Abstract
Hepatitis E virus (HEV) infection is one of the main causes of acute hepatitis worldwide. A recombinant hepatitis E vaccine, HEV 239, has been licensed in China for immunizing adults of 16 y old and above. The vaccine antigen contains pORF2 aa 368 - 606 of the HEV genotype 1 expressed in E. coli. The quality of the vaccine is controlled through a combination of biophysical, biochemical and immunochemical methods. The vaccine is well tolerated in adults. The efficacy of the HEV 239 vaccine against symptomatic and asymptomatic infection had been proven to be high during a Phase III clinical trial and long-term follow up. The safety and efficacy of HEV 239 vaccine in certain high-risk populations remains to be further investigated.
Introduction
Hepatitis E (HE) is caused by infection with the hepatitis E virus (HEV). The awareness of this disease has increased a lot in recent years.Citation1 HEV infection remains a serious threat to life and productivity in the developing world. In the developed countries, an increasing number of autochthonous HE cases have been reported, suggesting that this threat is not limited to developing regions. Patients with underlying liver disease and patients who are immunosuppressed or immunocompromised are at particular risk.Citation2
HEV is a non-enveloped, single-stranded, positive-sense RNA virus. It is the only member of the genus Hepevirus in the family Hepeviridae.Citation3 Four genotypes of HEV are associated with human diseases: genotypes 1 and 2 infect only humans, while genotypes 3 and 4 infect many animal species in addition to humans.Citation3,4 All 4 genotypes are characterized as one serotype, making the development of a univalent hepatitis E vaccine reasonable. Two candidate hepatitis E vaccines have been evaluated in clinical trials. The first of these, rHEV,Citation5 was developed by GlaxoSmithKine (GSK, Rixensart, Belgium). The other, HEV 239 (trade name Hecolin®),Citation6-8 was developed by Innovax (Xiamen, China) (). HEV 239 was launched in China in 2012. In this review, we focus on the development of the HEV 239 vaccine.
Table 1. Characteristics of the 2 existing hepatitis E vaccines that have been evaluated in clinical trials
Structural basis for the HEV 239 vaccine
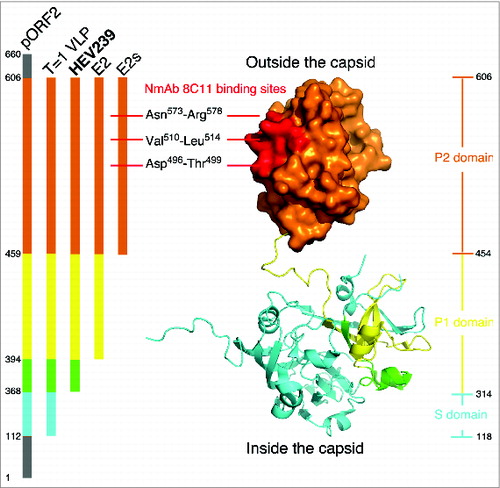
The HEV genome consists of 3 open reading frames (ORFs). ORF2 encodes a single structural protein (pORF2) for the HEV capsid. Structurally, the HEV capsid is arranged as a T = 3 icosahedral lattice, which assembles with the coordination of the HEV RNA genome.Citation9 The approximately 110 amino acid residues (aa) of the N-terminal region are responsible for RNA binding, given the positively charged arginine-clustered region here. With truncations in the 111 N-terminal aa and the 53 C-terminal aa, the protein can self-assemble into a T = 1 icosahedral VLP via a baculovirus / insect cell expression system.Citation10 The VLP form of genotypes 3 and 4 can be crystallized to allow the structural determination at a resolution of 3.5 Å.Citation11,12 The analysis of these structures has revealed 3 functional domains of pORF2, including the S domain (aa 118–313), which contributes to the 5-fold interaction and the formation of the basal shell; the P1 domain (aa 314–453), which is important for the 3-fold interaction; and the P2 domain (aa 454–606), which is involved in the 2-fold interaction and is responsible for host interactions.Citation11,12 The P2 domain, also called the E2s domain, was further analyzed, and its high-resolution crystal structure was determined. The crystal structure showed that two E2s form a tight homodimer through the strong hydrophobic interaction maintained by side chains of residues Val503, Trp548, Thr552, Ala555, Tyr557, Tyr561, Val598 and Val600 of both subunits of the dimer.Citation13 There is a unique groove region located on the most protrusive moiety along the 2-fold axis. This groove region was verified to be involved in virus-host interactions using site-directed mutagenesis and the binding of neutralizing mAbs.Citation13 The structure of the E2s domain in a complex with neutralizing mAb 8C11 further pinpointed the neutralization sites at Asp496-Thr499, Val510-Leu514, and Asn573-Arg578. Mutation and cell-model assays identified Arg512 as the most crucial residue for 8C11 interaction with and neutralization of HEV.Citation14 It should be noted that the epitope recognized by 8C11 represents a major immune dominant epitope. As such, it is capable of blocking naturally acquired anti-HEV from binding with the E2 antigen.Citation15
