Abstract
A major challenge in the development of effective therapies for rheumatoid arthritis (RA) is finding a method for the specific inhibition of the inflammatory disease processes without the induction of generalized immunosuppression. Of note, the development of therapeutic DNA vaccines and boosters that may restore immunological tolerance remains a high priority. pcDNA-CCOL2A1 is a therapeutic DNA vaccine encoding chicken type II collagen(CCII). This vaccine was developed by our laboratory and has been shown to exhibit efficacy comparable to that of the current “gold standard” treatment, methotrexate (MTX). Here, we used enzyme-linked immunosorbent assays with anti-CII IgG antibodies, quantified the expression levels of Th1, Th2, and Th3 cytokines, and performed flow cytometric analyses of different T-cell subsets, including Th1, Th2, Th17, Tc, Ts, Treg, and CD4+CD29+T cells to systemically evaluate humoral and cellular immune responses to pcDNA-CCOL2A1 vaccine in normal rats. Similar to our observations at maximum dosage of 3 mg/kg, vaccination of normal rats with 300 μg/kg pcDNA-CCOL2A1 vaccine did not induce the production of anti-CII IgG. Furthermore, no significant changes were observed in the expression levels of pro-inflammatory cytokines interleukin (IL)-1α, IL-5, IL-6, IL-12(IL-23p40), monocyte chemotactic protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, regulated on activation in normal T-cell expressed and secreted (RANTES), receptor activator for nuclear factor-κB ligand (RANKL), and granulocyte colony-stimulating factor (G-CSF) or anti-inflammatory cytokines IL-4 and IL-10 in vaccinated normal rats relative to that in controls(P > 0.05). However, transforming growth factor (TGF)-β levels were significantly increased on days 10 and 14, while interferon (IFN)-γ and tumor necrosis factor (TNF)-α levels were significantly decreased on days 28 and 35 after vaccination(P < 0.05). Similarly, there were no significant differences in the percentages of Tc, Ts, Th1/Th2, and Th17 cells between the 2 groups(P > 0.05), with the exception of Treg cells, which were significantly reduced on days 14 and 21 after vaccination (P < 0.05), and CD4+CD29+T cells, which were significantly increased on days 7 and 14 after vaccination(P < 0.05).Taken together, these results suggested that pcDNA-CCOL2A1 vaccine did not markedly affect the balance of immune system components in vaccinated normal rats, indicating that this DNA vaccine may have clinical applications in the treatment of RA.
Abbreviations:
- CCOL2A1
- chicken type II procollagen gene; CCII
- chicken type II collagen; RA
- rheumatoid arthritis; CIA
- collagen induced arthritis; MTX
- methotrexate; IL
- interleukin; MCP-1
- monocyte chemotactic protein-1; MIP-1α
- macrophage inflammatory protein-1 α; RANTES
- regulated on activation in normal T-cell expressed and secreted; RANKL
- receptor activator for nuclear factor-κB ligand; G-CSF
- granulocyte colony-stimulating factor; EAE
- experimental autoimmune encephalomyelitis; NOD
- spontaneous nonobese diabetic; MS
- multiple sclerosis; DMARD
- disease modifying antirheumatic drug
Introduction
Currently available treatments for rheumatoid arthritis (RA) are often ineffective in ameliorating the progression of the disease, particularly the invasive destruction of articular cartilage and bone; thus, RA remains incurable and highly incapacitating disease.Citation1-5 Accordingly, the exploration and development of new strategies, targets, and drugs for the treatment of RA are major focuses of many research groups. Moreover, while we have made great progress in improving our understanding of the pathogenesis of RA, the development of effective therapies for RA is still challenging as most agents that may suppress the inflammatory disease process also induce generalized immunosuppression.Citation2,6
