Abstract
To evaluate the safety and immunogenicity of 23-valent pneumococcal polysaccharide vaccine (PPV23), a randomized, double-blind and parallel controlled clinical trial was conducted in Yancheng, Jiangsu Province of China. There were 1200 subjects randomized into 2 groups with a 1:1 allocation. Subjects received 0.5 mL of tested PPV23 or control PPV23 by intramuscular injection in the deltoid, respectively. Results showed that seroconversion rates of all 23 types except type 3 were not significantly different between the 2 groups. The seroconversion rate of the Group T for type 3 (P = 0.0009) was significantly higher than the Group C. The post-vaccination GMCs of the Group T for types 1 (P = 0.0340), 3 (P = 0.0003), 9V (P = 0.0016), 11A (P = 0.0222) and 33F (P = 0.0344) were significantly higher than the Group C. The frequencies of local and general reactions were not significantly different and acceptable in both groups. In conclusion, The PPV23 showed a good immunogenicity and tolerability in 2 to 70 y old healthy people.
Introduction
Pneumococcal is the main pathogenic bacteria of pneumonia, bacteremia, meningitis, and otitis media. It has been established that 23-valent pneumococcal polysaccharide vaccine induces antibody production and that such antibody is effective in preventing pneumococcal disease. Clinical studies with 23-valent pneumococcal polysaccharide vaccine have demonstrated immunogenic responses.Citation1-3 It is very important to improve the vaccine coverage of prophylaxis against pneumococcal disease.
In this study, we examined the safety and immunogenicity of the 23-valent pneumococcal polysaccharide vaccine in healthy Chinese people. The results were described hereinafter.
Results
Study participants
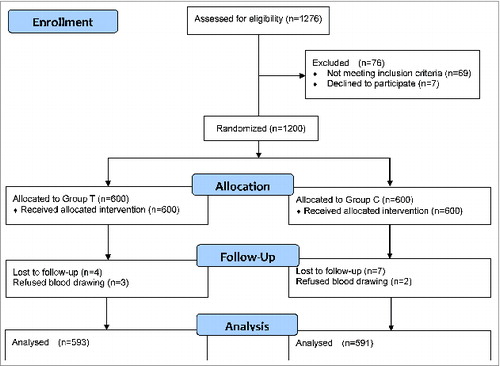
A total of 1276 volunteers were evaluated for the trial, of which 69 (5.41%) met the exclusion criteria, 7 (0.55%) were refused to blood drawing. There were 1200 subjects randomized into 2 groups with a 1:1 allocation. Age and gender were not significantly different between the 2 groups, the median age of Group T subjects was 30.29 y (range, 2–69 years), and the median age of Group C subjects was 30.29 y (range, 2–70 years); 301 (50.17%) of the subjects in the Group T and 313 (52.17%) of the subjects in the Group C were male.
Subjects received 0.5 mL of tested vaccine or control vaccine by an intramuscular injection in the deltoid, respectively. Serum was collected from 1184 subjects before and Day 28 post-vaccination (593 from Group T, 591 from Group C). The summary of results was presented in and .
Table 1. Summary of Subject Demographics
Immunogenicity
Both tested vaccine and control vaccine elicited significant immune responses. Pre- and post-vaccination GMCs, GMIs and seroconversion rates against each of the 23 pneumococcal types for each group were summarized in. The pre-vaccination GMCs of all 23 types except 6B and 14 were not significantly different between the 2 groups, the pre-vaccination GMCs of the Group C for type 6B (P = 0.0223) and 14 (P = 0.0421) were significantly lower.
Table 2. Serological Response of Subject to the 23-valent Pneumococcal Polysaccharide Vaccine
The post-vaccination GMC for type 3 was the highest (110.99 U/mL) in the Group T, type 6B was the lowest (30.53U/mL) in the Group C. The post-vaccination GMCs of the Group T for all 23 types except type 15B, 18C, 19A and 23F were higher than the Group C, but the differences were significant only for types 1 (P = 0.0340), 3 (P = 0.0003), 9V (P = 0.0016), 11A (P = 0.0222) and 33F (P = 0.0344). The post-vaccination GMC of the Group C only for type 18C (P = 0.0375) was significantly higher than the Group T.
