Abstract
To evaluate the impact of the universal infant HepB vaccination program on hepatitis B virus infection in Hangzhou, China. Hepatitis B incidences and HepB vaccination rates of Hangzhou from 1990 to 2003 were acquired from the historical paper-documents, while which were derived from China Information System for Diseases Control and Prevention and Zhejiang Information System for Immunization Program respectively from 2004 to 2013. A serology survey among person aged 0–59 y was conducted in Hangzhou in 2006. Participants were selected by stratified, multi-stage random sampling. Serum specimens were tested for HBsAg, anti-HBs , anti-HBc , HBeAg and anti-HBe by ELISA. For the past 24 years, hepatitis B incidence and mortality of Hangzhou declined dramatically (χ2 = 3.2 × 104; χ2 = 172.443; both P for trend < 0.001). Both urban and rural incidence descended (χ2urban = 1.904 × 104; χ2rural = 1.633 × 104 ; both P for trend < 0.001).Hepatitis B patients mainly concentrated in 20–40 y old; workers and farmers were the main infection occupations, which was varies in different years (χ2 = 1.619 × 103, P < 0.001). Significant association was found between incidence of hepatitis B and HepB vaccination rate (r = 0 .946, χ2 = 11.813, Pfor trend = 0.001). A total of 5605 participants aged 0–59 y included in this serological survey. The prevalence of HBsAg, anti-HBs, anti-HBc, HBeAg and anti-HBe were 6.19%, 45.83%, 57.25%, 0.62%, and 4.37%, respectively. Hangzhou has successfully integrated the HepB into routine immunization programs and this has had a significant impact on decreasing the incidence of hepatitis B infection.
Introduction
Hepatitis B is an infection of the liver caused by the hepatitis B virus (HBV), a double-stranded DNA virus of the hepadnaviridae family. The virus is transmitted by percutaneous or mucosal exposure to infected blood or body fluids.Citation1 HBV transmission can occur vertically from infected mother to child, sexually or horizontally (e.g. via injecting drug use, sharps injury or occupational exposure).Citation2-4 Most of the chronic hepatitis B infectors were resulted from HBV infecting during infants stage, lacking of effective treatment. One is likely to carry HBV for lifelong after the infection, which will lead to serious disease burden for family and the society. It is estimated that more than one-third of the world's population (or 2 billion people worldwide) has been infected by HBV, with more than 350 million as chronic carriers,Citation5 causing acute or chronic liver diseases, ranging from fulminant hepatitis to cirrhosis and eventually hepatocellular carcinoma (HCC),Citation5-8 accounting for between 520,000 and 1.2 million deaths annually.Citation9 Chronic HBV infection is the most prevalent cause of this tumor, accounting for 55% of global cases, and 89% of those in endemic regions for HBV infection.Citation9-10 In addition, up to 10% of liver transplant operations currently performed are necessitated caused by HBV.Citation10 Both HCC and cirrhosis are among the 10 most common causes of mortality in China; for both, HBV causes the majority of deaths.Citation11
HBV infection is a world-wide health problem. The prevalence of chronic HBV infection varies considerably in different populations and in different geographical regions.Citation10,12 It is most prevalent in the Asia-Pacific region, sub-Saharan Africa, and the Amazon Basin, where more than 8% of the population are chronic carriers of HBV, and between 70% and 98% show serological evidence of having been exposed to the virus. The Asia-Pacific region is home to approximately 75% of all global HBV carriers. As many as 45% of the world's population live in regions of high HBV epidemic, Japan, the Indian subcontinent, southern parts of Eastern, Central Europe, and the Middle-East have intermediate prevalence, with chronic carrier rates of 1 to 8% and exposure rates of 10 to 60%.Citation13 The data shows that HBV infection is also a serious health problem in China. In 1992, the national hepatitis serological survey revealed that the prevalence of hepatitis B surface antigen (HBsAg) was 9.75% in the population aged 1–59 years, and the rate in the 1–4 y age group was as high as the overall rate.Citation12,14,15
Preventing the HBV transmission is an important approach to limit hepatitis B and HBV-related diseases, such as chronic hepatitis, cirrhosis, and liver cancer.Citation16 Routine hepatitis B vaccine (HepB) vaccination through the national immunization program plays a vital role in decreasing subsequent HBV infection in children.Citation17-19 In order to prevent this serious health problem, a safe and effective HepB has been available in the United States since 1981, and universal vaccination of infants has been recommended since 1991.Citation20 The first vaccines were plasma-derived, which contained purified HBsAg obtained from the plasma of people with chronic HBV infection. Subsequently, recombinant DNA HepB has been developed, containing purified HBsAg obtained by culturing genetically engineered yeast or mammalian cells carrying the HBsAg gene. Currently recombinant DNA HepB is predominantly being used, while plasma-derived HepB are still being used in several low-income countries.Citation21 The HepB is safe, highly immunogenic, and effective at preventing hepatitis B occurrence. It also provides long-term protection against risks of infection encountered later in life.Citation22-23 ChangCitation24 highlights that the integration of the HepB vaccination program into the vaccination schedule for all infants will be the most urgent and important measure for controlling HCC in the world.
In view of various ways of HBV infection and the large possibilities of occurring within any general population, control of hepatitis B should be one of the highest priorities in China. China has made great efforts to establish universal infant immunization,Citation25-26 since perinatal transmission is a major mode of HBV transmission in China.Citation22,27 Between 1992 and 2005, there were 3 successive policies encouraging more parents to have their infants vaccinated.Citation28 The HepB was recommended for routine immunization of infants by the Ministry of Health in 1992 where parents paid for the vaccine and a user fee. HepB was then fully integrated into the routine immunization program from 2002 where parents only paid for the user fee while the vaccine was still freely available,Citation29-30 and infants have been vaccinated without any charge since June 1, 2005.Citation31-32 Furthermore, from 2009 to 2011, the government provided the vaccine free of charge to all children under 15 who had not completed 3 doses hepatitis B vaccination. Since HepB vaccine was marketed in China from 1987, as the most effective prevention strategy, the government introduced HepB vaccination program for newborns of highly infectious mothers in 1989 in Hangzhou. HepB was recommended for routine immunization of infants but parents had to pay for the vaccine. Under this program, every newborn infant was required to be vaccinated according to a 0, 1 and 6 months schedule regardless of the mothers’ HBV infection status.Citation33-34 In other words, the first vaccine dose was administered within 24 hours of birth and subsequent doses at 1 and 6 months.Citation31 Compared to other cities, Hangzhou, the capital of Zhejiang province, is highly developed economically and undergoes significant changes in the population density with many migrant workers and health conditions.Citation26 To evaluate whether the impact of the long-term HepB immunization program since 1989 that has been applied widely in Hangzhou where HBV prevalence was highly endemic before universal HepB vaccination is similar to the rest of the country, we investigated the prevalence of hepatitis B infection in 1990–2013. Since HBsAg prevalence rate was 10%, China was a high hepatitis B epidemic area, there were 120 million HBsAg carriers in our country. Due to such a huge number of HBsAg carriers and chronic hepatitis B patients, and tens of thousands of new hepatitis B cases appeared per year, it is necessary to further understand and to grasp HBV infection status, incidence trends and the immune level of the community crowd. In 2006, a serological survey was conduct to evaluate into state plan for immunization against to determine the prevalence of HBsAg, anti-HBs, and anti-HBc in a representative population aged from 0–59 in Hangzhou.