Advances in our understanding of the pathogenesis of RA have led to the clinical introduction of novel therapies, such as disease-modifying antirheumatic drugs (DMARDs), biological modifier therapies (e.g., recombinant regulatory cytokines, engineered molecules, and monoclonal antibodies targeting pro-inflammatory cytokines or lymphocyte cell-surface proteins), gene therapy, and therapeutic DNA vaccines.Citation2,4,6-8 Notably, the development of therapeutic DNA vaccines that may recover immunological tolerance through the induction of both CD4+CD25+FoxP3+regulatory T (Treg) and CD3+CD8+C28- suppressor T (Ts) cells and/or inhibition of both autoreactive CD4+CD28+Th1(Th1) and auto-antibody-producing B cellsremains a high priority. Indeed, many trials of therapeutic DNA vaccines in rodents and other small mammals have been highly successful at treating experimental RA, experimental autoimmune encephalomyelitis (EAE), pathologies in spontaneous nonobese diabetic (NOD) mice, and other autoimmune diseases. In particular, a therapeutic DNA vaccineen coding myelin basic protein was shown to be efficacious and safe in a phase I/II clinical study of multiple sclerosis (MS).Citation2,6,9,10
The pcDNA-CCOL2A1 vector is a novel therapeutic DNA vaccine encoding CCII that was developed in our laboratory for the treatment of RA. Notably, we found that this vaccine exhibited efficacy comparable to that of the current “gold standard” treatment, methotrexate (MTX), in established collagen-induced arthritis(CIA) in rats.Citation2,3 The development of the pcDNA-CCOL2A1 vaccine is supported by the function of type II collagen (CII) as a critical auto-antigen in RA, and by the effective treatment of RA with native chicken type II collagen (nCCII).Citation11-13 In a more recent study, we found that vaccination of normal rats with pcDNA-CCOL2A1 vaccine at a maximum dosage of 3 mg/kg was safe and well tolerated. This dosage also did not induce any abnormal clinical signs and had no adverse effects on normal physiological functions, including hematology, metabolism, and immunogenicity.Citation14
Therefore, based on these encouraging results, we systemically evaluated humoral and cellular immune responses to the pcDNA-CCOL2A1 vaccine in normal rats following a single intramuscular injection of the therapeutic dose(300 μg/kg). These studies are particularly important to evaluate the systemic effects of newly developed therapeutic DNA vaccines. As we all know well that these are also important indicators of vaccine quality, regardless of the use of traditional vaccines or DNA vaccines.
Results
Vaccination of normal rats with the pcDNA-CCOL2A1 vaccine did not induce production of anti-CII antibodies
Because our previous results indicated that serum from normal rats under normal physiological conditions did not contain any anti-CII antibodies,Citation2,3 we first examined whether vaccination with 300 μg/kg pcDNA3.1-CCOL2A1 vaccine induced the production of anti-CII antibodies in normal rats. Thus, we used enzyme-linked immunosorbent assays (ELISAs) to investigate serum levels of anti-CII antibodies from vaccinated normal rats after a single intramuscular injection of either pcDNA3.1-CCOL2A1 vaccine or NS control. Anti-CII antibodies were measured at various times after vaccination, and the results are shown in . In view of the facts that there are same respones to vaccination with the pcDNA-CCOL2A1 vaccine between male and female rats, we pooled data from male and female rats in the analysis of the experimental results. Indeed, we found that normal rats vaccinated with 300μg/kg pcDNA-CCOL2A1 did not induce the production of anti-CII antibodies (neither anti-rat nor anti-chicken CII antibodies) from day 3 to day 21 after vaccination.
Table 1. The plasma levels of anti-CII antibodies from vaccinated normal rats with pcDNA-CCOL2A1 vaccine at different time-points after a single intramuscular injection of therapeutic dosage of 300 ug/kg (Units/mL, n = 30).
Based on the results obtained using the therapeutic dosage of 300 μg/kg, we also measured plasma levels of the two types of anti-CII antibodies at 14 d after vaccination of normal rats with the maximum dosage of 3 mg/kg. Similar to the results at the therapeutic dosage of 300 μg/kg, vaccination of normal rats with 3 mg/kg pcDNA-CCOL2A1 vaccine resulted in only very weak production of anti-CII IgG antibodies (low levels outside the linear range of the standard curve) as compared to the control group. Interestingly, the levels of anti-rat CII antibodies in rats administered 3 mg/kg vaccine were significantly lower than those of the normal control group(0.07355 ± 0 .0558 versus 0.1077 ± 0 .0565, respectively; P < 0.05). In addition, anti-chicken CII antibodies were not detected at 14 d after vaccination with 3 mg/kg vaccine.