HEV 239 was designed as ORF2 aa368–606 fragment from a further N-terminal truncation of T = 1 VLP and was adapted to be expressed with a high efficiency in E.coli.Citation16 During the development of the HEV 239 vaccine, some historic constructs based on antigenicity and immunogenicity were produced, including E2s (aa459–606), E2(aa394-606), HEV239(aa368-606) and T=1 VLP(112-607). E2s forms a dimer with 8C11 binding activity (Fig. 1). E2 (aa394–606) forms a hexamer with considerable antigenicity and marked immunogenicity in rhesus monkeys. The N-terminal 26 aa extension of E2, called HEV 239, self-assembles into shrunken version of the virus-like particles of the ∼20 nm diameter. These particulate particles verified with plausible antigenicity and immunogenicity.Citation16, 17 Although HEV 239 (aa 368–606) protein is N-terminally shorter than T = 1 VLP (aa 112–606) in aa sequence, but they share the structural feature of outmost moiety of viral capsid (), which, E2s, or called P2 domain, is responsible for virus-host interaction and subsequently accounts for antigenicity and immunogenicity in vaccine design. Compared with the complex expression in insect cell, production in E.coli is easy to handle and scale-up. HEV 239 can self-assemble into particles in vitro. However, T = 1 VLP was only found forming particle in insect cell (rHEV vaccine,Citation5 GSK). In clinical trials, HEV 239 and T = 1 VLP conferred comparable hepatitis E protection in vaccine recipients.Citation5,7 In terms of nanoscale molecules used for antigen presenting cell processes, VLPs were verified to be the key characteristics for immune recognition.Citation17,18 Based on this, successful recombinant vaccines for hepatitis B and human papillomavirus were brought to market.Citation17,18 Thus, HEV 239 was considered for further preclinical study as a HEV vaccine.
Figure 1. Structural interpretation for HEV 239 vaccine. The structure of HEV ORF2 aa112–606 is rendering in surface mode (P2 domain) and cartoon mode (P1 and S domain), colored by key regions for several HEV historic constructs (left panel), i.e., aa112–367 in cyan, aa368–393 in green, aa394–458 in yellow and aa459–606 in orange. To elucidate the spatial configuration, 3 crystal structures were in superimposition to demonstrate major neutralization sites ( Neutralizing mAb 8C11 binding sites colored in red, by PDB (protein database) no. 3RKD), outmost protrusion domain (E2s domain in orange surface, by PDB no. 3GGQ) and P1&S domains (contributing for virus capsid assembly, by PDB no. 3HAG). The figure was prepared by the program PyMol.
The preclinical development of HEV 239 vaccine
HEV 239 was over expressed under the regulatory of T7 promoter in E. coli, the gene was cloned into a non-fusion plasmid vector pTO-T7 and subsequent expression was induced by IPTG. HEV 239 forms inclusion bodies in bacteria. These inclusion bodies were harvested by liquid-solid separation and pan-washing with Triton-X100 before chaotropic agent denaturation. After harvesting, the resulting ∼70% pure HEV 239 inclusion bodies were dissolved in 4 M urea. The HEV 239 protein was further purified to a final purity of 95–98% by 2 polishing chromatography steps and then subjected to particle reassembly with a scalable tangential flow technique, using a membrane cassette with a cutoff size of 30kD.Citation16 The reassembly condition was optimized for a robust range of pH, ion-strength and temperature. Essentially, HEV 239 can self-assemble in 4 M urea in the presence of salt, which facilitates the assembly control during this process.Citation19 Self-assembled HEV 239 was further fractionalized using capture chromatography, and the component with an average radius of ∼10 nm was collected and absorbed onto an aluminum adjuvant.Citation16
The pilot process scale was developed in 10 L fermentation batches, which generated several thousands of vials of vaccine for investigational new drug (IND) purposes. Briefly, the conditions for the serial Essentially, HEV 239 processing were gauged using a variety of biochemical and biophysical approaches, including SDS-PAGE, Western blotting, DLS (Dynamic Light Scattering), SEC HPLC (Size-Exclusion Chromatography), AFM (Atom Force Microscopy), AUC (Analytical Ultracentrifugation), DSC (Differential Scanning Calorimetry) and TEM (Transmission Electronic Microscopy).Citation20 Antigenicity was monitored using a sandwich ELISA with mAbs 8C11 and 8H3. This mAb couple identified the E2s moiety of HEV 239 on the groove region of the E2s crystal structure.Citation13 As a rare event in virology, the binding of 8C11 with an antigen will significantly enhance the binding affinity of another mAb 8H3 by 2 log in SPR (Surface Plasmon Resonance) assay. The synergistic action of this mAb couple also was discernible in authentic HEV.Citation15 All residual contaminants were evaluated and quantified under the regulatory limits issued by the China Food and Drug Administration (CFDA). The immunogenicity of the formulated HEV 239 was measured in vivo with the ED50 (50% effective dose) test in mice and with the anti-HEV levels in rhesus monkeys. The ED50 value for HEV 239 in mice was approximately 0.05 μg, demonstrating that HEV 239 has a high level of immunogenicity. Indeed, the immunogenicity of HEV 239 was 200 times greater than that of the E2 protein.Citation16,21 The protection efficacy of HEV 239 was primarily evaluated in the rhesus monkey model. Two doses of 5 μg, 10 μg or 20 μg HEV 239 were administered 4 weeks apart. The induced antibody response peaked at week 7 in all 3 dosage groups with comparable antibody titers of around 1384 IU. The anti-HEV