Abstract
Trivalent influenza vaccine (TIV) selects one of the 2 co-circulating influenza B lineages whereas quadrivalent influenza vaccine (QIV) includes both lineages. We examined potential cost-effectiveness of QIV versus TIV from perspectives of healthcare provider and society of Hong Kong. A decision tree was designed to simulate the outcomes of QIV vs. TIV in 6 age groups: 0–4 years, 5–9 years, 10–14 years, 15–64 years, 65–79 y and ≥80 years. Direct cost alone, direct and indirect costs, and quality-adjusted life-years (QALYs) loss due to TIV-unmatched influenza B infection were simulated for each study arm. Outcome measure was incremental cost per QALY (ICER). In base-case analysis, QIV was more effective than TIV in all-age population with additional direct cost per QALY (ICER-direct cost) and additional total cost per QALY (ICER-total cost) of USD 22,603 and USD 12,558, respectively. Age-stratified analysis showed that QIV was cost-effective in age groups 6 months to 9 y and ≥80 years from provider's perspective, and it was cost-effective in all age group except 15–64 y from societal perspective. Percentage of TIV-unmatched influenza B in circulation and additional vaccine cost of QIV were key influential factors. From perspectives of healthcare provider and society, QIV was the preferred option in 52.77% and 66.94% of 10,000 Monte Carlo simulations, respectively. QIV appears to be cost-effective in Hong Kong population, except for age group 15–64 years, from societal perspective. From healthcare provider's perspective, QIV seems to be cost-effective in very young (6 months-9 years) and older (≥80 years) age groups.
Background
In Hong Kong, one of 4 hospital admissions for influenza was caused by influenza B and influenza B admissions were highest among children and elderly.Citation1 For many years only one of the 2 influenza B lineages (Yamagata and Victoria), in addition to 2 subtypes of influenza A, was selected for the trivalent influenza vaccine (TIV). An epidemiology study in Hong Kong showed that, from 2000 to 2010, co-circulation of both influenza B subtypes occurred in 9 y and the percentage of TIV-unmatched influenza B lineage in clinical isolates ranged from 3.7% to as high as 96.4%.Citation1
Recently, quadrivalent influenza vaccine (QIV) including both influenza B/Yamagata and influenza B/Victoria, as well as 2 influenza A subtypes becomes available. QIV provides same coverage as TIV and it also includes protection against the TIV-unmatched influenza B lineage. An US epidemiology study demonstrated positive clinical benefits of QIV based upon the annual influenza B admission rate and distribution of co-circulated influenza B lineages in 1999–2009.Citation2 The economic impact projected from clinical outcomes of QIV was however highly subjected to the additional vaccine cost of QIV versus TIV as well as the perspective of cost analysis.Citation3 In order to facilitate the decision-making process of implementing QIV vaccination program, we compared the cost, clinical outcomes and quality-adjusted life-years (QALYs) of QIV vs. TIV in prevention of TIV-unmatched influenza B infection from perspectives of both healthcare providers (direct costs) and society (direct and indirect costs) of Hong Kong.
Results
Base-case analysis
Expected clinical outcomes in the base-case analysis were showed in, including influenza B (TIV-unmatched) infections, hospitalizations and deaths per 100,000. The incidence of clinical events was highest in extreme-age groups (6 month-4 y and ≥80 years) and lowest in adults (15–64 years).
Table 1. Expected clinical outcomes of QIV and TIV
showed the expected direct cost, total cost (including direct and indirect costs) and QALYs loss in each vaccine group. The highest QALY-savings occurred in 0–4 y (20.39 QALYs per 100,000) and lowest in 15–64 y (0.29 QALYs per 100,000). QIV was more effective than TIV in all-age population with additional direct cost per QALY saved (ICER-direct cost) and additional total cost per QALY saved (ICER-total cost) of USD22,603 and USD12,558, respectively. The direct costs of QIV were higher than those of TIV in all age groups and the highest additional direct cost occurred in elderly groups (65–79 y and ≥80 years). Total costs of QIV were higher than TIV costs in age groups 10–79 years, and QIV was cost-saving (USD 7,248–194,403 per 100,000 individuals) comparing to TIV in age groups 6 months-4 years, 5–9 y and ≥80 years.