Vaccination of normal rats with the pcDNA-CCOL2A1 vaccine did not alter the expression levels of most cytokines
Pro-inflammatory and anti-inflammatory cytokines are particularly important for modulating the balance between humoral and cell-based immune responses. To investigate the potential effects of vaccination with the pcDNA-CCOL2A1 vaccine on pro-inflammatory and anti-inflammatory cytokines, we systemically examined the expression levels of various cytokines in vaccinated normal rats on days 3 to 35 after a single intramuscular injection of pcDNA3.1-CCOL2A1 at the therapeutic dosage of 300 μg/kg. Because of the high variation among the levels of cytokines observed under normal physiological conditions, we were not able to compare the levels of cytokines at various times after vaccination with the normal control group. Therefore, we considered the cytokine measurements from the normal control group as the normal range (n = 30). The results of plasma cytokine analyses in vaccinated normal rats are shown in . These data showed that levels of pro-inflammatory cytokines, including interleukin (IL)-5, IL-6, IL-12, granulocyte colony-stimulating factor(G-CSF), macrophage inflammatory protein (MIP)-1α, monocyte chemotactic protein(MCP)-1, receptor activator for nuclear factor-κB ligand(RANKL), and regulated on activation in normal T-cell expressed and secreted (RANTES), were all within the normal range during the 35-day period after vaccination. Although the levels of IL-1α on the 28th day, tumor necrosis factor (TNF)-α on the 14th day, and interferon (IFN)-γ on the 10th and 14th days after vaccination were beyond the upper limit of the normal range, there were no significant differences compared with those found in normal control rats. Thereafter, TNF-α and IFN-γ levels were below the detection limit on days 28 and 35, while the levels of these cytokines were within the normal range on all other days tested.
Table 2. The plasma concentrations of inflammatory and anti-inflammatory cytokines from vaccinnated normal rats with pcDNA-CCOL2A1 vaccine at different time-points after a single intramuscular injection of therapeutic dosage of 300μg/kg(pg/mL, n = 30).
As shown in , among the measured anti-inflammatory cytokines, plasma IL-4 levels were within the normal range of fluctuation during the 35-day period after vaccination. Plasma IL-10 levels were beyond the upper limit of normal on day 35 after vaccination; however, no statistically significant differences were observed when compared with the maximum level in control rats. In contrast, plasma transforming growth factor (TGF)-β levels were significantly increased on days 10 and 14 after vaccination, returning to the normal range thereafter.
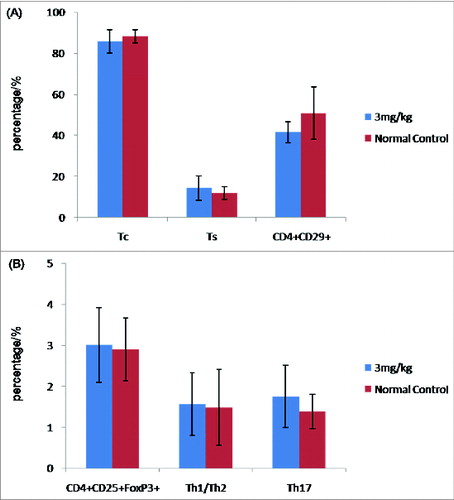
Next, we examined the effects of vaccination with 3 mg/kg pcDNA-CCOL2A1 on plasma levels of several key pro-inflammatory cytokines and anti-inflammatory cytokines on day 14 after vaccination. As shown in , pro-inflammatory cytokines, e.g., MIP-1α, MCP-1, and RANKL, and anti-inflammatory cytokines, e.g., IL-10 and TGF-β, were within the normal levels at day 14 after vaccination.
Table 3. The plasma concentrations of inflammatory and anti-inflammatory cytokines from vaccinnated normal rats with pcDNA-CCOL2A1 vaccine on the 14th day after a single intramuscular injection of maximum dosage of 3 mg/kg (pg/mL, n = 6).
Vaccination of normal rats with the pcDNA-CCOL2A1 vaccine did not alter most T-lymphocyte subsets
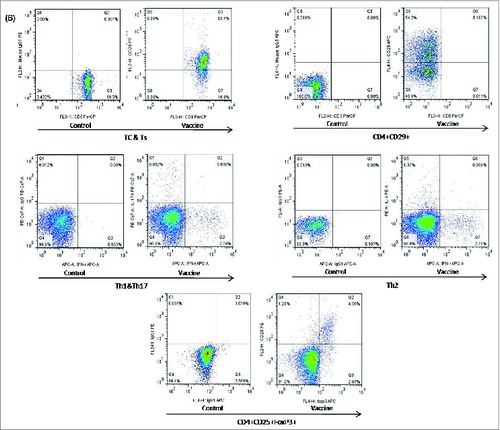
DNA vaccines have been shown to be remarkably effective at raising Tc and Th1/Th2 subsets.Citation2,6,15,16 For these reasons, we investigated whether there were changes in peripheral blood Tc, Ts, Treg, Th1/Th2, Th17, and CD4+CD29+T-cell subsets after vaccination of normal rats with 300 μg/kg pcDNA-CCOL2A1 vaccine. In order to exclude the spurious fluorescence, all matched isotype control antibodies including phycoerythrin (PE), phycoerythrin-Cy7 (PE-Cy7), allophycocyanin (APC), Alexa fluor-647 (AF-647) etc.-labeled IgG antibodies were used throughout as controls for non-specific cell surface binding in all fluorescence activated cell sorter (FACS)-based measurements. The left diagram of in each group shows the representative isotype-matched control and the corresponding analyzed T-lymphocyte subsets on the right diagram in . As shown in , there were no statistically significant differences in the percentage of Tc, Ts, Th1/Th2, and Th17 cells between the 2 groups(P > 0.05), with the exception of Treg cells, which were significantly lower on days 14 and 21 after vaccination(P < 0.05), and CD4+CD29+T cells, which were significantly higher on days 7 and 14 after vaccination (P < 0.05). Thereafter, levels of Treg cells and CD4+CD29+T cells recovered to normal levels by 28 and 35 d. In contrast, vaccination of normal rats with 3mg/kg pcDNA-CCOL2A1 vaccine did not cause changes in any of the T-lymphocyte subtypes examined in this study ().