Results
Hepatitis B prevalence
Hepatitis B incidence trends
Overall, hepatitis B incidence declined dramatically in Hangzhou (χ2 = 3.2 × 104 , P for trend< 0.001). The prevalence of hepatitis B infection decreased from 95.41 /100,000 to 70.13/100,000 from 1990 to 1993, and down to 7.62/100,000 in 2013. Details in this survey are presented in . Mortality of hepatitis B patient from 1990 to 2000 was around 0.2–0.5/100,000, which was controlled into lower than 0.05/100,000 from 2001 to 2013. The trend test showed that the mortality of hepatitis B decreased significantly for the past 24 y (χ2 = 172.443, P for trend< 0.001). It appears a growing trend (χ2 = 1.552 × 104 , P for trend< 0.001) for the hepatitis B patient proportion of the total hepatitis patient.
Table 1. Incidence and mortality of hepatitis b in Hangzhou city in 1990–2013
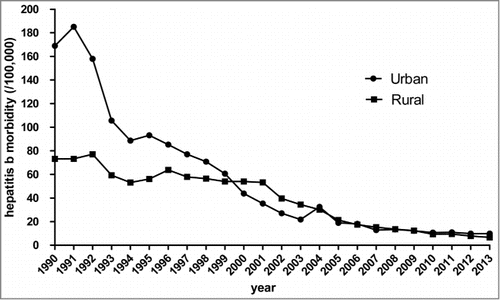
Urban-rural incidence of hepatitis B
There are 5 urban districts and 9 rural districts included in Hangzhou. Both urban and rural incidence of hepatitis B in Hangzhou appears a descending trend (χ2urban = 1.904 × 104 , P for trend< 0.001; χ2rural = 1.633 × 104 , P for trend< 0.001). Compared with 1990, urban incidence of hepatitis B dropped of 94.19%, while 91.05% in rural in Hangzhou in 2013. The ratio of annual incidence of urban to rural declined from 2.31 in 1990 to 1.50 in 2013. Decline of urban incidence of hepatitis B was sharper than rural (χ2 = 1.05 × 103 , P < 0.001). Details about the descending trend of urban-rural incidence of hepatitis B are presented in .
Population distribution of hepatitis B
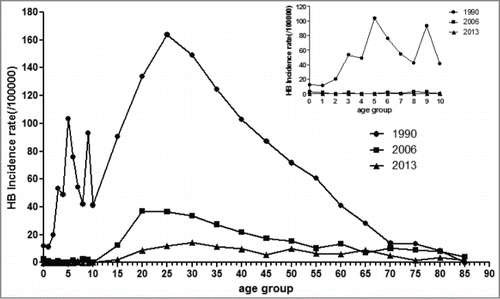
Age distribution of hepatitis B
In the 1990s, the age distribution of hepatitis B incidence in Hangzhou increased in the low age stage. There was a small peak at the “5 ∼” age group, while the highest peak was raised in “25 ∼” or “30 ∼” age group, and followed by a decreased tail. In recent years, the small peak at the “5 ∼” age group was flatting, and the incidence of hepatitis B under 10 y old age group was even lowed to zero. The age distribution of hepatitis B patients was mainly concentrated in 20–40 y old. showed the age distribution of hepatitis B in Hangzhou In 1990, 2006 and 2013 y
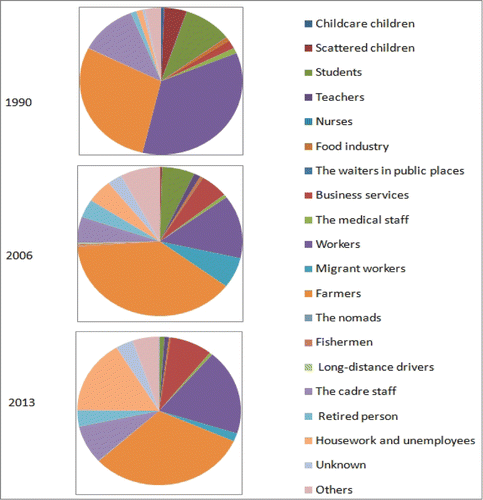
.Occupations distribution of hepatitis B
Professional types of hepatitis B were given priority to workers and farmers, distribution of which was varies in different years (χ2 = 1.619 × 103, P < 0.001). In 1990, hepatitis B infection was mainly occurred in workers which accounted for 34.91%, while the farmers incidence was 28.69%; in 2013, the workers incidence was 18.71% and the farmer was increased to 31.44%. Childcare children fell from 0.6% to 0.15%, scattered children declined from 4.5% to 0. The composition of students and the cadre staff reduced from 9.71% and 11.4% to 0.9% and 8.23% in 2013, respectively. The structure of the catering industry fell from 0.9% down to 0.3%, and medical staff composition reduced from 1.21% to 0.6%. Composition ratio of teachers, business services, retired person, house-worker and unemployees were all increased. ()
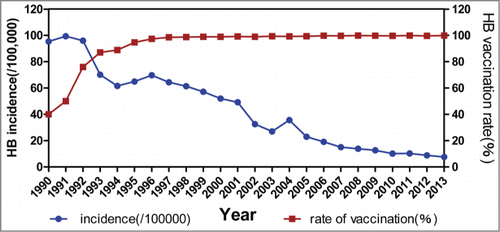
Hepatitis B vaccination in hangzhou
Since HepB was introduced into the EPI in Hangzhou in 1989, its vaccination rate of the targeted population who was included in routine immunization was increased from 87.02% in 1990 to 94.73% in 1993 and to 97% in 1996, following which has been above 98% from 1997 to 2013. showed hepatitis B incidence and HepB vaccination rate in Hangzhou in 1990–2013. Significant association was found between incidence of hepatitis B and rate of HepB vaccination (r = 0 .946, χ2 = 11.813, Pfor trend = 0.001).