Table 2. Base-case analysis results on expected direct cost, total cost and QALY loss per 100,000 individuals and ICER of QIV vs. TIV
Sensitivity analysis
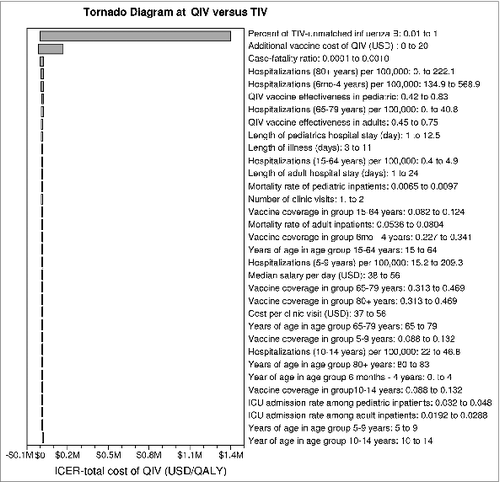
One-way sensitivity analysis was performed to identify influential factors and corresponding threshold values on ICERs of QIV using 1x gross domestic product (GDP) per capita of Hong Kong (USD 36,557) as the willingness-to-pay threshold of a cost-effective option. showed a tornado diagram on the impact of one-way variation of all parameters on ICER-total cost in all-age population. Two most critical factors identified were percent of TIV-unmatched influenza B lineage in circulation and additional vaccine cost of QIV versus TIV and threshold values were >28.0% and <USD5.7, correspondingly, for ICER-total cost to remain below USD36,557 per QALY. The tornado diagram on ICER-direct cost (not shown) was very similar to, and the threshold values were >35.0% TIV-unmatched influenza B and additional QIV cost <USD4.6 for ICER-direct cost of QIV to be less than USD 36,557 per QALY.
Figure 1. One-way sensitivity analysis (tornado diagram) of all parameters on ICER (total cost) of QIV in all-age population.
One-way sensitivity analysis () on age-stratified ICERs of QIV also identified the percent of TIV-unmatched influenza B lineage in circulation and additional vaccine cost of QIV vs. TIV to be the 2 most common influential factors affecting both ICERs (direct cost and total cost) of QIV in 5 of 6 age groups. Age-specific hospital admission rates for TIV-unmatched influenza B was another common critical factor found in 4 age groups. The number of influential factors identified was highest in age groups 10–14 y and 65–79 y.
Table 3. Influential factors and threshold values identified by one-way sensitivity analysis for 6 age groups
Table 4. Model inputs
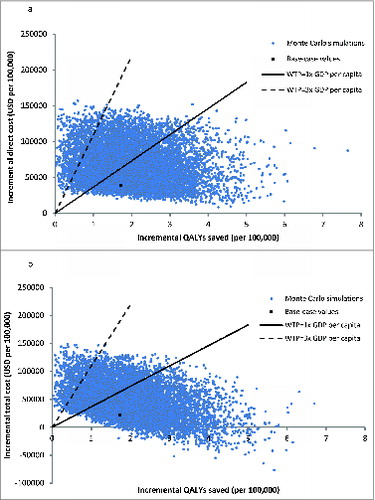
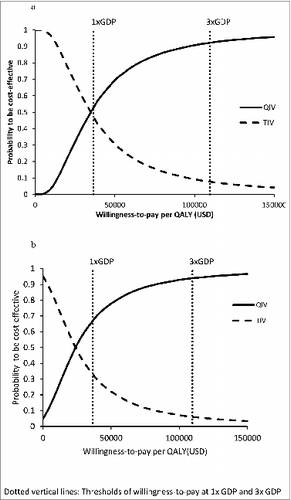
The probabilistic sensitivity analysis was performed by 10,000 Monte Carlo simulations on all-age population (). QIV was more costly than TIV with mean direct cost difference of USD 68,031 per 100,000 individual (95% CI 67,485–68,577) (p < 0.001) and mean total cost difference of USD47,051 per 100,000 individuals (95% CI 46,438–47,664) (p < 0.001). QIV saved 2.00 QALYs per 100,000 individuals (95% CI 1.98–2.02) (p < 0.001) comparing to TIV. ICERs-direct cost of QIV were <1x GDP per capita in 52.77% of 10,000 simulations, fell between 1x and 3x GDP per capita in 39.50%, and >3 × GDP per capita in 7.73%. Comparing total costs of QIV and TIV, QIV was cost-saving in 4.83% of 10,000 simulations. The ICERs-total cost of QIV were <1x GDP per capita in 62.11%, fell between 1x and 3x GDP in 27.02%, and >3x GDP per capita in 6.04% of time. The probabilities of each vaccine to be the preferred option were examined in the acceptability curves over a wide range of willingness-to-pay (0–150,000 USD/QALY) (). From perspectives of healthcare provider and society, QIV was the preferred option using 1x GPD per capita as willingness-to-pay in 52.77% and 66.94% of 10,000 Monte Carlo simulations, respectively.
