restricted to the 5-minute pre-test values only.
To use life table analysis to examine the time until HR criteria for POTS were reached during this test, and to estimate the proportion in whom the diagnosis of POTS would be missed by shorter periods of orthostatic challenge.
Methods
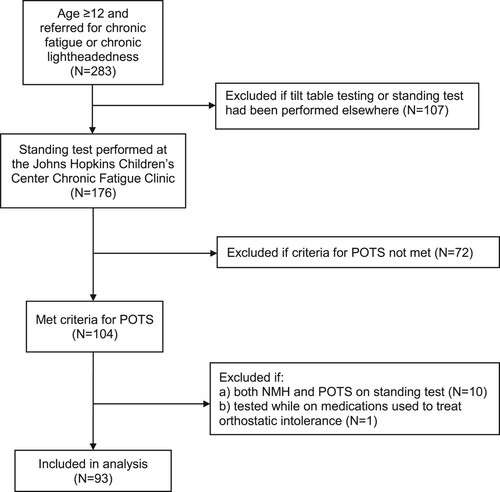
Eligible participants: We included consecutive patients age 12 years and older who were referred to the Johns Hopkins Chronic Fatigue Clinic for evaluation of chronic fatigue or orthostatic intolerance from 10/28/2008 to 11/17/2017, provided the passive standing test on their initial clinic visit was consistent with a diagnosis of POTS. We excluded individuals who (a) had undergone a head-up tilt table test or a standing test elsewhere under different conditions, (b) were already being treated for orthostatic intolerance, and (c) developed neurally mediated hypotension (NMH) during the standing test.
Exclusions a and b were intended in part to explore the hypothesis that the pre-test HR was higher due to apprehension about a new maneuver. Individuals whose other co-morbid conditions were being treated with selective serotonin-reuptake inhibitors (SSRI) or serotonin–norepinephrine re-uptake inhibitors (SNRI) for anxiety, mood, or pain, with oral or transdermal contraceptives or medroxyprogesterone for menstrual disorders or birth control, and stimulants for attention deficit disorder were included in the study. Exclusion c was based on the tendency for those with NMH to develop a cardio-inhibitory response at the time of hypotension, which would have caused an excessive reduction in the post-test supine HR that was not representative of the individual’s usual supine HR. The study was approved by the Institutional Review Board of the Johns Hopkins Medical Institutes. Informed consent was waived for a retrospective study using data collected as part of routine care.
Passive standing test methods: Orthostatic testing was conducted in a consistent manner over the study period by the same examiner, in the early afternoon, in a quiet room, using a modification of the PST of Hyatt and colleagues [Citation11]. An automated blood pressure cuff (Dynamap) was placed on the right upper limb, recording blood pressure and HR at 1-minute intervals throughout the test. After 5 min of supine posture, the patient stood with the heels 2–6 inches away from the wall, with the upper back leaning comfortably against the wall. The patient remained in this position for a maximum of 10 min, and was asked to minimize movement. At the end of the 5 min supine, and each minute during the PST, after the blood pressure and HR were recorded the patient was asked about the severity of orthostatic symptoms on a 0 to 10 scale. Otherwise, conversation was kept to a minimum. After 10 min upright, the patient returned to the supine position for an additional 2 min of blood pressure and HR recording. The PST was stopped early at the request of the patient, or in the event of severe presyncope. The case report form for this PST protocol (Appendix 1) can be downloaded from the ME/CFS link in the National Institute of Neurological Diseases and Stroke common data elements website, available at https://commondataelements.ninds.nih.gov.
Definitions: We used the 2011 consensus definition of POTS, which requires an increase in HR during a maximum of 10 min of standing of at least 30 beats per minute (bpm) in those greater than 19 years and at least 40 bpm in adolescents 12–19 years, compared to the lowest value recorded during the supine period [Citation7]. We diagnosed POTS only if there was no orthostatic hypotension within the first 3 min of upright posture, and only if there was a history of chronic orthostatic symptoms such as fatigue, lightheadedness, exercise intolerance, or cognitive dysfunction. Florid POTS was defined as a peak HR ≥ 120 bpm. For the purposes of this study, the lowest supine HR used in the calculation of the HR increment from supine to peak standing could be recorded during either the 5 min supine before standing or the 2 min supine after the completion of 10 min standing.