levels were measured by ELISA assay and calibrated against a World Health Organization (WHO) reference standard for anti-HEV (National Institute for Biological Standards and Control, NIBSC catalog no. 95/584), which was obtained from an recovered hepatitis E patients with an arbitrary assigned value of 100 IU.Citation16 The monkeys were then challenged with 104 or 107 genome equivalences of homologous HEV (genotype 1) or heterologous HEV (genotype 4). All of the HEV 239 vaccinated monkeys (n = 24 ) were completely protected from symptomatic hepatitis E and were also completely protected from subclinical infection by 104 HEV (n = 12 ). When challenged with 107 HEV, 9 of the 12 monkeys (75%) were protected from subclinical infection. Among the 3 monkeys with breakthrough HEV infection, 2 monkeys (with anti-HEV level of 368 IU and 196 IU) shed virus during the first week post infection and the third monkey (anti-HEV level 961 IU) had detectable HEV RNA in feces during week 3. The vaccine protection efficacy against homologous HEV (genotype 1) or heterologous HEV (genotype 4) infection was similar.Citation16 After the further safety evaluation of HEV 239 in animals, which including intramuscular irritation in rabbits, acute toxicity testing in KM (Kunming) mice, deformity testing in gravid white KM mice and repetitive immune toxicity testing in SD (Sprague dawley) rats, the HEV 239 vaccine was approved by the CFDA for further clinical trials.Citation8,22
Assessment the HEV 239 vaccine critical quality attributes (CQAs)
The product consistency of the HEV 239Citation8,20,23 vaccine was demonstrated using a combination of biophysical, biochemical and immunochemical methods. Results from these methods demonstrated that different vaccine batches have comparable antigen characteristics or CQAs.
The presence of clinically relevant epitopes can be used as a surrogate marker for vaccine potentcy.Citation17,24,25 A large panel of monoclonal antibodies (mAbs) against the HEV capsid protein were developed to improve the understanding of HEV virology and to analyze the CQAs of the vaccine during bioprocessing and in the lot release and stability testing.Citation14,15,20,26,27 Key epitopes of the particulate antigen were analyzed using a panel of murine anti-HEV mAbs against different epitopes, analogous to the approach used for other vaccines.Citation24,25,28-31 Epitope mapping using cross-inhibitory ELISA, surface plasmon resonance biosensor chip based binding assay, and sandwich ELISA using HEV 239 specific mAbs were used to assure vaccine potency.Citation20,26,27 Overall, our findings showed that the antigen production process is robust and scalable in Hecolin® manufacturing.
A successful and robust scale-up (i.e., 50-fold) in the production process (1.0 L, 10 L and 50 L) was demonstrated.Citation20 Multiple HEV 239 batches were produced at a commercial scale (50 L) and were characterized using several different methods ().
Table 2. Analytical toolbox for the comprehensive characterization of a recombinant HEV 239 antigen to demonstrate the process reproducibility and product consistency during Hecolin® production.Citation20,26,27
Biochemical methods
To verify the consistency of the primary amino acid sequence, analyses were carried out to determine the antigen purity, antigen integrity, molecular weight, pI and protein sequence.Citation20,27 The most prominent band on the SDS-PAGE gel showed an apparent molecular weight of 29.4 kDa. All three lots exhibited similar MWs by MALDI-TOF-MS, with a mean MW of 25,561.5 ± 1.5 Da (), which is consistent with the theoretical MW of HEV 239. In addition, LC-MS-based protein mapping with tryptic digestion was used to determine the peptide sequence. The overall amino acid sequence was confirmed with 100% coverage for all 3 batches.Citation20
Biophysical methods
Four different sizing methods (TEM, HPSEC, AUC and DLS) were used to monitor the particle size consistency (). The HEV 239-based VLPs were found to be 20–30 nm in diameter by TEM, with a certain degree of irregularity and heterogeneity.Citation20 HPSEC analysis resulted in nearly identical retention time for all 6 lots of HEV 239 aqueous products.Citation20 The AUC profiles demonstrated highly similar sedimentation properties for the 6 different lots, all of which peaked at approximately 21.5 s.Citation20
Both circular dichroism and UV spectroscopy methods verified the consistency in the secondary and tertiary protein structure among the different HEV 239 lots.Citation20
The thermal unfolding of the VLP antigen was analyzed using DSC by monitoring the Tm values of all HEV 239 lots, all of which ranged from ∼75–76°C. These results showed good cross lot product consistency as reflected by the thermal stability of the recombinant protein antigen during heat-induced unfolding.Citation20,27 In addition, the propensity for the protein to aggregate under thermal stress was assessed using cloud point analysis. The heat-induced unfolding or aggregation profiles of the VLPs were highly comparable among all 6 lots, as determined by both DSC and cloud point (Table 2).