Figure 1. (See previous page). The cellular immune response to the pcDNA3.1-CCOL2A1 vaccine(300 μg/kg) in normal rats. (A) The percentages of peripheral blood Tc, Ts, CD4+CD29+, CD4+CD25+Treg, Th1/Th2, and Th17 cells in vaccinated and unvaccinated normal rats. Rats were administered a single dose of 300 μg/kg pcDNA3.1-CCOL2A1 vaccine, and immune system cells were analyzed 3 to 5 d after injection. *P < 0.05 versus normal control (NC) using t-tests. (B) Representative data from flow cytometry analysis of peripheral blood CD4+CD25+Treg, CD4+CD29+, Th1/Th17,Th2, and Tc/Ts cells from vaccinated and unvaccinated normal rats 3 to 35 d after a single intramuscular injection of 300 μg/kg pcDNA3.1-CCOL2A1. The left diagram in each group shows the representative isotype-matched control and the corresponding analyzed T-lymphocyte subsets on the right diagram. These data are representative of 3 experiments. Three separate experiments yielded similar results.
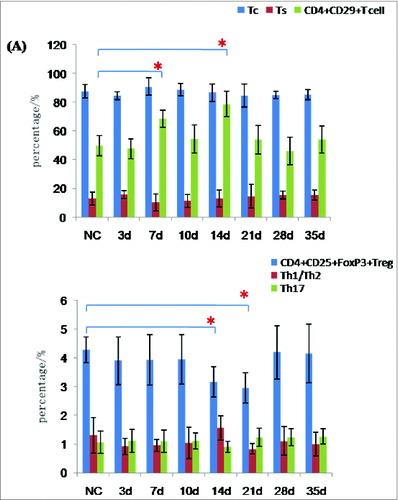
Figure 2. Cellular immune response to 3 mg/kg pcDNA3.1-CCOL2A1 vaccine in normal rats. (A) The percentages of peripheral blood Tc,Ts, and CD4+CD29+T cells in vaccinated and unvaccinated rats on day 14 after a single intramuscular injection of the vaccine. (B) The percentages of peripheral blood CD4+CD25+FoxP3+Treg, Th1/Th2, and Th17 cells from vaccinated and unvaccinated normal rats on day 14 after a single intramuscular injection of the vaccine. These data are representative of 3 experiments. Three separate experiments yielded similar results.
Discussion
Therapeutic DNA vaccines are a major breakthrough not only in the field of vaccination, but also in the field of autoimmune diseases, particularly for several important refractory autoimmune diseases, such as RA, MS, and insulin-dependent diabetes mellitus(IDDM). In theory, therapeutic DNA vaccines have several advantages over traditional vaccines and currently available treatments for autoimmune diseases.Citation2,6,9,15,17,18Unlike traditional or prophylactic vaccines that generally raise only humoral immune responses, such as different patterns of antibodies, therapeutic DNA vaccines possess the ability to simultaneously raise humoral and cellular immune responses.Citation16,19 Thus, it is particularly important to evaluate the systemic effects of newly developed therapeutic DNA vaccines on humoral and cellular immune responses. In the present study, our results clearly indicated that the pcDNA-CCOL2A1 vaccine did not markedly affect the balance of humoral and cellular immune responses in vaccinated normal rats.