Discussion
Our study examined the cost-effectiveness of QIV versus TIV for preventing TIV-unmatched influenza B infection in Hong Kong. The findings of base-case analysis demonstrated that QIV was cost-effective comparing to TIV for all-age individuals from perspectives of both healthcare provider and society, at a willingness-to-pay threshold of 1x GDP per capita. Age-stratified analysis further showed that QIV was only cost-effective in young children (6 months-9 years) and very old individuals ((≥80 years), but was not cost-effective in other age groups (10–79 years) from healthcare provider's perspective. From a societal perspective including direct medical cost and indirect cost resulting from loss of productivity due to illness or death, QIV was cost-effective (and even cost-saving in some age groups) in all but the adult group (15–64 years). The sensitivity analyses have suggested that the base-case analysis findings in all-age and 6 age groups were sensitive to the percent of TIV-unmatched influenza B lineage in circulation and additional vaccine cost of QIV vs. TIV. The 10,000 Monte Carlo simulations in all-age individuals estimated QIV to be cost-effective over 5090%– of time when threshold of willingness-to-pay varied from 1–3x GDP per capita from both perspectives of provider and society.
The present outcome analysis simulated age-stratified cost and QALY saved by QIV in Hong Kong. The cost analysis included direct costs alone (costs of vaccine and influenza treatment) and both direct and indirect costs (productivity loss of caregivers and patients). QIV was consistently more costly than TIV in all age groups when direct medical cost alone was considered from a perspective of healthcare provider. Our data suggested that the additional cost of QIV was not outweighed by the cost-saving in medical cost from reduction in clinical events. Similar findings in economic analysis of QIV were reported that QIV resulted in additional cost to third party payers unless the additional vaccine cost of QVI was USD0.40 or less.Citation3
From the societal perspective, QIV was highly cost-saving in age group 6 months-4 years, followed by 5–9 y and ≥80 years. As indicated by the clinical outcomes () of the 2 vaccines, pediatric groups with QIV had high reduction in hospitalizations and deaths. Prevention of a case of death in pediatric patients, despite the low mortality rate, could save the loss of life-long productivity and life-long QALYs. QIV vaccination in pediatric group was therefore both cost-saving and effective from a societal perspective. The clinical outcomes of QIV in age group ≥80 years showed second highest reduction of hospitalizations and highest reduction of deaths. Productivity loss due to death was calculated up to age 65 y (see Methods below) and therefore was not responsible for cost-saving in indirect cost of age group ≥80 years. The indirect cost (productivity loss by caregivers) saving from reducing a case of hospital admission of older patient by QIV outweighed the additional vaccine cost of QIV. Our results were consistent with findings on cost-effectiveness of influenza vaccination in Hong Kong elderly.Citation4 The base-case results (from both perspectives) of QIV to be either cost-effective or dominating in these 3 age groups (6 months-4 years, 5–9 y and ≥80 years) were only subjected to 3 key influential factors (percent of TIV-unmatched influenza B, additional vaccine cost, and hospital admission rate of influenza B) as indicated by the one-way sensitivity analysis.
Of the 3 other groups (10–79 years), age groups 10–14 y and 65–79 y showed conflicting base-case findings that QIV was cost-effective from societal perspective and not cost-effective from provider's perspective. The differences between the willingness-to-pay threshold (USD 36,557) and some base-case ICERs (USD 30,753, USD 36,716, USD 39,146) in these 2 groups were small. The base-case choice of preferred option became more sensitive to small change of ICER to cross the threshold of willingness-to-pay when this margin was narrow. The robustness of base-case results in these 2 age groups was therefore subjected to the variation of a number (up to 10) of influential factors. In the age group 15–64 years, QIV was not cost-effectiveness from both societal and provider's perspective and the ICERs of QIV were the highest. The base-case findings in age group 15–64 y were highly robust and were only sensitive to one factor that QIV would become cost-effective at extreme low additional vaccine cost (<USD1.1 from provider's perspective).