Immunochemical methods
The in vitro antigenicity of an antigen, as probed with different mAbs, correlates in general to the in vivo immunogenicity of a vaccine.Citation30-33 Therefore, a one-site binding and label-free assay (i.e., surface plasmon resonance or SPR)34-36 and a 2-site binding assay (i.e., a sandwich ELISA)Citation30,31,33 were carried out to evaluate the antibody binding activity of the HEV 239 antigen.
The label-free and real time sensor chip-based SPR method was used with 5 mAbs recognizing different epitopes (mouse IgGs-8C11, 8H3, 13D8, 12A10 and 16D7) to assess the immunoreactivity of the different batches.20 A sandwich ELISA was implemented in a manufacturing setting with the desired robustness and reproducibility to show product consistency.Citation20 A well-characterized neutralizing and protective mAb, 8C11, was selected as the capture antibody,Citation26 and 8H3 labeled with HRP was used as the detection antibody.
Immunological assessment
To measure the in vivo potency of the vaccine immunogenicity, 6 batches of HEV 239 were evaluated by measuring the ED50 in a mouse model after absorption onto an amorphous aluminum-based adjuvant. All six batches had comparable ED50 values in the range of 0.025–0.060 μg (). Two of the assays used here, the SDS-PAGE and the mouse potency assay (ED50 determination), are used for routine lot release testing on the final vaccine formulation and filled product. Based on assay results on recovered antigen, full antigen recovery was observed post adjuvant dissolution with no alterations on antigen characteristics.Citation27
Clinical development of HEV 239
All pre-approval clinical trials were carried out in China in accordance with guidelines and regulations issued by the CFDA and the provisions of the Declaration of Helsinki.
Efficacy of HEV 239
A randomized, double-blind, placebo-controlled, single-center Phase III clinical trial was conducted to assess the efficacy of the HEV 239 vaccine against symptomatic hepatitis E. Participants aged 16–65 (n = 112 604) were randomly assigned to receive 30 μg HEV 239 (absorbed to 0.8 mg of aluminum hydroxide suspended in 0.5 mL of buffered saline) or 5 μg of the hepatitis B vaccine control intramuscularly at 0, 1, and 6 months. The patients were followed for 19 months,Citation7 and symptomatic hepatitis E cases were identified though a sensitive surveillance system containing 205 local sentinels at the site. The efficacy was demonstrated to be 100% (95% CI, 72–100) in participants receiving all 3 doses of the HEV 239 vaccine or the control vaccine (PPS, per-protocol set) during the following 12 months, starting at 30 d after the receipt of the final dose (7 m–18 m). In the PPS cohorts, no HEV 239 vaccine recipients contracted hepatitis E, whereas 15 hepatitis E cases developed in the control group during the same year. For those who received at least one dose (ITT, intention to treat cohort), the efficacy was 96% (95% CI, 66–99) during the first 18 months.
In an extended efficacy study,Citation37 all participants in the Phase III trial were followed for another 3 y using the same local hepatitis surveillance system used during the Phase III trial. The long-term efficacy of HEV 239 for up to 4.5 y was demonstrated to be 93% (95% CI, 79 - 98) in the PPS cohort and 87% (95% CI, 71 - 94) in the ITT cohort. Due to the large cohorts of the Phase III trial, only participants in the immunogenicity subset were tested for anti-HEV IgG level at month 7, and as there had been no breakthrough hepatitis E cases in this immunogenicity subset with known anti-HEV level at month 7 until now, the protective level of anti-HEV can not be calculated.
As expected, 90% of the 29 HEV isolates from the identified hepatitis E patients were of genotype 4, which is the predominant HEV genotype circulating in China. The high efficacy of HEV 239, which is derived from HEV genotype 1, against HEV genotype 4 infection supported the concept that a hepatitis E vaccine containing one genotype might protect against infection from different HEV genotypes, which are capable of infecting mammals and share the same serotype.
The HEV 239 vaccine has also been shown to be effective in the prevention of subclinical HEV infection.Citation6,38 Asymptomatic HEV infection was identified by comparing paired serum samples from the same participant collected at 12 month intervals, starting 30 d after administration of the final dose when the antibody levels were supposed to have peaked. If the anti-HEV IgG level rose from negative to 0.154 Wu/mL (twice of the cut-off level) or increased by 4-fold or greater, patients were considered to have asymptomatic infection. The relative risk of HEV infection in the vaccine recipients decreased with increases in the anti-HEV IgG titer at month 7. Even marginal concentration of antibodies significantly lowered the infection risk. This might explain why the efficacy against subclinical HEV infection is similar in the PPS (79%) and ITT cohorts (77%).Citation38 Similar efficacy rates had been demonstrated in the Phase II trial, regardless of whether 3 doses (given at 0, 1 and 6 m) or 2 doses (given at 0, 1 and 6 m) of vaccine were given. In both groups, the tested vaccine contains only 20 μg of the HEV 239 antigen.