HBsAg prevalence rate among people in Hangzhou
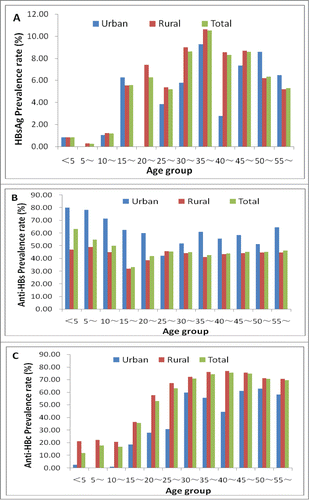
There were 5605 participants (urban: 622; rural: 4983) in the serological survey conducted in Hangzhou in 2006. Results shows that the weighted prevalences of HBsAg, anti-HBs, anti-HBc, HBeAg and anti-HBe for population aged 0–59 y were 6.19% (95%CI: 5.56% – 6.82%), 45.83% (95%CI: 44.53% – 47.13% ), 57.25% (95%CI: 55.95% – 58.55%), 0.62% (95%CI: 0.41% – 0.83%), 4.37% (95%CI: 3.83% – 4.91%), respectively. There were 17.23% population appeared only anti-HBs positive, While 24.96% appeared all of these 5 indicators negative. According to the national serological survey conducted in 1992,Citation35 HBsAg prevalence in Hangzhou was 10.31%, it was greatly diminished. HBsAg prevalence among children age <5 y was only 0.82%. showed the age-specific prevalence rate of (A) HBsAg, (B) Anti-HBs, (C) Anti-HBc and (D) HBV infection rate among people in 2006 in Hangzhou.
Discussion
HepB vaccination has been proved to be the most cost-effective method to control HBV infection and its sequelae in children as well as the adult population.Citation22,36,37 The successful introduction of HepB into the national immunization program has had a great impact on the decrease of HBsAg prevalence. A decline in the prevalence of chronic HBV infection among vaccinated people has been documented throughout the world, including Hong Kong, Taiwan, Italy, the Gambia, Alaska, and Senegal.Citation19,38-44 In the United States, which implemented routine HepB vaccination in 1991, the incidence of acute hepatitis B among children and adolescents has decreased by 89%.Citation45 In Taiwan, where routine infant HepB vaccination commenced in 1984, there has been a 75% decrease in the incidence of HCC among children.Citation18 These data are consistent with those of many studies in China,Citation28,46,47 which have shown the HBsAg prevalence has declined significantly since the introduction of the HepB vaccination. The national serological survey in 2006 shows that for population aged 1–59 years, the HBsAg carrier rate of person aged 0–59 y has decreased from 9.75% in 1992 to 7.18% in 2006 and for children <5 y is now only 1.0%.Citation31 Over the same period, the number of children <5 y who were infected fell from 6.02 million to 0.65 million.Citation31 To promote the equalization of public health service resources, our country provide the reseeding opportunity for children who under the age of 15, unvaccinated or unfinished 3 doses of HepB during 2009 to 2011. In May 2012, China has already reached both the national goal of less than 1% by 2010 and the WHO Western Pacific Regional goal of less than 2% by 2012, and has reached ahead of schedule that the WHO Western Pacific Regional requiring HBsAg prevalence to less than 1% among children <5 y by 2017. All efforts caused a significant dramatically decline in HBV prevalence and carrier rates of children born in Hangzhou. With multiple years of approximate 98% 3-dose HepB vaccination coverage, HBsAg prevalence has dropped below 1% in Hangzhou.
In our study, we found that HBV infection prevalence has declined in Hangzhou as a result of having a strict HepB vaccination programmer. In evaluating the effectiveness of the immunization program of HepB, background prevalence of HBsAg in the local population is one of the important factors. Before the HepB vaccination program, baseline survey data showed that Hangzhou was a highly endemic area for hepatitis B. In this study, we found that hepatitis B prevalence in our study was down to 7.62% in 2013. It appears a growing trend for the hepatitis B patient proportion of the total hepatitis patient, although the incidence of hepatitis B declined from 95.41/100,000 in 1990 down to 7.62/100,000 in 2013. The main reason might be that the most patient were hepatitis A patient in addition to the hepatitis B patient in total hepatitis patient, although there are 5 kinds of hepatitis including hepatitis A B C D and E. With the higher and higher rate of hepatitis A vaccination and the greatly improved of people's living habits, the incidence of hepatitis A infection was controlled effectively. The incidence of hepatitis A declined from 176.16/100,000 down to 1.12/100,000, which was declined much dramatically than hepatitis B. It led to a growing trend for the hepatitis B patient proportion of the total hepatitis patient in Hangzhou. We can explore the other reasons in further researches. We also found there was a small peak at the “5 ∼” age group in 1990s, while the highest peak was in "25 ∼30" age groups, and followed by a decreased tail. In recent years, the small peak at the “5 ∼” age group was flatting, and the incidence of hepatitis B under 10 y old age group was even down to zero. In fact, after 16 y (i.e., 2006), population in the 5∼ age group in the 1990s would be ∼25–30, and population in the 25–30 age group would be ∼41∼46, yet shows the highest peak was in the 25–30 age group but no peak in the 40–45 group. We speculated that the main reason was that shows the incidence of hepatitis B infection in Hangzhou rather than the prevalence. People who infected in 1990s would not be reported in 2006. This may be the reason why shows no peak in the 40–45 groups. In addition, only 10 children under 15 y old were reported each year. Patients’ age distribution of hepatitis B was mainly concentrated in 20–40 y old. These findings serve as an evidence of predominate mode of early childhood HBV transmission and an important role that a 3-dose infant vaccination schedule could reduce chronic HBV infection prevalence.Citation5 Considering the role played by birth dose vaccination in preventing mother-to-child transmission, it was unsurprising to find that HBV infection incidence of <10 years age group was even down to zero in recent years, comparing with HBV incidence rate in 1990. An another important factor could be that herd immunity played a role in decreasing the risk of HBV infection in this youngest age group since they had higher vaccination coverage and lower incidence rates than their older siblings. Now that 24 y have passed since the introduction of the HepB into the EPI in Hangzhou, it is urgent to carry on HepB vaccination for adolescent even adults. In addition, another cause was due to vaccination data quality, which may be un-homogeneous across all age groups. The older groups may be more vulnerable to recall bias or missing vaccination records. We also found composition ratio of teachers, business services, retired person, house-worker and unemployees were all increased. This suggests that it is necessary to focus on these groups in future, such as advice the no-vaccinated groups to receive hepatitis B vaccine, non-responders to re-vaccinated, and so on.