Statistical methods: We compared baseline characteristics between groups using t-tests, Chi-square tests, or Fisher’s exact tests depending on the type and distribution of the data. We used paired t-test to compare the difference in supine HR between pre-test and post-test.
The main comparison of interest for each subject for study objective 1 was the difference between the peak HR during 10 min of standing and either the lowest HR in the 5 min of supine posture before the test or the lowest HR in the 2 min supine after the completion of the standing test. We divided patients into three groups: group 1 were those who met HR criteria for POTS using the pre-test supine HR only; group 2 were those who met HR criteria for POTS using either the pre-test or the post-test supine HR data; group 3 were those who met HR criteria for POTS using the post-test supine HR only. We compared the group differences in pre-test supine HRs and the HR increments between the lowest supine value and the peak value standing using the one-way analysis of variance (ANOVA). Any significant differences between groups were then explored further using the post-hoc Bonferroni test. Differences in symptom reporting during standing were compared using non-parametric methods, as were sex differences between groups. We examined the paired pre- and post-test HR using the Pearson’s correlation coefficient and paired t-tests.
For the life table analysis in study objective 2, we considered the HR criteria for POTS to have been reached when the HR during standing first reached a 30 bpm increase (adults) or 40 bpm increase (adolescents) from the lowest value recorded during either the 5-minute pre-test supine period or the 2-minute post-test supine period. We used 95% confidence intervals (CI) for the estimates of the proportion of patients with POTS who would be missed if the test were stopped at each 1-minute interval. We analyzed differences in the time to POTS between males and females and between adolescents and adults (12–19 and > 20 years) using the Mantel-Haenszel hazard ratio. The analyses were conducted using SPSS version 25 and illustrations were prepared using GraphPad Prism version 7.00 for Windows, GraphPad Software, La Jolla, California, USA (www.graphpad.com).
Results
Participants: illustrates the derivation of the final study sample. Of the 93 included patients, 65 (70%) were female and 28 (30%) were male. All but one was Caucasian; the remaining individual was an Asian-Pacific Islander. There were no participants of Hispanic origin. The median age was 17 years (range, 12–48); 69 individuals were < 20 years, 19 were 20–30 years, and five were > age 30.
At the time of the standing test, no participant was being treated with any of the following for orthostatic intolerance: fludrocortisone, clonidine, midodrine, droxidopa, beta adrenergic antagonists, pyridostigmine bromide, disopyramide, angiotensin converting enzyme inhibitors, angiotensin receptor antagonists, desmopressin acetate, octreotide, ivabradine, or intravenous volume expansion. Thirty-five participants were being treated with a combination of (a) SSRI or SNRI medications for anxiety, depression, or pain (N = 21), (b) oral contraceptives or medroxyprogesterone for menstrual disorders or contraceptive purposes (N = 14), and (c) stimulants for attention deficit disorder (N = 7). The remaining 58 participants were not receiving any of the medications mentioned above.
The medication-naïve group of 58 did not differ from the 35 treated with these medications with regard to age (17.7 [6.0] vs. 18.4 [4.2] years; P = 0.56), male sex (31% vs. 29%; P = 0.80), Caucasian race (98% vs. 100%; P = 1.00), pre-test supine HR (66.7 [10.6] vs. 69.1 [8.9]; P = 0.27), peak HR (110.5 [21.1] vs. 115.5 [12.8]; P = 0.06), post-test supine HR (64.4 [11.1] vs. 67.9 [10.4]; P = 0.14), or median number of orthostatic symptoms provoked during the test (4 vs. 5; P = 0.10). Those in the medication-naïve group were less likely to fall into Group 2, defined as those meeting heart rate criteria for POTS using either the pre-test or the post-test supine HR data (66% vs. 91%; P < 0.01). We present results for the full sample of 93 and for the medication-naïve subset of 58.