Humoral immune responses, particularly antibody responses, are important indicators of vaccine quality, regardless of the use of traditional vaccines or DNA vaccines. Indeed, induction of the humoral immune response has become an important “gold standard” to determine the quality and effectiveness of newly developed vaccines.Citation15,19,20 For these reasons, we first systemically evaluated humoral immune response to the pcDNA-CCOL2A1 vaccine in normal rats. One of the findings from this study was that a single intramuscular injection of the pcDNA-CCOL2A1 vaccine (300 μg/kg or 3mg/kg) did not result in the production of anti-CII antibodies(neither anti-rat CII IgG antibodiesn nor anti-chicken CII IgG antibodies). This result was consistent with our recent findings demonstrating that 3 mg/kg pcDNA-CCOL2A1 vaccine does not induce the production of anti-CII IgG antibodies in normal rats. Indeed, vaccination of normal rats with 300 μg/kg vaccine did not inducethe production of anti-CII IgG antibodies from 3 to 35 d after vaccination. These results are also consistent with our previous studies showing that 300 μg/kg vaccine markedly reduces serum levels of anti-nCII antibodies in CIA rats.Citation2 However, these findings are inconsistent with established knowledge on the mechanisms of vaccine responses in humans and mammals; according to our prior understanding of the mechanisms of vaccines, all vaccines, including DNA vaccines, must be able to induce antibody responses or antibody production.Citation16,19 There are several explanations for our findings. One possible explanation is that antigenic type II collagen is sequestered, preventing the production of related anti-CII IgG antibodies under normal physiological conditions.Citation20,21 A more likely explanation is that administration of the pcDNA-CCOL2A1 vaccine in vivo in normal rats may only produce nonhydroxylated, nonglycosylated CCII; the lack of post-translational modifications (i.e., hydroxylation and glycosylation) would markedly affect the ability of the CII conformational epitopes to induce immune recognition and subsequent responses by B-lymphocyte.Citation2,21-24 Therefore, further studies are required to determine the cause of this interesting phenomenon.
The roles of different cytokines in controlling various aspects of the immune response suggest that particular cytokines may prove useful for optimizing vaccines against different diseases. Some cytokines have also been selected as adjuvants for DNA vaccines.Citation25,26 In our study, no significant changes were observed in most of the cytokines examined following administration of 300 μg/kg vaccine in normal rats. However, some changes were observed in the expression of the Th3-cytokine TGF-β and the Th1-cytokines IFN-γ and TNF-α on specific days after vaccination. Our findings here provide direct evidence that the pcDNA-CCOL2A1 vaccine did not result in global changes in cytokine networks in normal rats under normal physiological conditions. However, in a CIA rat model, 300 μg/kg pcDNA-CCOL2A1 vaccine upregulated the expression of the Th2-cytokine IL-10 and the Th3-cytokine TGF-β and downregulate the levels of IFN-γ and TNF-α (Th1 cytokines).Citation2 Thus, these results are critical for the potential use of this vaccine in the treatment of RA as the imbalance between pro-inflammatory Th1-cytokines and anti-inflammatory Th2 and/or Th3 cytokines which has been shown to be a major player in the immunopathogenesis of RA.Citation6,27,28
To design the most effective DNA vaccines, it is important to understand the types of immune responses required to prevent infection or alleviate autoimmune diseases.Citation16,19 In these cases, adaptive cellular immune responses comprising Tc, Ts, Treg, Th1/Th2, Th17, and CD4+CD29+T-cell subsets have been shown to be critical for maintaining normal immune function, controlling the pathogen, and protecting against disease progression. In the present study, our results indicated that the pcDNA-CCOL2A1 vaccine (300 μg/kg) had no effect on the percentages of Tc,Ts, Th1/Th2, and Th17 cells on days 3 to 35 after vaccination, with the exception of Treg cells and CD4+CD29+T cells, which exhibit transient changes in numbers. Interestingly, 3 mg/kg pcDNA-CCOL2A1 vaccine did not cause any significant changes in any of the examined T-cell subsets, indicating that the pcDNA-CCOL2A1 vaccine(at both 300 μg/kg and 3 mg/kg) did not affect the balance of adaptive cellular immune responses under normal physiological conditions. These findings are in contrast to those observed in our previous studies with established CIA rats, in which administration of the pcDNA-CCOL2A1 vaccine at the therapeutic dose could obviously increase the proportion of Treg cells and induced a shift from Th1 to Th2 cells.Citation2 These contradictory data (i.e., responses are observed under pathological conditions but not normal conditions) were unanticipated based on our understanding of the mechanisms of vaccines, particularly DNA vaccines, which are generally thought to raise both cytotoxic T cell(CTL) responses and different types of T-helper cell responses.Citation16,19 It is clear from these data that the exact mechanisms through which this DNA vaccine functions are not yet known. One possible explanation is that unlike DNA vaccines to immunize against infectious agents, which was predictable, the truly important part of DNA vaccines is not immunization of autoimmune diseases but rather the specific modulation of the immune system. The precise tweaking for unbalanced immune system provide by DNA vaccines may provide the opportunity to treat or even to cure autoimmune diseases.Citation9 We expect, of course, that these answers may be revealed satisfactorily in studies of the mechanism of immune tolerance under normal physiological conditions.