The clinical and economic impact of QIV was estimated previously in the US population.Citation2-3 Our analysis further differentiated the cost-effectiveness of QIV and identified influential factors in various age groups in Hong Kong. The clinical outcomes of present study were consistent with the reported findings that QIV reduced clinical events of TIV-unmatched influenza B.Citation2 Economic outcomes of our analysis found cost-saving only occurred in very young and older populations from societal perspective, and no cost-saving in any age group from provider's perspective. The current results supported the implementation of a QIV program in Hong Kong for the very young and older populations as QIV appears to be cost-effective in the extreme age groups from both perspectives of society and provider.
The present model was limited by projecting the influenza infection rate using local influenza hospital admission rate and a case-fatality ratio adopted from the western populations. The annual population-wide influenza infection rate in Hong Kong is not readily available, and thus an epidemiology method using case-fatality ratio was adopted.Citation2 Both case-fatality ratio and hospital admission rates were therefore examined over a wide range in the sensitivity analysis and threshold values were identified. Also, the present model simplified real-life outcomes of an influenza vaccination program. For instance, the impact of herd immunity generated by higher vaccine coverage rate as well as the additional cost to achieve higher vaccine coverage rate were not included in the current analysis. In conclusion, QIV appears to be cost-effective in Hong Kong population, except for the age group 15–64 years, when total costs were considered. When direct cost alone was included, QIV seems to be cost-effective in very young (6 months-9 years) and older (≥80 years) age groups. A QIV program for very young and older individuals is likely to be cost-effective from both perspectives of society and healthcare provider.
Methods
Model design
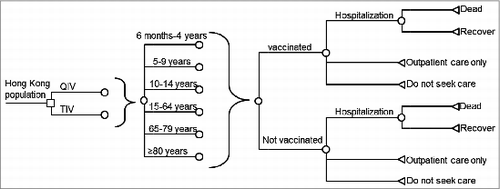
A decision tree was designed to simulate the outcomes of QIV versus TIV for prevention of seasonal influenza during one year in the Hong Kong population (). The Hong Kong population was stratified by age into 6 groups: 0–4 years, 5–9 years, 10–14 years, 15–64 years, 65–79 y and 80 y or older. Direct cost and total cost (including direct and indirect costs), infection rate, hospitalization rate, mortality rate and quality-adjusted life-years (QALYs) loss due to infection caused by TIV-unmatched influenza B lineage were simulated for each study arm.
Individuals in each age group might or might not receive influenza vaccination and they might be infected with the TIV-unmatched influenza B lineage. Furthermore, those who had the influenza infection might receive outpatient care only or inpatient care. All patients who were hospitalized might survive or die, with or without being admitted to the intensive care unit (ICU).
Clinical inputs
All model parameters were shown in. A literature search in MEDLINE over the period of 2000–2014 was performed using keywords including but not limited to “quadrivalent influenza vaccine,” “trivalent influenza vaccine,” “influenza vaccine effectiveness,” “vaccine coverage,” “influenza B,” “mortality," “case-fatality ratio,” “hospitalization," “duration of illness,” “sick days.” The selection criteria of clinical studies of influenza B infection were: (1) reports were written in English; (2) etiology of respiratory illnesses was identified, and (3) mortality rate and/or ICU admission rate were reported. All articles retrieved by this process were screened for relevance to our model. A manuscript was included if it had data pertaining to the model inputs.
The distribution of population in the 6 age groups was calculated using the mid-2013 population by age group statistics in Hong Kong.Citation5 The age-specific influenza vaccine coverage rates in Hong Kong were published by the Department of Health of Hong Kong.Citation6 The hospitalization rate for TIV-unmatched influenza B subtype was estimated using hospitalization rate of influenza B and percentage of TIV-unmatched lineages in influenza B isolates in Hong Kong patients from 2000 to 2010.Citation1 The ICU admission rate and mortality rate in hospitalized pediatric and adult patients were retrieved from outcome analyses of influenza in Hong Kong.Citation15,16 The overall infection rate of TIV-unmatched influenza B infection was further calculated using the case-fatality ratio of influenzaCitation17 and number of influenza-associated deaths. The rate of outpatient care was the difference between infection rate and hospitalization rate.