Heart rate results: As (a) illustrates, for the full sample there was a strong correlation between the paired supine HRs in the 5 min before and in the 2 min after the 10 min PST (r = 0.75; P < 0.001). The mean (SD) HR was significantly higher in the 5 min supine before the test than in the 2 min supine following the 10 min of standing (67.6 [10.0] versus 65.7 [10.9] beats per minute [bpm]; paired t-test, P = 0.01). The mean peak HR during standing was 112.3 (12.6) bpm, with a range of 84 to 145 bpm. Twenty-six percent of participants had a peak HR of 120 bpm or higher.
Figure 2. The correlation of paired pre-test and post-test supine heart rates for the full sample of 93 participants ((a)) and the medication-naïve subset of 58 ((b)).
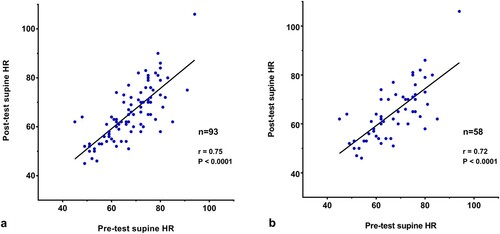
In the medication-naïve subset of 58, shown in (b), there was a similarly strong correlation between the paired supine HRs in the 5 min before and in the 2 min after the 10 min PST (r = 0.72; P < 0.001). The mean (SD) HR was significantly higher in the 5 min supine before the test than in the 2 min supine following the 10 min of standing (66.7 [10.6] versus 64.4 [11.1] beats per minute [bpm]; paired t-test, P = 0.03). The mean peak HR during standing was 112.0 (12.0) bpm, with a range of 88 to 145 bpm. Twenty-one percent of participants had a peak HR of 120 bpm or higher.
Diagnostic yield by HR group: In the full sample, 10 (11%) satisfied HR criteria for POTS using only the supine HR from the 5-minute pre-test period (Group 1), 70 (75%) met the HR criteria for POTS using either the pre-test or the post-test supine HRs (Group 2), and 13 participants (14%; 95% CI, 7–21%) satisfied the HR criteria for POTS using the supine HR from only the 2-minute post-test period of measurement (Group 3). As shown in (a), and as expected based on the way we defined the three groups, there was a significant difference by group in the pre-test supine HR. The mean HR increase (peak HR standing minus lowest HR supine) and peak HR standing also differed significantly by group ((a)). The HR increases were less robust for those meeting HR criteria for POTS using just the pre-test or just the post-test supine HR.
Table 1. Demographic and heart rate values during the standing test by HR group*.
Similar results were identified in the medication-naïve subset ((b)). Twelve participants (21%; 95% CI, 10–31%) satisfied the HR criteria for POTS using the supine HR from only the 2-minute post-test period of measurement.
The proportion with a peak HR ≥ 120 bpm was lower for those meeting the criteria for POTS in the pre-test or the post-test period only (Groups 1 and 3) compared to during both supine epochs (Group 2), (8% vs. 31%; P = 0.03, Fisher’s exact test for the full sample and 5% vs. 29%; P = 0.04 for the medication-naïve sample). There was no difference in age or sex by HR group in either the full study sample or the medication-naïve subset.
Provocation of orthostatic signs and symptoms during PST: For the full sample of 93, physician-observed increases in acrocyanosis occurred in 73%, and marked facial pallor was noted in 13%. For the medication-naïve sample of 58, increased acrocyanosis was noted in 76%, with marked facial pallor in 9%.
Self-reported increases in orthostatic symptoms during the 10 min upright are displayed in . The median number of orthostatic symptoms was 4 (range 1–9) for both the full sample and the medication-naïve sample; there was no difference between the Groups 1, 2, or 3 in the median number of orthostatic symptoms provoked (4 vs. 4 vs. 2, respectively; P = 0.18, Kruskal–Wallis test).
Table 2. Proportions reporting increased orthostatic symptoms during 10 min of passive standing.
Life table analysis: illustrates the time until HR criteria for POTS were reached during the 10 min of passive standing. The median time to HR criteria for POTS was 3 min for both the full sample ((a)) and the medication-naïve subset ((b)). The proportion of participants in whom the diagnosis of POTS would have been missed at different durations of orthostatic stress in the full sample is presented in . There was no difference in the survival curves by age or by sex. The median time to reaching HR criteria for POTS for males was 2 min versus 3 min for females (Hazard ratio [Mantel-Haenszel] 1.72; 95% CI, 0.97–3.1; P = 0.11). Those 12–19 years had a median time to reaching HR criteria for POTS of 3 min compared to 2 min for those ≥ 20 years (Hazard ratio 0.79; 95% CI, 0.47–1.30; P = 0.23).