In conclusion, this is the first study to systemically evaluate humoral and cellular immune responses to the pcDNA-CCOL2A1 vaccine in normal rats at both the therapeutic dose of 300 μg/kg and the maximum dose of 3 mg/kg. Our results suggested that the pcDNA-CCOL2A1 vaccine did not affect the balance of humoral and cellular immune system responses in vaccinated normal rats.
Materials and Methods
Animals
Inbred female and male Wistar rats (4–6 weeks old) were obtained from the Animal Breeding Center of the Academy of Military Medical Sciences (Beijing, China) and maintained under specific pathogen-free conditions. The experiments were performed under the supervision and guidelines of the Academy of Military Medical Sciences Animal Welfare Committee.
pcDNA-CCOL2A1 vaccine
The eukaryotic pcDNA-CCOL2A1 expression vector previously constructed in our laboratory contained a 4837-bp full-length cDNA encoding chicken type II procollagenCitation29 (Genbank databases ACCESSION:AY046949 and AF452711) with deletion of the N-propeptides, signal peptide sequence, and Kozak consensus sequence.Citation2 The resulting recombinant plasmid, pcDNA-CCOL2A1, was produced in Escherichia coli, purified using Endo-free Mega-prep kits (Qiagen, Valencia, CA, USA), and used as a DNA vaccine for the study of humoral and cellular immune responses.
Vaccination of normal rats with the pcDNA-CCOL2A1 vaccine
Rats were randomly divided into 3 groups, with at least 30 rats in each group (15 male rats, 15 female rats). Rats in group 1 received a single intramuscular (i.m.) injection into the left hind limb musculi biceps femoris with 300 μg/kg pcDNA3.1-CCOL2A1 vaccine; rats in group 2 were injected with normal saline (NS) as a negative control; and rats in group 3 were administered a single injection of 3 mg/kg pcDNA3.1-CCOL2A1 vaccine(n = 6).
Measurement of plasma anti-CII antibody levels ELISA
On days 3, 7, 10, 14, 21, 28, and 35 after vaccination with the pcDNA-CCOL2A1 vaccine (300 μg/kg or 3 mg/kg), plasma was obtained and stored at -20°C until use. The levels of plasma antibodies to CII were measured by ELISA, as previously described.Citation2,3,6 Antibodies against CII were detected in rat plasma with commercially available test kits (Rat-IgG Anti-Rat/Chicken Type II Collagen Antibody ELISA Kit, Chondrex,WA,USA). In this test assay, 8-well strips coated with type II collagen were mixed with rat plasma samples to allow specific binding of anti-CII antibodies. The secondary antibody (peroxidase-conjugated goat anti-rat IgG) was added, followed by addition of peroxidase, inducing the reaction with OPD-Urea H2O2. Antibody levels were quantified using 7 standard samples (containing 0.25–16 units/mL antibody). The tested plasma were diluted such that the measured optical density (OD) values were between standard samples one and 7. The presented values were calculated by multiplying the plasma dilution with the measured antibody level.
Cytokine assays for Th1, Th2, and Th3 cytokines
On days 3, 7, 10, 14, 21, 28, and 35 after vaccination with 300 μg/kg or 3 mg/kg pcDNA-CCOL2A1 vaccine, plasma or serum were obtained and stored at -20°C until use. The pro-inflammatory cytokines IL-1α, IL-5, IL-6, IL-12(IL-23p40), IFN-γ, TNF-α, MCP-1, MIP-1α, RANTES, RANKL, and G-CSF and the anti-inflammatory cytokines IL-4, IL-10, and TGF-β were quantified by high-throughput cytokine detection technology with multifunctional flow lattice(Luminex 200TM,TX,USA) using commercial kits (Affymetrix,Santa Clara, CA,USA)as previously described.Citation2,3,6
Flow cytometric analysis for T-cell subsets
Peripheral blood samples were collected from groups of rats on days3, 7, 10, 14, 21, 28, and 35 after vaccination with the pcDNA-CCOL2A1 vaccine with the therapeutic dose of 300 μg/kg or the maximum dose of 3 mg/kg. The percentage of CD3+CD8+CD28+cytotoxic T (Tc), CD3+CD8+C28-suppressor T (Ts), CD4+CD25+FoxP3+regulatory T (Treg), and CD4+CD29+T cells were detected with various specific, fluorescence-conjugated monoclonal antibodies(BD Biosciences, Franklin Lakes, NJ, USA; eBioscience, San Diego, CA, USA)by flow cytometry(BD FACSVerse, NJ, USA) as previously described.Citation2,3,6 Th1, Th2, and Th17 cells were identified using intracellular cytokine staining with a BD Cytofix/Cytoperm Plus Fixation/Permeabilization kit, and data are presented in a scatter diagram according to IFN-γ, IL-4, and IL-17A levels. Irrelevant isotype-matched anti-IgG (Gene) was run as negative controls to account for spurious fluorescence.
