Abstract
A 9-valent human papillomavirus (6/11/16/18/31/33/45/52/58) VLP (9vHPV) vaccine has recently been proven highly efficacious in preventing disease associated with vaccine HPV types in a pivotal Phase III study. The demonstration of lot-to-lot consistency to confirm the reliability of the manufacturing process is a regulatory requirement for vaccine licensure in the United States. A randomized trial was conducted to demonstrate that three lots of 9vHPV vaccine elicit equivalent antibody response for all 9 vaccine types. The study required thorough planning because it required success on 27 separate statistical comparisons. An innovative statistical approach was used taking into account between-lot variance for more conservative power calculations. The study demonstrated equivalence of three lots of 9vHPV vaccine for all 9 vaccine types.
Introduction
The 9-valent human papillomavirus (6/11/16/18/31/33/45/52/58) VLP (9vHPV) vaccine addresses the 4 HPV types covered by the licensed quadrivalent human papillomavirus (6/11/16/18) VLP (qHPV) vaccine (GARDASIL™/SILGARD™) plus five additional oncogenic types for increased cervical cancer coverage. A pivotal study in young women 16 to 26 years of age demonstrated that the 9vHPV vaccine is highly efficacious in preventing disease associated with vaccine HPV types.Citation1 The 9vHPV vaccine was licensed in 2014 in the United States under the name GARDASIL™9.
The demonstration of lot-to-lot consistency to support the reliability of the manufacturing process is generally an obligatory step in vaccine development.Citation2,3 Per regulatory guidance, consistency of manufacture needs to be demonstrated based on at least three manufacturing lots of the final manufacturing process (FMP).Citation4-6 As part of this requirement, a lot consistency clinical study must be performed to show that at least three FMP lots are equivalent with respect to immunogenicity.Citation7
A Phase III study was conducted to assess 9vHPV vaccine lot consistency in girls 9 to 15 years of age. This article summarizes the design and results of this study.
Methods
Study design
The V503-002 study (NCT #00943722) was designed to enroll 1800 girls 9 to 15 years of age, 600 boys 9 to 15 years of age, and 400 young women 16 to 26 years of age. The study was comprised of two immunogenicity substudies (): 1. An adult-adolescent immunobridging substudy to compare 9vHPV vaccine immunogenicity at Month 7 in girls versus young women, and boys versus young women; 2. A lot consistency substudy to demonstrate consistent immunogenicity at Month 7 in subjects randomized to three different vaccine lots of the final manufacturing process.
Figure 1. Subject distribution between the adult-adolescent substudy and the lot consistency substudy. The V503-002 study (NCT #00943722) was designed to enroll 1800 girls 9 to 15 years of age, 600 boys 9 to 15 years of age, and 400 young women 16 to 26 years of age. The study was comprised of two immunogenicity substudies: 1. An adult-adolescent immunobridging substudy to compare 9vHPV vaccine immunogenicity at Month 7 in girls versus young women, and boys versus young women; 2. A lot consistency substudy to demonstrate consistent immunogenicity at Month 7 in subjects randomized to three different vaccine lots of the final manufacturing process.
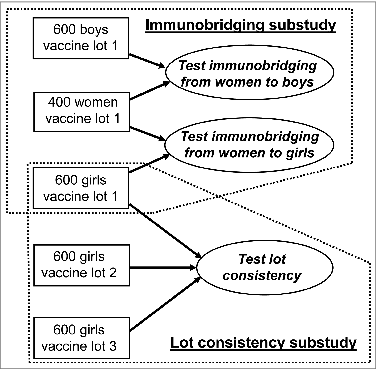
The lot consistency substudy was conducted in girls. All enrolled girls 9 to 15 years of age were equally randomized to three vaccine lots (termed lots 1, 2, and 3). Boys and young women did not participate in the lot consistency substudy and were all assigned to lot 1. As shown in , girls who received lot 1 participated in both substudies. This report is focused on the lot consistency substudy. The results of the adult-adolescent immunobridging substudy as well as the safety findings have been reported elsewhere.Citation8
Population
Subjects in the lot consistency substudy were enrolled from 66 sites in 17 countries (Austria, Belgium, Brazil, Chile, Colombia, Costa Rica, Finland, India, Peru, Poland, South Africa, South Korea, Spain, Sweden, Taiwan, Thailand and the United States). The study was conducted in accordance with principles of Good Clinical Practice and was approved by the appropriate institutional review boards and regulatory agencies. An external Data Monitoring Committee assessed safety findings throughout the study.