The administration of 2 doses of HEV 239 given at months 0 and 1 yielded efficacy rates of 100% (95% CI, 9–100) against hepatitis E during months 2 to 6, before the third shot.Citation7 These data justify the possibility of emergency vaccination during epidemics. Similarly, 2 doses of HEV 239 vaccine given at one month intervals provided satisfying protection against subclinical HEV infection for at least the following 5 months.Citation6
Immunogenicity of HEV 239
HEV 239 administration induced a vigorous anti-HEV IgG response in vaccine recipients, regardless of their initial antibody status. Of the baseline seronegative participants, 99.9% had seroconverted one month after the full vaccination course (7 m), and 87% remained seropositive at month 55. The geometric mean concentration (GMC) of antibody dropped quickly from a peak of 14.96 Wu/ml at month 7, which is higher than the level induced by natural HEV infection (0.6 Wu/ml) but lower than that induced after symptomatic hepatitis E disease (80.9 Wu/ml), to 1.47 Wu/ml for the first year.Citation37,38 The concentration then slowly decreased to 0.27 Wu/ml by month 55. Although the antibody decayed to a relative low level at this time point, the long-term efficacy until month 54 is still as high as 93% (95% CI, 79–98) in the PPS cohort, the need and timing for a booster dose of HEV 239 remains to be further investigated. For participants with pre-existing antibodies, the antibody response is more robust and sustained, with little difference between those who received 1, 2 or 3 doses of HEV 239 vaccine. This finding suggests that people with pre-existing HEV antibodies would benefit from even a single dose of the HEV 239 vaccine.Citation7,37 In a HBsAg positive cohort, the immunogenicity of HEV 239 was found to be similar to that of the general population.Citation39
Anti-HEV IgG levels induced by different dosage and immunization schedules of the HEV 239 vaccine were also evaluated in the Phase II trial, in which only subjects who were anti-HEV seronegative at baseline were enrolled. All participants who received 3 doses of the HEV 239 vaccine seroconverted by month 7, and the level of immune response increased as the dosage increase from 10 μg to 40 μg. The difference in the geometric mean antibody concentration (GMC) was significant between the 30 μg and the other higher doses (p < 0.05). However, the difference in the GMC between 20, 30 and 40 μg was not statistically significant. The study found that the 3 doses course was more potent than the 2 doses course.Citation6
Safety of HEV 239
The data from all clinical trials suggest that the HEV 239 vaccine is well tolerated.Citation40 In the Phase I trial, 44 healthy Chinese adults were enrolled, and all were anti-HEV IgG seronegative with normal serum biochemistry values. Then, 20 μg of the HEV 239 vaccine was administered at month 0 and 1. All participants were followed for 60 d after the first dose, and their serum biochemistry values were analyzed at day 0 and 60. No serious adverse events were reported. Furthermore, the serum biochemistry values of the participants, including fibrinogen (FBG), urea nitrogen (UREA), glucose (GLU), alanine aminotransferase (ALT), showed no abnormal changes from their baseline values by day 60.Citation8
The Phase III clinical trial had 112,604 participants. In this trial, data obtained from the “reactogenicity subset” (n = 2645) within the first 72 hours following each dose showed the occurrence of more frequent local adverse events in the HEV 239 group (13.5%) compared to the control hepatitis B vaccine group (7.1%). This might be partially due to the higher antigen dose in the HEV 239 vaccine (30 μg) than the hepatitis B vaccine (5 μg). Local adverse events of level 3 or higher, solicited systemic adverse events and unsolicited adverse events occurred at similar rates between the study groups. Serious adverse event (SAE) data were collected for all 112,604 participants and categorized according to the Medical Dictionary for Regulatory Activities (MedDRA). During the Phase III trial and the extended 4.5-year follow-up period, a comparable number of participants in both the vaccine and placebo groups experienced SAEs. None of the SAEs were believed to be related to the vaccination.Citation7,37
There were 37 women in the HEV 239 vaccine group and 31 women in the control group who were inadvertently administered the vaccine during pregnancy.Citation41 The rates of adverse events observed in the pregnant women were similar to those observed in matched non-pregnant women. The weights, body lengths and gestational ages of the newborns were comparable in both groups. Although this sample size is small and further study is needed, these data preliminarily suggest that the HEV 239 vaccine is well-tolerated in pregnant women. The safety of HEV 239 was also evaluated in HBsAg positive persons, and the resulting data are reassuring.Citation39
Future Challenges
To date, Hecolin® is only available in the Chinese market and been approved for use in people older than 16 years, it is indicated for vaccinating individuals at high risk of HEV infection, such as those involved in animal husbandry, students, members of the armed forces, food-handlers, women of childbearing age, and travelers to endemic regions. The Chinese CDC has an online reporting system to collect post-marketing adverse reactions/events, and this body has not identified any safety concerns with the vaccine. Although the existing 4.5 y of safety and efficacy data for Hecolin® are reassuring, data has not yet been collected in some special populations, including as pediatric subjects (< 16 y of age), the elderly (> 65 y of age), pregnant women, persons with underlying liver diseases or those who are immunosuppressed. These groups face a greater threat of HEV infection and are therefore in more urgent need of this vaccine.