The GMIs of all 23 types except types 9V and 18C were not significantly different between the 2 groups, the GMI of the Group T for type 9V (P<0.0001) was significantly higher than the Group C, the GMI of the Group C for type 18C (P=0.0081) was significantly higher than the Group T.
The seroconversion rate for type 9N was the highest (91.62%) in the Group T, type 5 was the lowest (37.61%) in the Group C. The seroconversion rates of all 23 types except type 3 were not significantly different between the 2 groups, the seroconversion rate of the Group T for type 3 (P = 0.0009) was significantly higher than the Group C.
Safety
There were 145 subjects reported adverse reactions in the Group T, and 160 in the Group C. There were no significant differences in the total incidence of adverse reactions between the 2 groups (P = 0.3533). No Grade 4 general or local adverse reaction was reported during the whole study. The observed adverse reactions were summarized in.
Table 3. Summary of adverse reactions
Solicited general symptoms
The total incidence of general adverse reactions in this study (Group T vs. Group C) was 11.17% vs. Thirteen.50%, No significant differences were detected between the 2 groups (P = 0.2537). The most common general adverse symptom was fever during the entire study, 53 subjects (8.83%) reported fever in the Group T and 59 subjects (9.83%) in the Group C (P = 0.6199). The more common general adverse symptoms were myalgia and headache in the post-vaccination. Fifteen subjects (1.25%) reported myalgia and 11 subjects (0.92%) reported headache. Most of the general adverse symptoms were Grade 1 intensity. Grade 1 fever symptom was reported by 67.0% of the total fever reports in the study, Grade 1 myalgia and headache symptoms were reported by 93.3% and 90.9% of the total myalgia and headache reports respectively. The general adverse reactions were not significantly different between the 2 groups, as shown in.
Table 4. Incidence and intensity grades of solicited general adverse reactions
Solicited local symptoms
The total incidence of local adverse reactions in this study (Group T vs. Group C) was 17.17% vs. Seventeen.00%, No significant differences were detected between the 2 groups (P = 1.000). The most common local adverse symptom was pain after vaccination. 95 subjects (15.83%) reported pain in the Group T and 98 subjects (16.33%) in the Group C (P=0.8752). The more common local adverse symptoms were swelling and redness after vaccination. Most of the local adverse reactions were Grade 1 intensity. Grade 1 pain symptoms were reported by 94.3% of the total pain reports in the study. Grade 1 swelling and redness were reported by 45.5% and 48.6% of the total swelling and redness reports respectively. The local symptoms of pain and swelling were not significantly different between the 2 groups, the redness of group T was significantly higher compared to the Group C (P=0.0381), as shown in.
Table 5. Incidence and intensity grades of solicited local adverse events
Unsolicited AEs and SAEs
In the study, 29 unsolicited adverse events (12 from Group T, 17 from Group C) were reported during the 28 d after vaccination. The adverse reactions of them were always mild and resolved with no sequelae. One female subject in the Group C reported SAE in the study, the subject fracture on Day 19 after vaccination and was hospitalized for 7 d No SAE was related to the vaccination.