Figure 3. Survival curves illustrating the time during the standing test at which point the HR criteria for POTS could be confirmed for those in the full sample ((a)) or the medication-naïve subset ((b)).
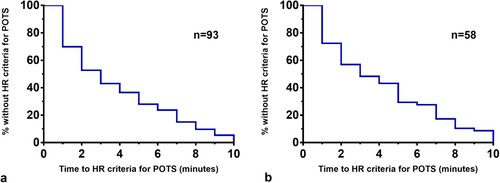
Table 3. Proportion of POTS diagnoses that would be missed at each minute of an abbreviated standing test (full sample, N = 93).
Of those whose peak standing HR was ≥ 120 bpm at any point during the 10-minute PST, 23/24 attained the age-appropriate HR increase for the diagnosis of POTS within the first 5 min upright, compared to 45/69 of those whose peak HR was < 120 bpm (P = 0.003).
Discussion
This study introduces two observations about the methods for conducting the PST for diagnosing POTS:
Measuring the supine HR for 2 min after a PST improves detection of POTS
Our data show that the supine HR before a 10-minute standing test was significantly higher than the post-test supine HR. In the full sample, an additional 14% more adolescents and young adults were categorized as having POTS when the definition of their lowest supine HR included a brief 2-minute post-test measurement along with the conventional pre-test measure. The 95% confidence interval around that estimate suggests that between 9 and 21% more individuals would be classified as having POTS by incorporating the post-test supine HRs in the calculation of the increment between the supine and standing HR. For the medication-naïve group, an additional 21% (95% CI, 10–31%) would meet criteria for POTS.
While our results might raise concerns about contributing to an over-diagnosis of POTS, all individuals were being evaluated for chronic symptoms consistent with the diagnosis, all had provocation of orthostatic symptoms during the period upright, and over 70% had provocation of or increases in acrocyanosis while standing. Moreover, those who met criteria for POTS based on their post-test supine HR values alone had significantly elevated pre-test supine HRs, suggesting that uncertainty or apprehension about what was about to occur during an unfamiliar test was associated with atypical pre-test cardiac acceleration above their usual supine baseline values. Consistent with this view, the post-test supine HR values in this group were similar to the pre-test supine HR in the remainder of the study population.
Several comments about the study methods are germane. We elected to exclude individuals who developed a neurally mediated drop in blood pressure during the PST because the relative bradycardia after a vaso-vagal event would not be representative of the true baseline supine HR. Including patients who developed a cardio-inhibitory response would have had the potential to over-diagnose and mis-categorize individuals as having POTS.
In the full sample, 35 of 93 participants were being treated for other conditions with medications that can be used to treat orthostatic intolerance, including SSRI/SNRI medications, contraceptives, or stimulants. We retained these individuals in the analysis because our main interest was in measuring whether the pre-test and post-test heart rates would differ for each individual. Among those who had not undergone a 10-minute or longer orthostatic challenge, we felt the pre- and post-test HR data would not be affected by the specific medication used, especially if the mechanism of the higher HR pre-standing was related to apprehension about the upcoming procedure. The 93 participants comprise a practical sample, similar to patients encountered in clinical settings, as these medications are in common use among those who have symptoms consistent with POTS and ME/CFS. However, to test whether the study findings differed among those not being treated with medications used for managing orthostatic intolerance, we also report the results for the group of 58 medication-naïve participants. While the smaller sample had a lower statistical power to detect differences between the heart rate groups, the main study results were similar regardless of medication status.
Other standing test protocols specify a longer period of time supine to obtain baseline measures. In the study by Hyatt and colleagues, for example, participants remained supine for at least 7 h before the standing test [Citation11]. In a study of active standing, Plash and colleagues positioned subjects supine for at least an hour and in many instances, the entire night [Citation13]. We elected to use an abbreviated period supine in order to make this test more practical for usual outpatient clinical settings. It was only after instituting this PST protocol that we observed informally that some individuals had elevated HRs in the pre-test supine period, generating the questions that we tested in the current study. We acknowledge that a 5-minute pre-test and a 2-minute post-test period of supine HR monitoring might not be optimal. Future studies could examine whether a longer period of time supine before the standing test (e.g. 10 min) would reduce the difference in HR between the pre-test and post-test periods. Similarly, further work is needed to explore whether a longer period supine after the test (e.g. 5–10 min) would change its diagnostic yield.