On the other hand, although epidemics of hepatitis E with thousands of cases and hundreds or tens of deaths occurred now and then in low-resource countries, HEV 239 vaccine are not available to these people until it be prequalified by WHO. The deep gap between the vaccine and those who are in urgent need of this vaccine still waits to be filled.Citation42
In conclusion, the availability of Hecolin® represents an important milestone in the control and prevention of HEV. However, there is still long way to go.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The work was supported by National Major Scientific and Technological Special Project for "Significant New Drugs Development" (2013ZX09101017), National Natural Science Foundation of China (81373061), National High-tech Research & Development Program (863 Program) (2012AA02A408).
References
- Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, Dalton HR. Hepatitis E. Lancet 2012; 379(9835):2477-88; PMID:22549046; http://dx.doi.org/10.1016/S0140-6736(11)61849-7
- Krain LJ, Nelson KE, Labrique AB. Host immune status and response to hepatitis E virus infection. Clin Microbiol Rev 2014; 27(1):139-65; PMID:24396140; http://dx.doi.org/10.1128/CMR.00062-13
- Purcell RH, Emerson SU. Hepatitis E: an emerging awareness of an old disease. J Hepatol 2008; 48(3):494-503; PMID:18192058; http://dx.doi.org/10.1016/j.jhep.2007.12.008
- Huang FF, Sun ZF, Emerson SU, Purcell RH, Shivaprasad HL, Pierson FW, Toth TE, Meng XJ. Determination and analysis of the complete genomic sequence of avian hepatitis E virus (avian HEV) and attempts to infect rhesus monkeys with avian HEV. J Gen Virol 2004; 85(Pt 6):1609-18; PMID:15166445; http://dx.doi.org/10.1099/vir.0.79841-0
- Shrestha MP, Scott RM, Joshi DM, Mammen MP, Jr., Thapa GB, Thapa N, Myint KS, Fourneau M, Kuschner RA, Shrestha SK, et al. Safety and efficacy of a recombinant hepatitis E vaccine. N Engl J Med 2007; 356(9):895-903; PMID:17329696; http://dx.doi.org/10.1056/NEJMoa061847
- Zhang J, Liu CB, Li RC, Li YM, Zheng YJ, Li YP, Luo D, Pan BB, Nong Y, Ge SX, et al. Randomized-controlled phase II clinical trial of a bacterially expressed recombinant hepatitis E vaccine. Vaccine 2009; 27(12):1869-74; PMID:19168109; http://dx.doi.org/10.1016/j.vaccine.2008.12.061
- Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, Wang H, Yang CL, Jiang HM, Cai JP, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 2010; 376(9744):895-902; PMID:20728932; http://dx.doi.org/10.1016/S0140-6736(10)61030-6
- Wu T, Li SW, Zhang J, Ng MH, Xia NS, Zhao Q. Hepatitis E vaccine development: a 14 year odyssey. Hum Vaccin Immunother 2012; 8(6):823-7; PMID:22699438; http://dx.doi.org/10.4161/hv.20042
- Xing L, Li TC, Mayazaki N, Simon MN, Wall JS, Moore M, Wang CY, Takeda N, Wakita T, Miyamura T, et al. Structure of hepatitis E virion-sized particle reveals an RNA-dependent viral assembly pathway. J Biol Chem 2010; 285(43):33175-83; PMID:20720013; http://dx.doi.org/10.1074/jbc.M110.106336
- Li TC, Yoshimatsu K, Yasuda SP, Arikawa J, Koma T, Kataoka M, Ami Y, Suzaki Y, Mai le TQ, Hoa NT, et al. Characterization of self-assembled virus-like particles of rat hepatitis E virus generated by recombinant baculoviruses. J Gen Virol 2011; 92(Pt 12):2830-7; PMID:21865442; http://dx.doi.org/10.1099/vir.0.034835-0
- Guu TS, Liu Z, Ye Q, Mata DA, Li K, Yin C, Zhang J, Tao YJ. Structure of the hepatitis E virus-like particle suggests mechanisms for virus assembly and receptor binding. Proc Natl Acad Sci U S A 2009; 106(31):12992-7; PMID:19622744; http://dx.doi.org/10.1073/pnas.0904848106
- Yamashita T, Mori Y, Miyazaki N, Cheng RH, Yoshimura M, Unno H, Shima R, Moriishi K, Tsukihara T, Li TC, et al. Biological and immunological characteristics of hepatitis E virus-like particles based on the crystal structure. Proc Natl Acad Sci U S A 2009; 106(31):12986-91; PMID:19620712; http://dx.doi.org/10.1073/pnas.0903699106