The lot consistency substudy was designed to enroll girls 9 to 15 years of age. Participants were required to be generally healthy and sexually naïve at enrollment and throughout the vaccination period (i.e., through month 7). Reasons for exclusions from the study included: pregnancy (determined by urine or serum β-human chorionic gonadotropin testing), known allergy to any vaccine component, thrombocytopenia, immunosuppression or prior immunosuppressive therapy, and previous receipt of an HPV vaccine.
Randomization and vaccination
Following informed consent and determination that all inclusion criteria and none of the exclusion criteria were met, eligible subjects received an allocation number and were randomized to a vaccination group. An Interactive Voice Response System (IVRS) was used to allocate study subjects and balance randomization between sites. The IVRS assigned the subject an allocation number from an allocation schedule. Girls were enrolled in two age strata (9 to 12 years of age and 13 to 15 years of age at enrollment) in approximately a 2:1 ratio to ensure that the immunogenicity and safety profile of the vaccine in younger subjects would be clearly defined. Girls enrolled in the lot consistency substudy were equally randomized in a double-blinded manner to three FMP vaccine lots. The randomized allocation schedule was based on balanced randomization blocks of size 6.
All subjects were administered a 3-dose regimen of 9vHPV vaccine (at day 1, month 2, and month 6). All subjects received the same formulation of 9vHPV vaccine administered (in the deltoid) as a 0.5-mL intramuscular injection. A 0.5-mL dose of 9vHPV vaccine contains 30 μg HPV 6, 40 μg HPV 11, 60 μg HPV 16, 40 μg HPV 18, 20 μg HPV 31, 20 μg HPV 33, 20 μg HPV 45, 20μg HPV 52, 20 μg HPV 58, and 500 μg of amorphous aluminum hydroxyphosphate sulfate (AAHS). All participants were required to be afebrile (oral temperature <37.8 °C) within 24 hours before each injection. All participants underwent pregnancy testing that was based on urine or serum analyses for β-human chorionic gonadotropin before each vaccination. Participants who were found to be pregnant were not to be vaccinated. No subject was found pregnant in this study.
Assessment
All participants were assessed for antibody titers to all 9 vaccine HPV types at day 1 and month 7. Seropositivity at day 1 was not a reason for exclusion from the study. However, the results of this testing were part of the criteria to define the per protocol analysis population.
Serum samples obtained at day 1 and month 7 from all subjects were tested for anti-HPV 6, anti-HPV 11, anti-HPV 16, anti-HPV 18, anti-HPV 31, anti-HPV 33, anti-HPV 45, anti-HPV 52, and anti-HPV 58 by HPV 6, 11, 16, 18, 31, 33, 45, 52, 58 competitive Luminex immunoassay (HPV-9 cLIA).Citation9 A subject was defined to be anti-HPV 6, anti-HPV 11, anti-HPV 16, or anti-HPV 18 seropositive if the anti-HPV serum cLIA level was ≥ 30, ≥ 16, ≥ 20, or ≥ 24 mMU/mL, respectively. A subject was defined to be seropositive to HPV Types 31, 33, 45, 52, or 58 if the anti-HPV serum cLIA level was ≥ 10, ≥ 8, ≥ 8, ≥ 8, or ≥ 8 mMU/mL, respectively.Citation9
All participants were observed for at least 30 minutes after each vaccination for any immediate reaction, with particular attention to any evidence of a hypersensitivity reaction. All subjects received a vaccination report card (VRC) at the day 1, month 2, and month 6 study vaccination visits. On the VRC, the parent/guardian of the subject was asked to record adverse experiences. Safety findings for this study have been reported.Citation8
Statistical methodology
The primary approach to the analyses of immunogenicity was per-protocol. Each vaccine component was analyzed separately. To be included in the primary immunogenicity analysis for the HPV 6 and HPV 11 components, subjects had to be seronegative to both HPV 6 and 11 at day 1 (because of extensive cross-reactivity due to the high aminoacid sequence identity [92%] between HPV 6 and HPV 11 L1 proteins.Citation10 To be included in the primary immunogenicity analysis for the other vaccine HPV types, subjects were only required to be seronegative at day 1 for the HPV type being analyzed. In addition, subjects had to receive all 3 doses of the correct clinical material within acceptable day ranges (dose 2: days 36 to 84 and dose 3: days 148 to 218, relative to day 1) and have at least 1 post-dose 3 serology result within acceptable day ranges (days 21 to 49 post-dose 3) and not violate the protocol.