There were several limitations of this study. First, we did not investigate factors associated with the slightly higher pre-test HRs among those undergoing a first standing test. While we speculate that apprehension or uncertainty about what is involved in the test could cause slightly higher pre-test HRs, we did not ask specifically about pre-test apprehension, nor did we include a measure of anxiety. If pre-test apprehension is a factor, one would expect the difference between pre-test and post-test HRs to diminish with repeat testing, as individuals become more familiar with the test procedures. It is possible that the decrease in HR following the test is instead due to a physiologic post-test change in vagal tone, which could be measured in future studies and compared to results in healthy controls. It is also possible that the more frequently HR is checked, the more likely it is that there will be a regression to the mean or to the most representative HR value. Second, it is now well established that there is a diurnal variation in HR among those with POTS, with higher standing HRs being noted in the morning compared to the afternoon [Citation14,Citation20]. It is unclear if the differences we observed would be present if testing was performed in the morning. Third, it is unclear whether the participants in this study are representative of all adolescents and young adults with POTS, as they were being evaluated in a tertiary care chronic fatigue clinic. In pediatric and adult studies, individuals with ME/CFS and POTS have a greater symptom burden than those with POTS alone [Citation21,Citation22]. Confirmation of the same pattern of response in cardiology or neurology clinics would help define whether the phenomena we report are generally present in everyone with POTS or are more common in those with a prominent degree of fatigue.
As with any diagnostic test, an important goal is to correctly identify individuals who truly have the condition in question, in part to make effective therapies available, and to avoid mistakenly exposing those who do not have the condition to unnecessary treatment and possible harm. While the data above suggest that the additional patients diagnosed with our study procedures had symptoms and signs suggestive of POTS, strictly speaking we cannot exclude the possibility that this PST or other methods of identifying POTS are leading to an unsupported increase in the diagnosis of individuals who do not need treatment. Definitive evidence would need to come from a randomized trial showing a similar rate of response to POTS therapy among those diagnosed with POTS using the post-test HR versus just the pre-test HR.
Less than 10 min upright misses a substantial proportion of POTS diagnoses
Our results show that 53% (95% CI, 43–63%) of those meeting the 2011 Consensus definition of POTS would be missed by a 2-minute PST and that 27% (95% CI, 19–37%) would be missed during a 5-minute test. On the basis of these findings, we recommend that a full 10 min remain the standard duration of upright posture for diagnosing POTS in both clinical and epidemiologic studies.
Several points about this portion of the study deserve further discussion. First, a 5-minute test would identify all but one of those who reached a peak HR ≥ 120 bpm at some point during the 10-minute test, and thus would enrich the rate of diagnosing florid POTS. Those meeting POTS criteria during the final 5 min of the test had significantly lower peak HR values. It would be reasonable to assume that those with higher HR increments with standing, or a peak HR ≥ 120, would have worse overall health. However, we are not aware of data to suggest that there is a strong correlation between the HR increase during orthostatic testing and health-related quality of life (HRQOL) for patients with POTS. In a retrospective series of 94 adults with POTS assessed during a 5-minute head-up tilt test, Benrud-Larson and colleagues found no difference in HRQOL between those who did or did not have a sustained HR ≥ 120 during the test [Citation23]. Moon and colleagues examined 107 mainly adult patients whose POTS was diagnosed with a 10-minute active standing test. They found no correlation between the maximal orthostatic HR increment and either orthostatic symptoms or HRQOL [Citation24]. In both studies, a major determinant of HRQOL was symptom severity. These two studies would suggest that the individuals diagnosed in the last 5 min of the standing test are no less likely to have impaired HRQOL than those with earlier and more robust hemodynamic changes. Until we have data from randomized trials, we have no evidence to suggest that the group diagnosed in the final 5 min of the test is less likely to benefit from treatment.