- Li S, Tang X, Seetharaman J, Yang C, Gu Y, Zhang J, Du H, Shih JW, Hew CL, Sivaraman J, et al. Dimerization of hepatitis E virus capsid protein E2s domain is essential for virus-host interaction. PLoS Pathog 2009; 5(8):e1000537; PMID:19662165; http://dx.doi.org/10.1371/journal.ppat.1000537
- Tang X, Yang C, Gu Y, Song C, Zhang X, Wang Y, Zhang J, Hew CL, Li S, Xia N, et al. Structural basis for the neutralization and genotype specificity of hepatitis E virus. Proc Natl Acad Sci U S A 2011; 108(25):10266-71; PMID:21642534; http://dx.doi.org/10.1073/pnas.1101309108
- Zhang J, Gu Y, Ge SX, Li SW, He ZQ, Huang GY, Zhuang H, Ng MH, Xia NS. Analysis of hepatitis E virus neutralization sites using monoclonal antibodies directed against a virus capsid protein. Vaccine 2005; 23(22):2881-92; PMID:15780737; http://dx.doi.org/10.1016/j.vaccine.2004.11.065
- Li SW, Zhang J, Li YM, Ou SH, Huang GY, He ZQ, Ge SX, Xian YL, Pang SQ, Ng MH, et al. A bacterially expressed particulate hepatitis E vaccine: antigenicity, immunogenicity and protectivity on primates. Vaccine 2005; 23(22):2893-901; PMID:15780738; http://dx.doi.org/10.1016/j.vaccine.2004.11.064
- Zhao Q, Li S, Yu H, Xia N, Modis Y. Virus-like particle-based human vaccines: quality assessment based on structural and functional properties. Trends Biotechnol 2013; 31(11):654-63; PMID:24125746; http://dx.doi.org/10.1016/j.tibtech.2013.09.002
- Grgacic EV, Anderson DA. Virus-like particles: passport to immune recognition. Methods (San Diego, Calif) 2006; 40(1):60-5; PMID:16997714; http://dx.doi.org/10.1016/j.ymeth.2006.07.018
- Yang C, Pan H, Wei M, Zhang X, Wang N, Gu Y, Du H, Zhang J, Li S, Xia N. Hepatitis E virus capsid protein assembles in 4M urea in the presence of salts. Protein Sci 2013; 22(3):314-26; PMID:23281113; http://dx.doi.org/10.1002/pro.2213
- Zhang X, Wei M, Pan H, Lin Z, Wang K, Weng Z, Zhu Y, Xin L, Zhang J, Li S, et al. Robust manufacturing and comprehensive characterization of recombinant hepatitis E virus-like particles in Hecolin((R)). Vaccine 2014; 32(32):4039-50; PMID:24892250; http://dx.doi.org/10.1016/j.vaccine.2014.05.064
- Wu T, Wu XL, Ou SH, Lin CX, Cheng T, Li SW, Ng MH, Zhang J, Xia NS. Difference of T cell and B cell activation in two homologous proteins with similar antigenicity but great distinct immunogenicity. Mol Immunol 2007; 44(12):3261-6; PMID:17408743; http://dx.doi.org/10.1016/j.molimm.2007.01.002
- Zhang J, Shih JW, Wu T, Li SW, Xia NS. Development of the hepatitis E vaccine: from bench to field. Semin Liver Dis 2013; 33(1):79-88; PMID:23564392; http://dx.doi.org/10.1055/s-0033-1338116
- Zhao Q, Zhang J, Wu T, Li SW, Ng MH, Xia NS, Shih JW. Antigenic determinants of hepatitis E virus and vaccine-induced immunogenicity and efficacy. J Gastroenterol 2013; 48(2):159-68; PMID:23149436; http://dx.doi.org/10.1007/s00535-012-0701-1
- Deschuyteneer M, Elouahabi A, Plainchamp D, Plisnier M, Soete D, Corazza Y, Lockman L, Giannini S, Deschamps M. Molecular and structural characterization of the L1 virus-like particles that are used as vaccine antigens in Cervarix, the AS04-adjuvanted HPV-16 and -18 cervical cancer vaccine. Hum Vaccin 2010; 6(5):407-19; PMID:20953154; http://dx.doi.org/10.4161/hv.6.5.11023
- Mulder AM, Carragher B, Towne V, Meng Y, Wang Y, Dieter L, Potter CS, Washabaugh MW, Sitrin RD, Zhao Q. Toolbox for non-intrusive structural and functional analysis of recombinant VLP based vaccines: a case study with hepatitis B vaccine. PloS one 2012; 7(4):e33235; PMID:22493667; http://dx.doi.org/10.1371/journal.pone.0033235
- Wei M, Zhang X, Yu H, Tang ZM, Wang K, Li Z, Zheng Z, Li S, Zhang J, Xia N, et al. Bacteria expressed hepatitis E virus capsid proteins maintain virion-like epitopes. Vaccine 2014; 32(24):2859-65; PMID:24662711; http://dx.doi.org/10.1016/j.vaccine.2014.02.025
- Zhang Y, Li M, Yang F, Li Y, Zheng Z, Zhang X, Lin Q, Wang Y, Li SW, Xia NS, et al. Comparable quality attributes of hepatitis E vaccine antigen with and without adjuvant adsorption-dissolution treatment, Hum Vaccin Immunothare, 2015, In Press.