Discussion
In this study, tested vaccination with PPV23 was well tolerated, fever was observed in 53 (8.83%) subjects, pain at the injection site was observed in 95 (15.83%), and redness in 24 (4.00%). These local and general adverse reactions were always transient and mild. The result was in agreement with that reported in the literature.Citation4
Both the tested and control vaccine elicited significant immune responses. The seroconversion rates of the Group T for types 2, 8, 9N, 9V, 18C and 33F were higher than 70%, type 9N of which was the highest (89.26%). The seroconversion rates of the Group T for types 1, 3, 4, 5, 10A, 12F and 14 was lower than 50%, type 5 of which was the lowest (37.61%). The result was in agreement with that reported in the literature.Citation4
The pre-vaccination GMCs of the Group T for types 3, 5, 7F, 11A, 12F, 14, 15B, 19A and 20 were higher than other types, type 3 of which was the highest (34.35 U/mL). The pre-vaccination GMCs of the Group T for types 8, 9N, 18C and 33F were lower than other types, type 9N of which was the lowest (7.21U/mL). It is interesting that the pre-vaccination GMCs in this study seemed to correlates with the situation of serotype distribution in China.Citation5,6
In conclusion, the tested PPV23 was demonstrated to be non-inferior to the control PPV23 on safety and immunogenicity. The tested PPV23 showed a good immunogenicity and tolerability in 2 to 70 y old healthy people. There could be possibility of PPV23 widely use in Chinese people after the tested PPV23 licensed in China.
Materials and Methods
Study design
The randomized, double-blind and parallel controlled clinical trial was conducted in Yancheng, Jiangsu Province of China from September 2011 to December 2012. The trial protocol was reviewed and approved by the Ethics Committee of Jiangsu Province Centers for Disease Control and Prevention. The trial was registered at www.clinicaltrials.gov (NCT01451086).
Vaccines
The Group T received a 0.5mL dose of tested PPV23 (Beijing Minhai Biotechnology Co., Ltd, Beijing, China). The Group C received a 0.5mL dose of licensed PPV23 (Chengdu Institute of Biological Products, Chengdu, China). Vaccinations were given intramuscular injection in the deltoid.
Subjects
Subjects were excluded if any of the following conditions were displayed: subjects with any pneumococcal vaccine before vaccination; history of pneumococcal infection; women in pregnancy or lactation period in trial duration; allergic history or any serious adverse event (SAE) after vaccination, such as allergy, urticaria, dyspnea, angioedema, celialgia; known or suspected immune dysfunction, including persons with congenital immunodeficiency or persons with HIV positive; functional or anatomic asplenia; patients treated with chemotherapy in past 5 y or administered with immunosuppressive agents, cytotoxicity factors or corticosteroids in the 6 months preceding the vaccine trial; receipt of blood products in the 3 months preceding vaccination; participation in another clinical study investigating a vaccine, drug in the 30 d preceding vaccination; receipt of any live virus vaccine in the 30 d preceding vaccination; receipt of any subunit vaccine and inactivated vaccine in the 14 d before vaccination; thrombocytopenia, bleeding disorder; history of asthma,angioneurotic edema,diabetes mellitus or malignancy tumor; history of thyroid gland excision or treatment for thyroid gland disease in last 12 months; hypertension, blood pressure still above 145/95mmHg even with drug treatment; history of eclampsia, epilepsia, psychosis; febrile illness (temperature ≥ 38°C) in the 3 d or any acute illness/infection in the 7 d preceding vaccination; in progress of anti-tuberculosis prophylaxis or therapy; those cannot fulfill the protocol or cannot sign the informed consent form for any medical, psychological, social, occupational and other reasons, according to investigator judgment.
Twelve hundred healthy subjects who were between the ages of 2 to 70, were selected and randomized to tested group or control group (ratio 1:1) in Yancheng, China.
Assessment of immunogenicity
Venous blood samples (up to 4 mL) were collected before and 4 weeks after immunization. Sera were separated immediately and stored at −20°C until they were assayed. The concentrations of antibody against 23 pneumococcal types were measured by ELISA at the National Institutes for Food and Drug Control, Beijing, China. The procedure of the ELISA is described previously.Citation7 Purified pneumococcal capsular polysaccharides were provided by Beijing Minhai Biotechnology Co., Ltd. C-polysaccharides (C-Ps) were obtained from the State Serum Institute (Denmark). Reference serum from pneumococcal vaccinated adults was designated to contain 100U antibody per milliliter. Antibody concentrations were represented as percent of the reference serum from the National Institutes for Food and Drug Control.