Based on , we found that the HBV incidence rate in urban districts was much greater than that in rural districts before 1999. The main reason may be due to that people in urban districts health consciousness was higher than who in rural districts. Thus, rate of visiting the doctors was higher in urban districts once people were in sick. In addition, level of medical diagnosis in urban districts hospitals were high than in rural districts. All these may lead to the HBV incidence rate in urban districts was much greater than that in rural districts. An additional finding from this survey was that the decline of urban incidence of hepatitis B is sharper than rural. It suggests that it is necessary to search for possible reasons. In 1989, HepB was recommended for routine immunization of infants but parents had to pay for the vaccine in Hangzhou. Therefore vaccination coverage was higher in urban and high socioeconomic areas and lower in rural and lower socioeconomic areas. It may be unaffordable for people who live in remote conditions rural in visiting to a vaccination center both in terms of transport costs and time. Strategies for strengthen the access to vaccination are important for improving immunization coverage in Hangzhou, especially in rural. If economic conditions permit, increased government subsidies should be considered, especially in rural districts.
Professional types in this study were given priority to workers and farmers, distribution of which was various in different years. HepB infection was mainly occurred in workers (34.91%) and farmers (28.69%) in 1990; while workers down to 18.71% and the farmers upper to 31.44% in 2013. In light of the evidence presented here, it is strongly indicates that there may be several occupation-related factors (such as socioeconomic, family status, education, etc.) needed to be further researched. Results from this survey provide valuable direction on how to proceed to strengthen an already highly successful program.
Results of this serology survey carried out in Hangzhou in 2006 shows that the prevalence of HBsAg, anti-HBs, anti-HBc, HBeAg and anti-HBe for population aged 0–59 y were 6.19%, 45.83%, 57.25%, 0.62%, 4.37%, respectively. There were 17.23% population appeared only anti-HBs positive, While 24.96% appeared all of these 5 indicators negative. HBsAg prevalence was greatly diminished among those age <15 y compared to that found in the 1992 national serological survey, and among children age <5 y was only 0.82% (90% reduction).
There are more than 130 million chronic HBV carriers in China, 3050%– of who are thought to have acquired HBV infection from mother-to-child transmission.Citation48-50 Based on some studies, genotype C might be a risk factor for HBV mother to child transmission.Citation51 It should be recommended that pregnant women who are HBeAg positive and have a high viral load be tested for HBV genotypes. However, there are still a large number of persons un-avoided from hepatitis B infection in spite of the high HepB vaccination coverage. The possible reasons might be varies vaccination doses, varies vaccination programs, varies the first needle inoculation time, immune-related genetic factors. In this survey, we did not take into account of other confounding factors, such as poverty level, education level, persons with high-risk behaviors for HBV infection. A Swedish study revealed that parents with a high level of knowledge about the disease had a more positive attitude about having their children vaccinated. Ertekin etc. found there was no difference in vaccination rates with respect to gender and age of the children; however, the mothers’ educational level, socio-economic status, and number of siblings were important factors affecting HBV vaccination of their children. Herd immunity induced by mass or nation-wide vaccination programs is well documented.Citation52-54 Preliminary studies have shown that lamivudine Citation55-56 and telbivudine Citation57 during pregnancy may reduce vertical transmission of HBV. Maternal to infant transmission may occur during the antenatal, intrapartum, or postnatal period. It was recommended that passive-active HepB vaccination should be administered as soon as possible after birth.
It has been reported that different doses of vaccine could offer similar levels of serological protection rates within 5 y after immunization.Citation58-59 However, participants receiving 20 μg of vaccine have been found to have higher antibody levels than those receiving 10 μg vaccine in some studies.Citation60-61 Conflicting reports from previous studies comparing the efficacy of HepB have been observed. However, Gilbert et al. observed that vaccination with 10 μg of recombinant HBV vaccine may provide a clinically effective alternative to the standard 20 μg dose in healthy adolescents.Citation62 Despite several research endeavors, there is very little knowledge about the long-term serological protection following different doses of vaccine. Our previous vaccine intervention trial also demonstrated that both 10 μg and 20 μg doses could elicit similar protective response lasting for 11 y In the current study, the long-term immunogenicity and serological protection rates in both 10 μg and 20 μg dose plasma-derived HepB groups were similar during 23 y of follow-up in participants from highly HBV-endemic areas.Citation63 No significant differences between these vaccinated groups were identified during this study. This result was consistent with the findings from previous research studies.Citation58,64 Furthermore, as MiddlemanCitation65 suggested, more studies are needed to elucidate the consequences of noncompliance with the recommended vaccination schedule.
There are several strengths in our study. First, our study has recorded both hepatitis B incidence and HepB vaccination rate in Hangzhou from 1990 to 2013, a total of 24 y In addition, our study has the strength of using a population-based sampling method with a large sample size of 5605 participants, and the survey was administered in a face-to-face interview on randomly selected subjects in Hangzhou. Nevertheless, with a decent sample size and face-to-face interview, the results are reliable. It confirmed that HBV infection is prevalent among Hangzhou population. One point must be stressed was that the misclassification between acute and chronic HepB was very common in the China National Notifiable Disease Reported System (NNDRS). Just as the WHO program conducted in China, only 33% of acute HepB cases reported in the NNDRS are of the 'true' acute case.Citation66 However, the situation was much better in Hangzhou. The diagnostic accordance rate of acute HepB cases reported in the NNDRS was emphasized greatly and improved effectively since 1990s in Hangzhou. Infectious Disease Control and Prevention Section of Hangzhou Center for Disease Control and Prevention have reviewed the diagnostic accordance rate of acute Hepatitis cases reported in the NNDRS, and confirmed that the diagnostic accordance rate of acute HepB cases reported in the NNDRS was sustained more than 95%, although the reporting quality of the NNDRS has varied greatly over the last decade.
There are also several limitations in our study that need to be addressed. First, the data across different eras as the sensitivity of the HBV testing may not be the same. Thus, we could not explore the temporal aspect of the observed relationships. Second, we could not validate the information by checking with medical records on the self-reported HBV status. Third, vaccination data quality is not homogenous across groups (e.g., age, areas, and occupations). For example, the older groups were more vulnerable to poor record retrieval and less accurate caregiver recall. Because of lacking of data about any benefit of an incomplete vaccination series in our study, thus, the actual effectiveness of HepB vaccination might be higher than we reported in this article. Although there are no data on the duration of protection with fewer than 3 doses of vaccine, one and 2 doses should provide at least short-term protection during a time when the risk of developing chronic infection is greatest (i.e., infancy and early childhood). We also did not take into account the potential impact of herd immunity from infant HepB vaccination.Citation67 Although no data are available to estimate the proportion of a population that must be immune from HBV infection for there to be an effect from herd immunity, recent experience with routine infant HepB vaccination among Alaska Natives and in Taiwan suggest that once HBV transmission is eliminated among young children there is a reduction in infection pressure in the general population.Citation19,68 The data of hepatitis B cases reported through the China National Notifiable Disease Reporting System were classified into acute and chronic cases. In the current study, we used the data of acute cases to document the effect of hepatitis B vaccination. However, it may be underreported of hepatitis B cases, and it may be risky in terms of the definitiveness of the conclusions that can be drawn.