- Towne V, Zhao Q, Brown M, Finnefrock AC. Pairwise antibody footprinting using surface plasmon resonance technology to characterize human papillomavirus type 16 virus-like particles with direct anti-HPV antibody immobilization. J Immunol Methods 2013; 388(1-2):1-7; PMID:23159495; http://dx.doi.org/10.1016/j.jim.2012.11.005
- Zhao Q, Potter CS, Carragher B, Lander G, Sworen J, Towne V, Abraham D, Duncan P, Washabaugh MW, Sitrin RD. Characterization of virus-like particles in GARDASIL(R) by cryo transmission electron microscopy. Hum Vaccin Immunother 2014; 10(3):734-9; PMID:24299977; http://dx.doi.org/10.4161/hv.27316
- Shank-Retzlaff M, Wang F, Morley T, Anderson C, Hamm M, Brown M, Rowland K, Pancari G, Zorman J, Lowe R, et al. Correlation between mouse potency and in vitro relative potency for human papillomavirus Type 16 virus-like particles and Gardasil vaccine samples. Hum Vaccin 2005; 1(5):191-7; PMID:17012876; http://dx.doi.org/10.4161/hv.1.5.2126
- Schofield T. In vitro versus in vivo concordance: a case study of the replacement of an animal potency test with an immunochemical assay. Dev Biol 2002; 111:299-304; PMID:12678253
- Sitrin RD, Zhao Q, Potter CS, Carragher B, Washabaugh MW. Recombinant protein based viral vaccines, In Vaccine Analysis: Strategies, Principles and Control. Brian Nunnally et al. (eds.) Springer Verlag Berkub Heidelberg, 2015 . Chapter 3, Recombinant Virus-like Particle Protein Vaccines; p.81-112 (DOI: 10.1007/978-3-662-45024-6-3)
- Shank-Retzlaff ML, Zhao Q, Anderson C, Hamm M, High K, Nguyen M, Wang F, Wang N, Wang B, Wang Y, et al. Evaluation of the thermal stability of Gardasil. Hum Vaccin 2006; 2(4):147-54; PMID:17012891; http://dx.doi.org/10.4161/hv.2.4.2989
- Zhao Q, Wang Y, Abraham D, Towne V, Kennedy R, Sitrin RD. Real time monitoring of antigenicity development of HBsAg virus-like particles (VLPs) during heat- and redox-treatment. Biochem Biophys Res Commun 2011; 408(3):447-53; PMID:21527246; http://dx.doi.org/10.1016/j.bbrc.2011.04.048
- Zhao Q, Towne V, Brown M, Wang Y, Abraham D, Oswald CB, Gimenez JA, Washabaugh MW, Kennedy R, Sitrin RD. In-depth process understanding of RECOMBIVAX HB(R) maturation and potential epitope improvements with redox treatment: multifaceted biochemical and immunochemical characterization. Vaccine 2011; 29(45):7936-41; PMID:21871939; http://dx.doi.org/10.1016/j.vaccine.2011.08.070
- Zhao Q, Modis Y, High K, Towne V, Meng Y, Wang Y, Alexandroff J, Brown M, Carragher B, Potter CS, et al. Disassembly and reassembly of human papillomavirus virus-like particles produces more virion-like antibody reactivity. Virol J 2012; 9:52; PMID:22356831; http://dx.doi.org/10.1186/1743-422X-9-52
- Zhang J, Zhang X-F, Huang S-J, Wu T, Hu Y-M, Wang Z-Z, et al. Long-term efficacy of a hepatitis E vaccine. N Engl J Med 2015;372(10):914-22; PMID: 25738667; http://dx.doi.org/10.1056/NEJMoa1406011.
- Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, Yao X, Liang ZL, Wu T, Li JX, Yan Q, et al. Protection against hepatitis E virus infection by naturally acquired and vaccine-induced immunity. Clin Microbiol Infect 2014; 20(6):O397-405; PMID:24118636; http://dx.doi.org/10.1111/1469-0691.12419
- Wu T, Huang SJ, Zhu FC, Zhang XF, Ai X, Yan Q, Wang ZZ, Yang CL, Jiang HM, Liu XH, et al. Immunogenicity and safety of hepatitis E vaccine in healthy hepatitis B surface antigen positive adults. Hum Vaccin Immunother 2013; 9(11):2474-9; PMID:23887167; http://dx.doi.org/10.4161/hv.25814
- WHO Weekly epidemiological record, 2014, 89, 321-336.
- Wu T, Zhu FC, Huang SJ, Zhang XF, Wang ZZ, Zhang J, Xia NS. Safety of the hepatitis E vaccine for pregnant women: A preliminary analysis. Hepatology 2012; 55(6):2038; PMID:22161542; http://dx.doi.org/10.1002/hep.25522
- Nelson KE, Shih JW, Zhang J, Zhao Q, Xia N, Ticehurst J, et al. Hepatitis E vaccine to prevent morbidity and mortality during epidemics. Open Forum Infect Dis 2014: 1(3): ofu098; PMID:25714510; PMID: 25734166; http://dx.doi.org/10.1093/ofid/ofu098