The relevant parameters for assessing lot consistency are geometric mean titers (GMTs) and seroconversion rates. The primary immunogenicity objective of the lot consistency substudy was to demonstrate that the FMP results in lots of the 9vHPV vaccine induce consistent Month 7 GMTs for serum anti-HPV 6, anti-HPV 11, anti-HPV 16, anti-HPV 18, anti-HPV 31, anti-HPV 33, anti-HPV 45, anti-HPV 52, and anti-HPV 58. The statistical criterion for equivalence required that the two-sided 95% confidence interval (CI) of GMT ratio for each of the three pairs of lots (lot 1 vs. lot 2, lot 1 vs. lot 3, and lot 2 vs. lot 3) be contained entirely within the interval (0.5, 2.0) for each HPV type. The secondary immunogenicity objective of the lot consistency substudy was to demonstrate that the FMP results in lots of the 9vHPV vaccine that induce consistent Month 7 seroconversion percentages to HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58. These tests were conducted using the method of Miettinen and Nurminen.Citation11 The statistical criterion for equivalence required that the two-sided 95% CI of the difference between seroconversion percentages for each of three pairs of lots (lot 1 vs. lot 2, lot 1 vs. lot 3, and lot 2 vs. lot 3) be contained entirely within the interval (-5%, 5%) for each HPV type.
Margin for lot consistency
The primary analysis of lot consistency required 27 pairs of tests of equivalence for the 3 manufacturing lots and 9 HPV types. Study success was defined as meeting all 27 pairs of equivalence tests. To determine the sample size, the study power calculation was based on total variance (i.e., between-lot variance as well as within-lot variance). Between-lot variability has been generally ignored in vaccine lot consistency studies. The critical importance of considering between-lot variability in study power determination has been recently recognized.Citation12,13 Some between-lot variability is expected under any manufacturing practices and, although small, it can have substantial impact on the sample size and power.Citation12 Therefore, the study was designed to account for between-lot variability. For example, even with a small between-lot variance of less than 1% of the total variance, an equivalence margin of 1.5-fold would require a sample size up to 6 times greater than that utilizing an equivalence margin of 2-fold. Thus, to account for between-lot variability and conduct the study with an acceptable sample size and power, a 2-fold equivalence margin was found to be appropriate for this lot consistency substudy. Lot consistency is concluded if the two-sided 95% confidence interval of the ratio of geometric means of titers (GMTs) for each of the three pairs of lots is contained within the interval (0.5, 2.0).
Under the assumptions of approximately 20% exclusion rate for the per-protocol population in this age group and 2.0-fold equivalence margin for GMT ratio, the lot consistency substudy with 600 subjects per group would provide above 90% power to show consistency for the 3 lots for all 9 HPV types at an overall one-sided, 2.5% alpha-level as long as the between-lot variance is not above 1.2% of the total variance (between-lot variance and within-lot variance).
Results
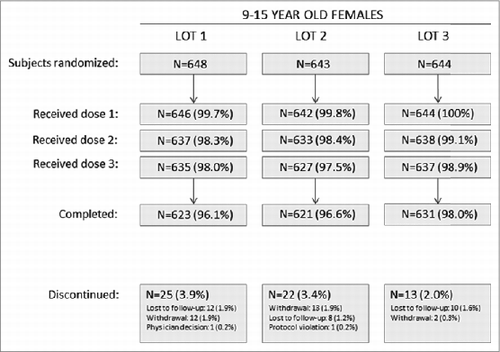
A total of 1935 subjects were randomized from 66 sites in Africa (South Africa), Asia (India, Korea, Taiwan, Thailand), Europe (Austria, Belgium, Finland, Poland, Sweden, Spain), Latin America (Brazil, Chile, Colombia, Costa Rica, Peru) and North America (United States). A summary of the number of subjects who were randomized, vaccinated, who completed or discontinued during the study can be seen in . No subject discontinued study vaccination due to an adverse event. A summary of baseline subject characteristics is provided in . All vaccination groups were diverse with respect to geographic region, race, and ethnicity.
Table 1. Subject characteristics
Figure 2. Accounting of subjects in the lot consistency substudy. A total of 1935 subjects were randomized from 65 sites in Africa (South Africa), Asia (India, Korea, Taiwan, Thailand), Europe (Austria, Belgium, Finland, Poland, Sweden, Spain), Latin America (Brazil, Chile, Colombia, Costa Rica, Peru) and North America (United States). Withdrawal = patient withdrew consent.