With the heavy disease burden, urgent efforts are needed to provide these so-easy-to-use vaccines to children in order to save millions of lives. Better public health strategies may be needed to deliver the HepB to those who do not comply with the vaccination schedule in the community. What is more important, anti-HBs negative person should be tested for HBsAg, and educated about the needs to address all HBV exposures so that exposed patients can be given prevention for HBV in time. The remaining unexposed ones should be given a full 3 dose course of HepB vaccination, and be tested for anti-HBs level one month after receiving the final dose.Citation69-70 Non-responders should be re-vaccinated with another 3 doses. Related organizations should clearly place the responsibility on the health education institution/employer to ensure that all high risk groups are educated about knowledge of HBV transmission in the healthcare setting, and are offered vaccination, testing, and appropriate counseling or career guidance if necessary.
In conclusion, the universal infant vaccination program is helpful in reducing the prevalence of HBsAg significantly. Universal HepB vaccination will further reduce the incidence of chronic hepatitis B in Hangzhou in the future. Monitoring HBsAg in children who have received HepB vaccination will be helpful for early detection of HBV infection in this population. It is gratifying that the findings from this study can be used to support application of the new HepB vaccination program to protect other high- and middle- endemic regions of China and other countries against HepB.
Materiels and Methods
Population and data collection
We included Hangzhou residents, new immigrants or non-residents in Hangzhou. Information System was used qualified since 2004, hepatitis B incidence and HepB vaccination rate from 1990 to 2003 acquired from the historical paper-documents of Hangzhou, while basic socio-demographic information and vaccination history of all subjects from 2004 to 2013 derived from China Information System for Diseases Control and Prevention and Zhejiang Information System for Immunization Program respectively. In this System, people less than 10 y old were divided into one group per year, people between 10 to 85 y old were divided into one group per 5 years, and people who above 85 y old were all in one group. There are 26 age groups for the age distribution in total. Data was entered accuracy into the Information System by the Legal Reporter, and checked strictly several times by department of Municipal and Provincial CDC. A child was considered vaccinated based on either affirmative historical documentation or data in the Information System, and was considered to have a missing vaccination status if no records were available to review. The data of hepatitis B cases reported through the China National Notifiable Disease Reporting System were classified into acute and chronic cases. In the current study, we used the data of acute cases to document the effect of hepatitis B vaccination.
Vaccination programs
In order to reduce HBV infection rate in Hangzhou, HepB was introduced into the Expanded Program on Immunization by the government in 1989. At the beginning of this program, HepB was asked for all newborns, but costs of vaccines and vaccination should be paid by parents, with which was developed into free for all newborns from June 1, 2005.Under this vaccination program, the first vaccine dose was administered within 24 hours of birth and subsequent doses were at 1 and 6 months respectively.Citation31 Hepatitis B immunoglobulin (HBIG) was not included in the 1992 vaccination program. It was begun to implement booster immunization for 6 y old child in 1998. A recombinant DNA HepB at a concentration of 5 ug/dose was adopted. Supplementary immunization activities were conducted for children born after January 1, 1992 who did not get vaccination or finish the program. Vaccinate three stitches more for 6 y old child who was unable to be determined whether primary immunization was success or not was also launched in 2005.To further reduce the overall HBV infection rate, from 2009 to 2011, the government provided the vaccine free of charge to all children under 15 who had not been vaccinated. Under this vaccination program, a higher vaccine dose (10 ug) was recommended. The vaccine was given on the same schedule as in “1992 Program” for infants, and an identical immunization program was adopted for adults negative for HBV antigens and antibodies.
Population serological survey
The 2006 national hepatitis B serological survey was conducted in 160 national disease surveillance sites, and only one district of Hangzhou City (Xia Cheng District) was included. In order to present the situation of hepatitis B infection in Hangzhou scientifically, another district of Hangzhou City (Chun An District) was also included at the same time. These two districts survey were carried out at the same time via unified method. The results of Chun An District was on behalf of the urban areas and Xia Cheng District was on behalf of the rural areas. Thus, it can present the situation of hepatitis B infection in Hangzhou scientifically. The target population was aged 0–59 y local residents and migrant who living in Hangzhou for more than 3 months (including those who were born in Hangzhou, who were not but permanent Hangzhou residents, new immigrants or non-residents in Hangzhou). Participants in this serological survey were selected by multi-stage random sampling. It was divided into 2 major regional groups (urban and rural) for further sampling in this serological survey. According to the survey plan, we used cluster system sampling in the first stage, and simple random sampling in the second stage. After receiving appropriate training by leading researchers for this survey, the physicians of each participating hospital began to conduct medical examinations, interviews, and laboratory tests on subjects who volunteered for the free medical and health examinations. Together, approximately 5605 participants (urban: 622; rural: 4983) were invited by the Department of Health of Hangzhou City to engage in the recruitment of participants, interviewing, and medical examinations. Demographics and HepB vaccination history were collected by questionnaire and review of vaccination records. Blood samples (5 ml per person) were collected from each participant included for population. All these sera collected in 2006. Serum was separated and stored at −20°C for application. Serum tested for HBsAg, anti-HBs, anti-HBc, HBeAg and anti-HBe by ELISA. To ensure the reliability and accuracy of the results, these 2 major regional groups (urban and rural) used unified reagents, methods, judgment standards, and testing protocol. ELISA reagents for HBsAg, anti-HBs, anti-HBc, HBeAg and anti-HBe testing were purchased from Xiamen Xinchuang biotechnology co., ltd. and Shanghai Kehua biotechnology co., ltd. HBsAg, anti-HBs, anti-HBc, HBeAg and anti-HBe upper to 1ng/ml, 10mIU/ml, 10mIU/ml, 10mIU/ml and 10mIU/m may be identified, respectively. Detailed quality-control measures were made during all stages of this serological survey. The sample collected must be strictly carried out in accordance with the "sampling plan." All investigators received appropriate training by leading researchers. Questionnaire response rate must be above 80%, complete rate must be above 99%, and the rate of blood samples must be more than 95%. If not meet the above criteria all should be supplementary investigation timely. The survey was approved by the Ethics Committee of The First Affiliated Hospital at the School of Medicine of Zhejiang University.