Although the absence of continuous HR monitoring during standing might be viewed as a weakness, we were examining common, practical methods of measurement available in any clinic as opposed to procedures available mainly in dedicated autonomic or cardiovascular laboratories. In support of this method, Kirbis and colleagues found no differences in mean HR increases when comparing continuous monitoring and discrete HR measurements at specific time intervals during either passive tilt or active standing [Citation15].
A strength of this study is that the data were collected in the same manner by a single examiner, thus eliminating variability due to inter-examiner differences in conducting the PST. A limitation of our study was that we conducted the PSTs in the afternoon, whereas POTS is more likely to be identified during orthostatic testing conducted in the morning [Citation14,Citation20]. It remains to be seen whether differences in the time to POTS would be the same for testing performed in the morning. Because many individuals with POTS and chronic fatigue have worse function earlier in the day, and might not be able to get to clinic evaluations until later in the day, our findings from early afternoon testing are likely to apply to a substantial proportion of individuals, particularly those with ME/CFS.
POTS can be treated with increased sodium and fluid, postural counter-measures, compression garments, and other non-pharmacological interventions, as well as with medications, all of which would have the potential to improve daily function and HRQOL as well as prevent further deterioration in function. A test with less than 10 min of orthostatic stress would overlook this potential in a substantial proportion of patients.
Conclusions
We conclude that a minor methodological modification of the 10 min in-clinic passive standing test—namely the inclusion of a 2-minute supine post-test HR measure to help ascertain the representative supine HR—has the potential to improve the rates of diagnosing POTS among those with chronic orthostatic symptoms, and thus would increase the proportion of individuals who would qualify for treatment. Given the substantially lower diagnostic yield of abbreviated standing tests, we recommend a full 10 min of standing for the diagnosis of POTS.
Acknowledgments
We thank the patients and families whose participation made this study possible. This study was supported by philanthropic contributions to the Chronic Fatigue Program at the Johns Hopkins Children’s Center. Dr. Rowe is supported by the Sunshine Natural Wellbeing Foundation Professorship of Chronic Fatigue and Related Disorders.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Maria Roma
Maria Roma has been a summer research assistant in the Johns Hopkins Pediatric Chronic Fatigue Clinic for the past two years. She has ME/CFS, POTS, and Ehlers-Danlos Syndrome (EDS). At present, she is an undergraduate student at Binghampton University, Binghampton, NY, USA.
Colleen L. Marden
Colleen L. Marden is the Research Coordinator for the Johns Hopkins Pediatric Chronic Fatigue Program. Her initial experience with ME/CFS came through caring for her daughter, who developed the illness at age 12, and is now fully recovered.
Peter C. Rowe
Peter C. Rowe is a Professor of Pediatrics and the Director of the Johns Hopkins Pediatric Chronic Fatigue Clinic. For the past 25 years, he has been active in the clinical care of those with ME/CFS, EDS, and orthostatic intolerance, and in the clinical investigation of these conditions.
References
- Arnold AC, Ng J, Lei L, et al. Autonomic dysfunction in cardiology: pathophysiology, investigation, and management. Canadian J Cardiology. 2017;33:1524–1534. doi: https://doi.org/10.1016/j.cjca.2017.09.008
- Sheldon RS, Grubb BP, Olshansky B, et al. Heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015;12:e41–e63. doi: https://doi.org/10.1016/j.hrthm.2015.03.029
- Stewart JM, Boris JR, Chelimsky G, et al. Pediatric disorders of orthostatic intolerance. Pediatrics. 2018;141:e20171673. doi: https://doi.org/10.1542/peds.2017-1673
- Institute of Medicine. Committee on the diagnostic criteria for myalgic encephalomyelitis/chronic fatigue syndrome. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. Washington, D.C. The National Academies Press, February 10, 2015.