At enrollment, serum antibody titers for HPV 6, 11, 16, 18, 31, 33, 45, 52, or 58 that were greater than the predefined seropositive threshold values for one or more HPV type, indicative of previous exposure to the respective vaccine HPV types, were detected in 8.5% (163 of 1,920) of the study participants.
displays the results of the statistical analyses of lot equivalence of month 7 HPV cLIA GMTs for each vaccine HPV type in the per-protocol immunogenicity (PPI) population. The lower bound of two sided 95% CI of GMT ratio for each of three pairs of lots (lot 1 vs. lot 2, lot 1 vs. lot 3, and lot 2 vs. lot 3) for all 9 vaccine types (representing a total of 27 comparisons) was contained entirely within the interval (0.5, 2.0) for each HPV type, with p-values <0.001, indicating cLIA GMT responses in groups who received the three vaccine lots were equivalent., The criterion for equivalent antibody responses for this secondary endpoint was met as well (). Overall, 99.6% of participants seroconverted by month 7 to all 9 HPV types.
Table 2. Statistical analysis of equivalence of geometric mean titers at month 7
Table 3. Statistical analysis of equivalence of proportions of subjects who seroconverted by month 7
Discussion
This study was designed to demonstrate manufacturing lot consistency of the 9vHPV vaccine. Three groups of girls 9 to 15 years of age were randomized to three lots of the FMP process. At one month after the completion of a 3-dose vaccination regimen, statistical equivalence of the GMTs between the 3 lots was established for all 9 vaccine types. Seroconversion rates were over 99% in all three groups and found to be statistically equivalent. These results support the conclusion that the 9vHPV vaccine from three lots of the FMP induces consistent antibody responses to all 9 vaccine HPV types.
A few subjects did not seroconvert at month 7. Because there is no known immune correlate of protection for HPV vaccines (e.g., minimum level of antibody that predicts protection against infection or disease), the absence of detectable antibodies is not equivalent to absence of protection. The minimum antibody titer needed for protection is not known but animal studies suggest that very low antibody titers (e.g., up to 100-fold lower than the threshold of detection of a standard pseudovirion-based neutralizing assay [PBNA]) may be protective.Citation14 This result is also relevant to cLIA (the immunoassay used in this study) since PBNA and cLIA are highly correlated and similarly sensitive.Citation15
Lot consistency demonstration is generally required to support vaccine registration.Citation1-3 This entails a randomized clinical trial to establish that three different manufacturing lots provide equivalent immunogenicity. The specific requirements of each lot consistency study should be determined based on a thorough investigation considering the particulars of the study, as sample size and risk of failure can be dramatically increased if requirements are too stringent. Study parameters to consider include number of vaccine genotypes, desired power to demonstrate lot consistency, assumed within-lot variance for each genotype, assumed between-lot variance for each genotype, and lot consistency criteria.
It is known that an increase in sample size reduces the within-lot variance of the mean, but does not affect the between-lot variance.Citation10 Even under the best manufacturing practices, small variations between lots are expected. A small between-lot variance can greatly impact sample size and compromise study success as was the case in a previous lot consistency study of a meningococcal vaccine.Citation10,11 In the present study, these issues were compounded by the need to demonstrate equivalence of three vaccine lots, each containing antigens from nine HPV types, which represents 27 separate comparisons. Based on a comprehensive investigation the study design described herein was found to be appropriate for demonstration of lot consistency. These considerations may be applicable to other vaccine development programs and may aid in designing efficient and successful vaccine lot consistency studies.
Disclosure of Potential Conflicts of Interest
Edson Moreira reports grants and personal fees from Merck & Co., Inc. Alain Luxembourg, Xiao Sun, Roger Maansson, Susan Christiano Erin Moeller and Joshua Chen are employees of Merck & Co., Inc. and may hold stock and/or stock options. Rudiwilai Samakoses and Kyung-Hyo Kim report no conflicts of interest.
Acknowledgements
Investigators (who are not also authors): T. Acuna, R. Aguilar, K. Ahmed, A. Amaresh, W. Andrews Jr., D. Apter, A. Arguedas, A. Basta, L. Bekker, N. Bhatla, S. Bianchi, S. Block Jr., N. Brodski, A. Campaner, X. Castellsague, X. Cerda, C. Chambers, S. Chaterjee,C. Cho, R. Debski, D. Ferris, C. Fisher Jr., N. Fraser, G. Gray, G. Gutierrez, L. Hammes, K. Hoppenbrouwers, L. Huang, E. Joura, J. Kang, T. Karppa, D. Kim, P. Knapp, T. Kojaoghlanian,J. Kratzer, K. Kuismanen, J. Kumar, S. Lalwani, J. Leader, S. Marwah, L. Nilsson, S. Olsson, P. Oszukowski, L. Roca, M. Pietruszka, P. Pitisuttithum, T. Poling, O. Reich, Reina, J., K. Reisinger, J. Restrepo, P. Rogge, A. Rosich, D. Schmeidler, L. Sher, C. Sanchez, M. Simon, D. Singh, H. Tsai, E. Tulkki, M. Varman, K. Vega, M. Victoria, M. Virta.