Statistical analysis
Categorical variables were expressed as frequencies (percentages), and Pearson's chi-square test was used to determine group differences. Trend test was used to analyze the associations between hepatitis B incidence rate and HepB vaccination rate adjusted for potential confounders (e.g. age and gender). Participants included in this sero-survey were from one urban area and one rural area of Hangzhou City. In view of the representativeness of the anticipants in the survey, HBsAg, anti-HBs, anti-HBc, HBeAg and anti-HBe for population aged 0–59 y were standardized by age and sex groups according to the 2005 Hangzhou population structure.Citation71 Data were double entered independently and checked for accuracy using Epidata software, Version 3.1. All statistical analyses were performed using SPSS for Windows, version 17.0 (SPSS Inc., Chicago, IL, USA). P-values ≤ 0 .05 (2-sided) were considered to be statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Immunisation against infectious disease–1996 Edward Jenner Bicentenary Edition. Commun Dis Rep CDR Wkly 1996; 6(39):339; PMID:8897732
- Aspinall EJ, Hawkins G, Fraser A, Hutchinson SJ, Goldberg D. Hepatitis B prevention, diagnosis, treatment and care: a review. Occup Med (Lond) 2011; 61(8):531-40; PMID:22114089; http://dx.doi.org/10.1093/occmed/kqr136
- Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006; 45(4):529-38; PMID:16879891; http://dx.doi.org/10.1016/j.jhep.2006.05.013
- Evans AA, Chen G, Ross EA, Shen FM, Lin WY, London WT. Eight-year follow-up of the 90,000-person Haimen City cohort: I. Hepatocellular carcinoma mortality, risk factors, and gender differences. Cancer Epidemiol Biomarkers Prev 2002; 11(4):369-76; PMID:11927497
- Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol 2005; 34(6):1329-39; PMID:16249217; http://dx.doi.org/10.1093/ije/dyi206
- Bozorgi SH, Ramezani H, Nooranipour M, Ahmadi M, Baghernejad A, Mostajeri A, Kargar-Fard H, Sadri M, Alavian SM. Risk factors of viral hepatitis: yet to explore. Transfus Apher Sci 2012; 47(2):145-9; PMID:22858443; http://dx.doi.org/10.1016/j.transci.2012.06.023
- Vitaliti G, Pratico AD, Cimino C, Di Dio G, Lionetti E, La Rosa M, Leonardi S. Hepatitis B vaccine in celiac disease: yesterday, today and tomorrow. World J Gastroenterol 2013; 19(6):838-45; PMID:23430309; http://dx.doi.org/10.3748/wjg.v19.i6.838
- Chen DS. From hepatitis to hepatoma: lessons from type B viral hepatitis. Science 1993; 262(5132):369-70; PMID:8211155; http://dx.doi.org/10.1126/science.8211155
- de Franchis R, Hadengue A, Lau G, Lavanchy D, Lok A, McIntyre N, Mele A, Paumgartner G, Pietrangelo A, Rodés J, et al. EASL international consensus conference on hepatitis B. 2002 Geneva, Switzerland. Consensus statement (long version). J Hepatol 2003; 39 Suppl 1:S3-25; PMID:14708673
- Ganem D, Prince AM. Hepatitis B virus infection–natural history and clinical consequences. N Engl J Med 2004; 350(11):1118-29; PMID:15014185; http://dx.doi.org/10.1056/NEJMra031087
- He J, Gu D, Wu X, Reynolds K, Duan X, Yao C, Wang J, Chen CS, Chen J, Wildman RP, et al. Major causes of death among men and women in China. N Engl J Med 2005; 353(11):1124-34; PMID:16162883; http://dx.doi.org/10.1056/NEJMsa050467
- Custer B, Sullivan SD, Hazlet TK, Iloeje U, Veenstra DL, Kowdley KV. Global epidemiology of hepatitis B virus. J Clin Gastroenterol 2004; 38(10 Suppl 3):S158-68; PMID:15602165
- Muthuphei MN. Childhood liver diseases in ga-rankuwa hospital, South Africa. East Afr Med J 2000; 77(9):508-9; PMID:12862144
- Huy TT, Abe K. Molecular epidemiology of hepatitis B and C virus infections in Asia. Pediatr Int 2004; 46(2):223-30; PMID:15056256; http://dx.doi.org/10.1046/j.1442-200x.2004.01867.x
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 2004; 11(2):97-107; PMID:14996343; http://dx.doi.org/10.1046/j.1365-2893.2003.00487.x
- Kohn MA, Farley TA, Scott C. The need for more aggressive follow-up of children born to hepatitis B surface antigen-positive mothers: lessons from the Louisiana Perinatal Hepatitis B Immunization Program. Pediatr Infect Dis J 1996; 15(6):535-40.
- Baytan B, Gunes AM, Gunay U. Efficacy of primary hepatitis B immunization in children with acute lymphoblastic leukemia. Indian Pediatr 2008; 45(4):265-70; PMID:18451443
- Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med 1997; 336(26):1855-9; PMID:9197213; http://dx.doi.org/10.1056/NEJM199706263362602
- Lin HH, Wang LY, Hu CT, Huang SC, Huang LC, Lin SS, Chiang YM, Liu TT, Chen CL. Decline of hepatitis B carrier rate in vaccinated and unvaccinated subjects: sixteen years after newborn vaccination program in Taiwan. J Med Virol 2003; 69(4):471-4; PMID:12601753; http://dx.doi.org/10.1002/jmv.10333
- Poland GA, Jacobson RM. Clinical practice: prevention of hepatitis B with the hepatitis B vaccine. N Engl J Med 2004; 351(27):2832-8; PMID:15625334; http://dx.doi.org/10.1056/NEJMcp041507
- Goldstein ST, Alter MJ, Williams IT, Moyer LA, Judson FN, Mottram K, Fleenor M, Ryder PL, Margolis HS. Incidence and risk factors for acute hepatitis B in the United States, 1982-1998: implications for vaccination programs. J Infect Dis 2002; 185(6):713-9; PMID:11920288; http://dx.doi.org/10.1086/339192
- Lee C, Gong Y, Brok J, Boxall EH, Gluud C. Effect of hepatitis B immunisation in newborn infants of mothers positive for hepatitis B surface antigen: systematic review and meta-analysis. BMJ 2006; 332(7537):328-36; PMID:16443611; http://dx.doi.org/10.1136/bmj.38719.435833.7C
- Van Herck K, Van Damme P. Benefits of early hepatitis B immunization programs for newborns and infants. Pediatr Infect Dis J 2008; 27(10):861-9; PMID:18776823; http://dx.doi.org/10.1097/INF.0b013e318173966f