Statistical analysis
Data analysis was performed using SPSS software version13.0. For descriptive analyses, mean ± standard deviations are reported. Student's t-tests were used to compare the differences in cytokines levels and the percentages of various T-cell subsets between the vaccinated group and the negative control group. Differences with P-values of less than 0.05 were considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported in part by grant from the National Major Scientific and Technological Special Project for “Significant New Drugs Development” (No.2009ZX09103-624 to Xi YZ). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Solomon DH, Bitton A, Katz JN, Radner H, Brown EM, Fraenkel L. Review: treat to target in rheumatoid arthritis: fact, fiction, or hypothesis? Arthritis Rheumatol 2014; 66:775-82; PMID:24757129; http://dx.doi.org/10.1002/art.38323
- Song X, Liang F, Liu N, Luo Y, Xue H,Yuan F, Tan L, Sun Y, Xi C, Xi Y.Construction and characterization of a novel DNA vaccine that is potent antigen-specific tolerizing therapy for experimental arthritis by increasing CD4+CD25+Treg cells and inducing Th1 to Th2 shift in both cells and cytokines.Vaccine 2009; 27:690-700; PMID:19095031
- Song XQ, Liang F, Liu N, Luo Y, Yuan F, Xue H, Tan LX, Long J, Zhao X, Sun YY, et al. Therapeutic efficacy of experimental rheumatoid arthritis with low-dose methotrexate by increasing partially CD4+CD25+Treg cells and inducing Th1 toTh2 shift in both cells and cytokines. Biomedicine & Pharmacotherapy 2010; 64 :463-71; PMID:20359858
- McInnes IB, Schett G. Mechanisms of disease:The pathogenesis of rheumatoid arthritis. NEJM 2011; 365:2205-19; PMID:2215003; http://dx.doi.org/10.1056/NEJMra1004965
- Holmdahl R, Malmström V, Burkhardt H. Autoimmune priming, tissue attack and chronic inflammation - the three stages of rheumatoid arthritis. Eur J Immunol 2014; 44:1593-9; PMID:24737176
- Xue H, Liang F, Liu N, Song XQ, Yuan F, Luo Y, Zhao X, Long J, Sun YY, Xi YZ. Potent antirheumatic activity of a new DNA vaccinetargeted to B7-2/CD28 costimulatory signalingpathway in autoimmune arthritis. Hum Gene Ther 2011; 22:65-76; PMID:20695769; http://dx.doi.org/10.1089/hum.2010.110
- Ramiro S, Gaujoux-Viala C, Nam JL, Smolen JS, Buch M, Gossec L, van der Heijde D, Winthrop K, Landewé R. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2013 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis 2014; 73:529-35; PMID:24401994
- Gaujoux-Viala C, Nam J, Ramiro S, Landewé R, Buch MH, Smolen JS, Gossec L. Efficacy of conventional synthetic disease-modifying antirheumatic drugs, glucocorticoids and tofacitinib: a systematic literature review informing the 2013 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis 2014; 73:510-5; PMID:24395555
- Mallon S. DNA vaccines: Treatment options for autoimmune diseases. Microbiol Mol Gent 2008; 4:99-103
- Robinson WH, Garren H, Utz PJ, Steinman L. Millennium Award. Proteomics for the development of DNA tolerizing vaccines to treat autoimmune disease. Clin Immunol 2002; 103:7-12; PMID:11987980
- Kim WU, Cho ML, Jung YO, Min SY, Park SW, Min DJ, Yoon JH, Kim HY. Type II collagen autoimmunity in rheumatoid arthritis. Am J Med Sci 2004; 327:202-11; PMID:15084916
- Trentham DE, Dynesius-Trentham RA, Orav EJ. Effects of oral administration of type II collagen on rheumatoid arthritis. Science 1993;261:1727-30; PMID:8378772; http://dx.doi.org/10.1126/science.8378772