The authors also thank David Ege, Brad Holstine, Michael Kosinski, Thomas Linden, and Dave Wohlpart for the manufacture and supply of the vaccine lots, and Scott Vuocolo, Ph.D. for assistance in editing this manuscript.
Funding
Funding was provided by Merck & Co., Inc., Whitehouse Station, NJ, USA.
References
- Joura E, Giuliano A, Iversen O-E, Bouchard C, Mao C, Mehlsen J, Moreira E, Ngan Y, Petersen L, Lazcano E, et al. Efficacy of a 9-valent HPV vaccine against genital infection and intraepithelial neoplasia in women. New Engl J Med 2014; 372(8):711-23; PMID: 25693011.
- Lachenbruch PA, Rida W, Kou J. Lot consistency as an equivalence problem. J Biopharm Stat 2004; 14:275-90; PMID:15206526; http://dx.doi.org/10.1081/BIP-120037179
- Marshall V, Baylor NW. Food and Drug Administration regulation and evaluation of vaccines. Pediatrics 2011; 127 Suppl 1:S23-S30; PMID:21502242; http://dx.doi.org/10.1542/peds.2010-1722E
- US Food and Drug Administration CfBR. Guidance for industry for the evaluation of combination vaccines for preventable disease: production, testing and clinical studies. http://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/UCM175909.pdf. 1997.
- World Health Organization. Clinical considerations for evaluation of vaccines for prequalification. http://www.who.int/immunization_standards/vaccine_quality_considerations_oct10.pdf. 2010.
- European Medicines Agency - Committee for human medicinal products. Note for guidance on the clinical evaluation of vaccines. http://www.ema.europa.eu/docs/en_GB/document_library/scientific_guideline/2009/09/WC500003875.pdf. 2005.
- Wang WW, Mehrotra DV, Chan IS, Heyse JF. Statistical considerations for noninferiority/equivalence trials in vaccine development. J Biopharm Stat 2006; 16:429-41; PMID:16892905; http://dx.doi.org/10.1080/10543400600719251
- Van Damme P. Immunogenicity and safety of a novel 9-valent hpv vaccine in girls 9-15 years of age compared to the quadrivalent vaccine. EUROGIN 2013, November 3-6 Available at http://www.eurogincom/2013/indexphp/abstracts/eurogin-2013-abstracts2014. Accessed January 12, 2014. 2014.
- Roberts C, Hess E, Matys K, Brown M, Haupt R, Luxembourg A, Vuocolo S, Saah A, Antonello J, Green T. Development of a human papillomavirus competitive luminex immunoassay for nine HPV types. Hum Vaccin Immunother 2014; 10(8):2174-103; PMID:25424920
- Brown MJ, Seitz H, Towne V et al. Development of neutralizing monoclonal antibodies for oncogenic human papillomavirus types 31, 33, 45, 52, and 58. Clin Vaccine Immunol 21:587–593, 2014
- Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–226.
- Ganju J, Izu A, Anemona A. Sample size for equivalence trials: a case study from a vaccine lot consistency trial. Stat Med 2008; 27:3743-54; PMID:18416439; http://dx.doi.org/10.1002/sim.3273
- Sun X, Li X, Chen J. Comments on 'Sample size for equivalence trials: a case study from a vaccine lot consistency trial' by J. Ganju, A. Izu and A. Anemona. Stat Med 2012; 31:1652-3; PMID:22711252; http://dx.doi.org/10.1002/sim.4408
- Stanley M, Pinto LA, Trimble C. Human papillomavirus vaccines–immune responses. Vaccine 2012;30 Suppl 5:F83–F87.
- Brown D, Muller M, Sehr P et al. Concordance assessment between a multiplexed competitive Luminex immunoassay, a multiplexed IgG Luminex immunoassay, and a pseudovirion-based neutralization assay for detection of human papillomavirus types 16 and 18. Vaccine 32:5880–5887, 2014.