- Chang MH. Decreasing incidence of hepatocellular carcinoma among children following universal hepatitis B immunization. Liver Int 2003; 23(5):309-14; PMID:14708890; http://dx.doi.org/10.1034/j.1478-3231.2003.00865.x
- Lai CL, Ratziu V, Yuen MF, Poynard T. Viral hepatitis B. Lancet 2003; 362(9401):2089-94; PMID:14697813; http://dx.doi.org/10.1016/S0140-6736(03)15108-2
- Beasley RP. Development of hepatitis B vaccine. JAMA 2009; 302(3):322-4; http://dx.doi.org/10.1001/jama.2009.1024
- Global progress toward universal childhood hepatitis B vaccination, 2003. MMWR Morb Mortal Wkly Rep 2003; 52(36):868-870; PMID:12970620
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al. Evaluation of the impact of hepatitis B vaccination among children born during 1992-2005 in China. J Infect Dis 2009; 200(1):39-47; PMID:19469708; http://dx.doi.org/10.1086/599332
- Zhou YH, Wu C, Zhuang H. Vaccination against hepatitis B: the Chinese experience. Chin Med J (Engl) 2009; 122(1):98-102; PMID:19187625
- Lu FM, Zhuang H. Prevention of hepatitis B in China: achievements and challenges. Chin Med J (Engl) 2009; 122(24):2925-7; PMID:20137475
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al. Epidemiological serosurvey of hepatitis B in China–declining HBV prevalence due to hepatitis B vaccination. Vaccine 2009; 27(47):6550-7; PMID:19729084; http://dx.doi.org/10.1016/j.vaccine.2009.08.048
- Progress in hepatitis B prevention through universal infant vaccination–China, 1997-2006. MMWR Morb Mortal Wkly Rep 2007; 56(18):441-5; PMID:17495790
- Andersson A, Qvarfordt I, Laan M, Sjostrand M, Malmhall C, Riise GC, Cardell LO, Linden A. Impact of tobacco smoke on interleukin-16 protein in human airways, lymphoid tissue and T lymphocytes. Clin Exp Immunol 2004; 138(1):75-82; PMID:15373908; http://dx.doi.org/10.1111/j.1365-2249.2004.02580.x
- Shen LP, Zhang Y, Wang F, Zhang S, Yang JY, Fang KX, Yu T, Wang XY, Zhang WY, Bi SL. Epidemiological changes in hepatitis B prevalence in an entire population after 20 years of the universal HBV vaccination programme. Epidemiol Infect 2011; 139(8):1159-65; PMID:21156099; http://dx.doi.org/10.1017/S0950268810002827
- Xia G.L., Li C.B., Cao H.L., et al. Prevalence of hepatitis B and C virus infections in the conventional Chinese population: results from a nationwide cross-sectional seroepidemiologic study of hepatitis A, B, C, D and E virus infections in China, 1992. Int Hepatol Commun (1996) 5:12.
- Puro V, De Carli G, Cicalini S, Soldani F, Balslev U, Begovac J, Boaventura L, Campins Martí M, Hernández Navarrete MJ, Kammerlander R, et al. European recommendations for the management of healthcare workers occupationally exposed to hepatitis B virus and hepatitis C virus. Euro Surveill 2005; 10(10):260-4; PMID:16282641
- Ni YH, Huang LM, Chang MH, Yen CJ, Lu CY, You SL, Kao JH, Lin YC, Chen HL, Hsu HY, et al. Two decades of universal hepatitis B vaccination in taiwan: impact and implication for future strategies. Gastroenterology 2007; 132(4):1287-93; PMID:17433322; http://dx.doi.org/10.1053/j.gastro.2007.02.055
- Mele A, Tancredi F, Romano L, Giuseppone A, Colucci M, Sangiuolo A, Lecce R, Adamo B, Tosti ME, Taliani G, et al. Effectiveness of hepatitis B vaccination in babies born to hepatitis B surface antigen-positive mothers in Italy. J Infect Dis 2001; 184(7):905-8; http://dx.doi.org/10.1086/323396
- Wu JS, Hwang LY, Goodman KJ, Beasley RP. Hepatitis B vaccination in high-risk infants: 10-year follow-up. J Infect Dis 1999; 179(6):1319-25; PMID:10228050; http://dx.doi.org/10.1086/314768
- Huang LM, Chiang BL, Lee CY, Lee PI, Chi WK, Chang MH. Long-term response to hepatitis B vaccination and response to booster in children born to mothers with hepatitis B e antigen. Hepatology 1999; 29(3):954-9; PMID:10051503; http://dx.doi.org/10.1002/hep.510290349
- Liao SS, Li RC, Li H, Yang JY, Zeng XJ, Gong J, Wang SS, Li YP, Zhang KL. Long-term efficacy of plasma-derived hepatitis B vaccine: a 15-year follow-up study among Chinese children. Vaccine 1999; 17(20-21):2661-6; PMID:10418916; http://dx.doi.org/10.1016/S0264-410X(99)00031-6
- Whittle H, Jaffar S, Wansbrough M, Mendy M, Dumpis U, Collinson A, Hall A. Observational study of vaccine efficacy 14 years after trial of hepatitis B vaccination in Gambian children. BMJ 2002; 325(7364):569; PMID:12228132; http://dx.doi.org/10.1136/bmj.325.7364.569
- Coursaget P, Leboulleux D, Soumare M, le Cann P, Yvonnet B, Chiron JP, Coll-Seck AM, Diop-Mar I. Twelve-year follow-up study of hepatitis B immunization of Senegalese infants. J Hepatol 1994; 21(2):250-4; PMID:7989718; http://dx.doi.org/10.1016/S0168-8278(05)80404-0
- McMahon BJ, Bruden DL, Petersen KM, Bulkow LR, Parkinson AJ, Nainan O, Khristova M, Zanis C, Peters H, Margolis HS. Antibody levels and protection after hepatitis B vaccination: results of a 15-year follow-up. Ann Intern Med 2005; 142(5):333-41; PMID:15738452; http://dx.doi.org/10.7326/0003-4819-142-5-200503010-00008
- Acute hepatitis B among children and adolescents–United States, 1990-2002. MMWR Morb Mortal Wkly Rep 2004; 53(43):1015-1018; PMID:15525899
- Liu C, Li H, Gao L, Li F, Liang X, Yang K. Hepatitis B immunisation leads to the decline of hepatitis B virus prevalence in Gansu province, China. Aust N Z J Public Health 2011; 35(1):91-2; PMID:21299712; http://dx.doi.org/10.1111/j.1753-6405.2010.00670.x
- Zhang L, Xu A, Yan B, Song L, Li M, Xiao Z, Xu Q, Li L. A significant reduction in hepatitis B virus infection among the children of Shandong Province, China: the effect of 15 years of universal infant hepatitis B vaccination. Int J Infect Dis 2010; 14(6):e483-488; PMID:19939719; http://dx.doi.org/10.1016/j.ijid.2009.08.005