- Xu SQ, Liu S, Zhang W, Shen J, Liu HX, Wang RC. A multicenter, double-blind, randomized, controlled phase III clinical trial of chicken type II collagen in rheumatoid arthritis. Arthritis Res Ther 2008; 11:R180-189; PMID:19951408; http://dx.doi.org/10.1186/ar2870
- Long J, Zhao X, Yun S, Zhang Z, Jin J, Yu K, Hao L, Dai D, Ding L, Tan L, et al. Safety and immunogenicity of a novel therapeutic DNA vaccine encoding chicken type II collagen for rheumatoid arthritis in normal rats. Immunol Res 2014( to be published)
- Luo Y, Liang F, Liu N, Xue H, Song XQ, Yuan F, Long J, Zhao X, Sun YY, Xi YZ. Potent control of acute graft-versus-host disease lethality after immunization with a novel DNA vaccine targeted to B7-1/CD28 costimulatory signaling pathway. J Immunother 2013; 36: 82-92; PMID:23377669; http://dx.doi.org/10.1097/CJI.0b013e31827a6e3e
- Robinson HL. DNA vaccines: basic mechanism and immune responses. Int J Mol Med 1999; 4:549-55; PMID:10534580
- Edlmayr J, Niespodziana K, Focke-Tejkl M, Linhart B, Valenta R. Allergen-specific immunotherapy: towards combination vaccines for allergic and infectious diseases. Curr Top Microbiol Immunol 2011; 352:121-40; PMID:21626347
- Fioretti D, Iurescia S, Fazio VM, Rinaldi M. DNA vaccines: developing new strategies against cancer. J Biomed Biotechnol 2010; 2010:174378; PMID:20368780; http://dx.doi.org/10.1155/2010/174378
- Robinson HL, Torres CAT. DNA vaccines. Semin Immunol 1997; 9:271-83; PMID:9327522
- Arens R. Rational design of vaccines: learning from immune evasion mechanisms of persistent viruses and tumors. Adv Immunol 2012; 114:217-43; PMID:22449784; http://dx.doi.org/10.1016/B978-0-12-396548-6.00009-3
- Myllyharju J, Kivirikko KI.Collagens and collagen-related diseases. Ann Med 2001; 33: 7-21; PMID:11310942; http://dx.doi.org/10.3109/07853890109002055
- Myers LK, Brand DD, Ye XJ, Cremer MA, Rosloniec EF, Bodo M, Myllyharju J, Helaakoski T, Nokelainen M, Pihlajaniemi T, et al. Characterization of recombinant type II collagen: arthritogenicity and tolerogenicity in DBA/1 mice. Immunology 1998; 95: 631-39; PMID:9893056; http://dx.doi.org/10.1046/j.1365-2567.1998.00637.x
- Nokelainen M. Recombinant human collagens:Characterization of type II collagen expressed in insect cells and production of types I-III collagen in the yeast pichia pastoris. Acta Universitatis Ouluensis D Medica 604; 2000:1-70.
- Myers LK, Seyer JM, Stuart JM, Terato K, David CS, Kang AH. T cell epitopes of type II collagen that regulate murine collagen-induced arthritis. J Immunol 1993; 151:500-5; PMID:7686947
- Lisziewicz J, Calarota SA, Lori F. The potential of topical DNA vaccines adjuvanted by cytokines. Expert Opin Biol Ther 2007; 7:1563-74; PMID:17916048
- Barouch DH, Letvin NL, Seder RA. The role of cytokine DNAs as vaccine adjuvants for optimizing cellular immune responses. Immunol Rev 2004; 202:266-74; PMID:15546399
- Yoshida K, Hashimoto T, Sakai Y, Hashiramoto A. Involvement of the circadian rhythm and inflammatory cytokines in the pathogenesis of rheumatoid arthritis. J Immunol Res 2014;2014:282495; PMID:25229267
- Astry B, Harberts E, Moudgil KD. A cytokine-centric view of the pathogenesis and treatment of autoimmune arthritis. J Interferon Cytokine Res 2011; 31:927-40; PMID:22149412
- Xi CX, Liu N, Liang F, Guo SQ, Sun YY, Yang F, Xi YZ. Molecular cloning, characterization and localization of chicken type II procollagen gene. Gene 2006;366, 67-76; PMID:16297573; http://dx.doi.org/10.1016/j.gene.2005.06.032