Assessment of safety
After vaccination, nurses observed subjects at the study site for 30 minutes for immediate adverse reactions. Subjects/participant's parents were given a transparent sheet with concentric circles apart to measure the local reactions (redness and swelling) at the injection sites, a thermometer to measure the axilla temperature, and diary cards to record solicited and unsolicited adverse events (AEs). Solicited AEs were recorded within 7 d following vaccination and unsolicited AEs within 28 d post-vaccination. Symptom intensity was defined as: Grade 1 (mild); Grade 2 (moderate); Grade 3 (serious) and Grade 4 (life-threatening).
Statistical analysis
Primary objective outcome was seroconversion (fold2- responses) rate of subjects on Day 28 post-vaccination.
Secondary objective outcome were the antibody geometric mean concentration (GMC), geometric mean antibody fold increase (GMI) on Day 28 post-vaccination, and the incidence of systemic and local adverse reactions in healthy subjects within 28 d post-vaccination.
The GMC of each type between the Group T and Group C were compared using paired Student's t-test. The seroconversion rates and frequency of adverse reactions between the Group T and Group C were compared using Pearson χ2 test or Fisher's exact tests. All statistical analyses were used in SAS9.2 software.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Vila-Córcoles Angel, Ochoa-Gondar Olga, Hospital Imma, Ansa Xabier, Vilanova Angels, Rodrı´guez Teresa, Llor Carl. Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: the EVAN-65 study. Clin Infect Dis 2006; 43: 860-8; PMID:16941367; http://dx.doi.org/10.1086/507340
- Miernyk Karen M, Butler Jay C, Bulkow Lisa R, Singleton Rosalyn J, Hennessy Thomas W, Dentinger Catherine M, Helen V Peters, Barbara Knutsen, Parkinson Alan J. Immunogenicity and reactogenicity of pneumococcal polysaccharide and conjugate vaccines in alaska native adults 55-70 years of age. Clin Infect Dis 2009; 49: 241-8; PMID:19522655; http://dx.doi.org/10.1086/599824
- Lee Hoan-Jong, Kang Jin-Han, Henrichsen Jørgen, Konradsen Helle Bossen, Jang Seong-Hee, Shin Hee-Young, Ahn Hyo-Seop, Choi Yong, Hessel Luc, Nam Sung-Woo. Immunogenicity and safety of a 23-valent pneumococcal polysaccharide vaccine in healthy children and in children at increased risk of pneumococcal infection. Vaccine 1995; 13: 1533-8; PMID:8578838; http://dx.doi.org/10.1016/0264-410X(95)00093-G
- WHO. Twenty-three-valent pneumococcal polysaccharide vaccine. WHO position paper. Wkly Epidemiol Rec 2008; 83: 373-84; PMID:18927997.
- Ding Shaoqing, Ye Renbang, Yuan Zhenglin, Du Zongli. Serotype distribution and influence of streptococcus pneumonia in china. Bullet Med Res 1989; 18: 28-9.
- Yao Kaihu, Lu Quan, Deng Li, Yu Sangjie, Zhang Hong, Deng Qiulian, Tong Yuejuan, Gao Wei, Yuan Lin, Shen Xuzhuang, et al. Serotype distribution and resistance to β-lactams of Streptococcus pneumoniae isolated from children in beijing, shanghai and guangzhou, 2000–2002. China J Pediat 2006; 44: 928-32; PMID:17254463.
- Konradsen HB, Sorensen UB, Henrichsen J. A modified enzyme-linked immunosorbent assay for measuring type-specific anti-pneumococcal capsular polysaccharide antibodies. J Immunol Methods 1993; 164: 13-20; PMID:8360502; http://dx.doi.org/10.1016/0022-1759(93)90270-H