- Cacciola I, Cerenzia G, Pollicino T, Squadrito G, Castellaneta S, Zanetti AR, Mieli-Vergani G, Raimondo G. Genomic heterogeneity of hepatitis B virus (HBV) and outcome of perinatal HBV infection. J Hepatol 2002; 36(3):426-32; PMID:11867188; http://dx.doi.org/10.1016/S0168-8278(01)00295-1
- Papadakis MA, Elefsiniotis IS, Vlahos G, Daskalakis G, Barbatis C, Antsaklis A. Intrauterine-transplacental transmission of hepatitis B virus (HBV) from hepatitis B e antigen negative (precore mutant, G1896A) chronic HBV infected mothers to their infants. Preliminary results of a prospective study. J Clin Virol 2007; 38(2):181-3; PMID:17142098; http://dx.doi.org/10.1016/j.jcv.2006.10.008
- Zhang SL, Yue YF, Bai GQ, Shi L, Jiang H. Mechanism of intrauterine infection of hepatitis B virus. World J Gastroenterol 2004; 10(3):437-8; PMID:14760774
- Ding Y, Sheng QJ, Ma L, Dou X. Chronic HBV infection among pregnant women and their infants in Shenyang, China. Virology Journal, 2013, 10(1):17; PMID:23294983
- Garland SM, Skinner SR, Brotherton JM. Adolescent and young adult HPV vaccination in Australia: achievements and challenges. Prev Med 2011; 53 Suppl 1:S29-35; PMID:21962468
- Kim TH, Johnstone J, Loeb M. Vaccine herd effect. Scand J Infect Dis 2011; 43(9):683-9; PMID:21604922
- Trotter CL, Maiden MC. Meningococcal vaccines and herd immunity: lessons learned from serogroup C conjugate vaccination programs. Expert Rev Vaccines 2009; 8(7):851-61; PMID:19538112; http://dx.doi.org/10.1586/erv.09.48
- van Zonneveld M, van Nunen AB, Niesters HG, de Man RA, Schalm SW, Janssen HL. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat 2003; 10(4):294-7; PMID:12823596; http://dx.doi.org/10.1046/j.1365-2893.2003.00440.x
- Xu WM, Cui YT, Wang L, Yang H, Liang ZQ, Li XM, Zhang SL, Qiao FY, Campbell F, Chang CN, et al. Lamivudine in late pregnancy to prevent perinatal transmission of hepatitis B virus infection: a multicentre, randomized, double-blind, placebo-controlled study. J Viral Hepat 2009; 16(2):94-103; http://dx.doi.org/10.1111/j.1365-2893.2008.01056.x
- Han GR, Cao MK, Zhao W, Jiang HX, Wang CM, Bai SF, Yue X, Wang GJ, Tang X, Fang ZX. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. J Hepatol 2011; 55(6):1215-21; http://dx.doi.org/10.1016/j.jhep.2011.02.032
- Duval B, Boulianne N, De Serres G, Laflamme N, De Wals P, Massé R, Trudeau G, Delage G, Desjardins L. Comparative immunogenicity under field conditions of two recombinant hepatitis B vaccines in 8-10-year-old children. Vaccine 2000; 18(15):1467-72; PMID:10618544; http://dx.doi.org/10.1016/S0264-410X(99)00422-3
- Goh KT, Tan KL, Kong KH, Oon CJ, Chan SH. Comparison of the immune response of four different dosages of a yeast-recombinant hepatitis B vaccine in Singapore children: a four-year follow-up study. Bull World Health Organ 1992; 70(2):233-9; PMID:1600584
- Chiaramonte M, Majori S, Ngatchu T, Moschen ME, Baldo V, Renzulli G, Simoncello I, Rocco S, Bertin T, Naccarato R, et al. Two different dosages of yeast derived recombinant hepatitis B vaccines: a comparison of immunogenicity. Vaccine 1996; 14(2):135-7; PMID:8852410; http://dx.doi.org/10.1016/0264-410X(95)00148-T
- ul-Haq N, Hasnain SS, Umar M, Abbas Z, Valenzuela-Silva C, Lopez-Saura P. Immunogenicity of 10 and 20 microgram hepatitis B vaccine in a two-dose schedule. Vaccine 2003; 21(23):3179-85; PMID:12804846; http://dx.doi.org/10.1016/S0264-410X(03)00232-9
- Schiff GM, Sherwood JR, Zeldis JB, Krause DS. Comparative study of the immunogenicity and safety of two doses of recombinant hepatitis B vaccine in healthy adolescents. J Adolesc Health 1995; 16(1):12-7; PMID:7742331; http://dx.doi.org/10.1016/1054-139X(94)00105-N
- Wu Q, Zhuang GH, Wang XL, Hou TJ, Shah DP, Wei XL, Wang LR, Zhang M. Comparison of long-term immunogenicity (23 years) of 10 mug and 20 mug doses of hepatitis B vaccine in healthy children. Hum Vaccin Immunother 2012; 8(8):1071-6; PMID:22854666; http://dx.doi.org/10.4161/hv.20656
- Most J, Larcher C, Vogetseder W, Prodinger WM, Huemer HP, Ebenbichler CF, Dierich MP. Recombinant versus plasma-derived hepatitis B vaccine: comparison of immunogenicity in medical students. Vaccine 1992; 10(11):740-1; PMID: 1308114; http://dx.doi.org/10.1016/0264-410X(92)90507-G
- Middleman AB. Hepatitis B immunization among adolescents. Curr Opin Pediatr 1995; 7(4):366-70; http://dx.doi.org/10.1097/00008480-199508000-00004
- Cui F, Drobeniuc J, Hadler SC, Hutin YJ, Ma F, Wiersma S, et al. Review of hepatitis B surveillance in China: Improving information to frame future directions in prevention and control. Vaccine 31S:J79-84, 2013; http://dx.doi.org/10.1016/j.vaccine.2013.05.054
- Fine PE. Herd immunity: history, theory, practice. Epidemiol Rev 1993; 15(2):265-302; PMID:8174658
- Harpaz R, McMahon BJ, Margolis HS, Shapiro CN, Havron D, Carpenter G, Bulkow LR, Wainwright RB. Elimination of new chronic hepatitis B virus infections: results of the Alaska immunization program. J Infect Dis 2000; 181(2):413-8; PMID:10669320; http://dx.doi.org/10.1086/315259
- Are booster immunisations needed for lifelong hepatitis B immunity? European Consensus Group on Hepatitis B Immunity. Lancet 2000; 355(9203):561-5; PMID:10683019; http://dx.doi.org/10.1016/S0140-6736(99)07239-6
- Gunson RN, Shouval D, Roggendorf M, Zaaijer H, Nicholas H, Holzmann H, de Schryver A, Reynders D, Connell J, Gerlich WH, et al. Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections in health care workers (HCWs): guidelines for prevention of transmission of HBV and HCV from HCW to patients. J Clin Virol 2003; 27(3):213-30; PMID:12878084; http://dx.doi.org/10.1016/S1386-6532(03)00087-8
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al. Epidemiological serosurvey of Hepatitis B in China-Declining HBV prevalence due to Hepatitis B vaccination. Vaccine, 2009 27:6550-7; PMID:19729084; http://dx.doi.org/10.1016/j.vaccine.2009.08.048