- Rowe PC, Barron DF, Calkins H, et al. Orthostatic intolerance and chronic fatigue syndrome associated with Ehlers-Danlos syndrome. J Pediatr. 1999;135:494–499. doi: https://doi.org/10.1016/S0022-3476(99)70173-3
- De Wandele I, Rombaut L, Leybaert L, et al. Dysautonomia and its underlying mechanisms in the hypermobility type of Ehlers-Danlos syndrome. Semin Arthritis Rheum. 2014;44:93–100. doi: https://doi.org/10.1016/j.semarthrit.2013.12.006
- Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. doi: https://doi.org/10.1007/s10286-011-0119-5
- Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–137. doi: https://doi.org/10.1212/WNL.43.1_Part_1.132
- Kanjwal Y, Kosinski D, Grubb BP. The postural tachycardia syndrome: definitions, diagnosis, and management. PACE. 2003;26:1747–1757. doi: https://doi.org/10.1046/j.1460-9592.2003.t01-1-00262.x
- Low PA, Opfer-Gehrking TL, Textor SC, et al. Postural tachycardia syndrome (POTS). Neurology. 1995;45:S19–S25.
- Hyatt KH, Jacobson LB, Schneider VS. Comparison of 70° tilt, LBNP, and passive standing as measures of orthostatic tolerance. Aviat Space Environ Med. 1975;46:801–808.
- Streeten DHP, Thomas D, Bell DS. The roles of orthostatic hypotension, orthostatic tachycardia, and subnormal erythrocyte volume in the pathogenesis of chronic fatigue syndrome. Am J Med Sci. 2000;320:1–8. doi: https://doi.org/10.1016/S0002-9629(15)40790-6
- Plash WB, Diedrich A, Biaggioni I, et al. Diagnosing postural tachycardia syndrome: comparison of tilt testing compared with standing haemodynamics. Clin Science. 2012;124:109–114. doi: https://doi.org/10.1042/CS20120276
- Moon J, Lee HS, Byun J-I, et al. The complexity of diagnosing postural orthostatic tachycardia syndrome: influence of diurnal variability. J Am Soc Hypertension. 2016;10:263–270. doi: https://doi.org/10.1016/j.jash.2016.01.011
- Kirbis M, Grad A, Meglic B, et al. Comparison of active standing test, head-up tilt test and 24-hour ambulatory heart rate and blood pressure monitoring in diagnosing postural tachycardia syndrome. Funct Neurol. 2013;28:39–45.
- Winker R, Prager W, Haider A, et al. Schellong test in orthostatic dysregulation:a comparison with tilt-table testing. Wien Klin Wochenschr. 2005;117:36–41. doi: https://doi.org/10.1007/s00508-004-0288-5
- Arnold AC, Ng J, Raj SR. Postural tachycardia syndrome—diagnosis, physiology, and prognosis. Auton Neurosci: Basic. 2018. doi:https://doi.org/10.1016/j.autneu.2018.02.005.
- Braune S, Wrocklage C, Schutle-Monting J, et al. Diagnosis of tachycardia syndromes associated with orthostatic symptoms. Clin Autonom Res. 1999;9:97–101. doi: https://doi.org/10.1007/BF02311766
- Hoad A, Spickett G, Elliott J, et al. Postural orthostatic tachycardia is an under-recognized condition in chronic fatigue syndrome. Q J Med. 2008;101:961–965. doi: https://doi.org/10.1093/qjmed/hcn123
- Brewster JA, Garland EM, Biaggioni I, et al. Diurnal variability in orthostatic tachycardia: implications for the postural tachycardia syndrome. Clin Science. 2012;122:25–31. doi: https://doi.org/10.1042/CS20110077
- Stewart JM, Gewitz MH, Weldon A, et al. Orthostatic intolerance in adolescent chronic fatigue syndrome. Pediatrics. 1999;103:116–121. doi: https://doi.org/10.1542/peds.103.1.116
- Okamoto LE, Raj SR, Peltier A, et al. Neurohumoral and haemodynamic profile in postural tachycardia and chronic fatigue syndromes. Clin Science. 2012;122:183–192. doi: https://doi.org/10.1042/CS20110200
- Benrud-Larson LM, Dewar MS, Sandroni P, et al. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc. 2002;77:531–537. doi: https://doi.org/10.4065/77.6.531
- Moon J, Kim D-Y, Byun J-I, et al. Orthostatic intolerance symptoms are associated with depression and diminished quality of life in patients with postural tachycardia syndrome. Health Qual Life Out. 2016;14:144. doi: https://doi.org/10.1186/s12955-016-0548-x