The TIV-unmatched influenza B event rate with QIV was calculated by rearranging the following vaccine efficacy formula.Citation18
Vaccine efficacy = (disease attack rate without vaccine – disease attack rate with vaccine) / (disease attack rate without vaccine).
Disease attack rate with vaccine = Disease attack rate without vaccine x (1 - vaccine efficacy).
The QIV effectiveness against TIV-unmatched influenza B lineage was assumed to be similar to the TIV effectiveness against the TIV-selected influenza B lineage. Model input of vaccine effectiveness in adults were pooled from clinical trials over seasons 2005–2011.Citation7-14 Vaccine effectiveness in children were retrieved from a surveillance study on influenza over seasons 2009–2013.Citation19
Utility inputs
The QALYs lost by each infected individual were calculated using the utility loss and the duration of time-spent in each of the 4 statuses: (1) Outpatient care, (2) hospitalization without ICU admission, (3) ICU care, and (4) death. The utility loss of each health status was estimated from findings of health-related quality of life studies.Citation20-26 The time-spent in outpatient care and hospitalization were the duration of illness and length of hospital stay, correspondingly.Citation15,16,27,28 The QALYs loss as a result of deaths occurred during the model year were calculated using life expectancy in Hong Kong and age-specific utility values, and the future loss of QALYs due to death were discounted by 3% per year.Citation5,29
Cost inputs
The economic analysis was conducted from the perspectives of healthcare provider (direct cost only) and society (direct medical cost and indirect cost) of Hong Kong. Direct medical cost included cost of vaccine and costs of influenza treatment. The current cost of TIV in Hong Kong was USD7.7 (USD1 = HKD7 .8). The additional cost of QIV vs. TIV in Hong Kong was assumed to be +USD3 (range +USD1–20). Influenza treatment included outpatient care and hospitalization (with and without ICU care). The number of clinic visit for outpatient care was assumed to be one time (range 1–2 visits). The cost per general outpatient clinic visit, and cost per hospital day (in general medical ward or ICU) were estimated from the 2014 charges of Hospital Authority. Hospital Authority is the largest, non-profit-making public health organization in Hong Kong providing primary to tertiary care. The charges listed represent only the cost components (including labor costs) with no addition of profits, the cost per general outpatient visit and daily cost of hospitalization were therefore approximated using the Hospital Authority charges.Citation30
The productivity loss of adult patients and caregivers of pediatric patients were included in indirect cost and these items were estimated by the median daily income (non-gender specific) of the population in Hong KongCitation5 and length of hospitalization for hospitalized cases. For those who only received outpatient care, the duration of productivity loss was estimated using the number of days attending outpatient clinic. The proportion of employed patients and caregivers was estimated by the percentage of Hong Kong population in the labor force and the employment rate among the labor force.Citation5 The productivity loss of patients who died was estimated by lifetime earnings between age 18 y to 65 y using the median income of Hong Kong.
Cost-effectiveness analysis and Sensitivity Analysis
If the QIV group had a lower cost and resulted in higher QALYs saved, it would dominate the TIV group. If it cost more to save QALYs, the incremental cost of QIV per QALY saved (ICER) was calculated using the following equation: (CostQIV−CostTIV)/(QALY lossTIV−QALY lossQIV). As recommended by the World Health Organization (WHO), ICER less than 1x GDP per capita was considered as highly cost-effective and less than 3x GDP per capita was considered as cost-effective. 1x GDP per capita was therefore used as the threshold of willingness-to-pay per QALY.Citation31 The GDP per capita of Hong Kong was USD36,557 in 2013.Citation5 A scenario with ICER less than USD36,557 was considered as cost-effective.
Sensitivity analysis was performed by TreeAge Pro 2009 (TreeAge Software, Inc.., Williamstown, MA, USA) and Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA) to examine the robustness of the model results. All the parameters were examined over the upper and lower limits of the variables, if available. Otherwise, a range of variation by ± 20% of the base-case value was used. One-way sensitivity analysis on all model inputs was performed to screen for potential influential factors. To evaluate the impact of uncertainty in all variables simultaneously, a probabilistic sensitivity analysis was performed using Monte Carlo simulation. The direct cost, total cost and QALYs of each study arm were recalculated 10,000 times by randomly drawing each of the model input from a triangular probability distribution. The probability of each vaccine to be the preferred option was determined over a range of 1–3x GDP per capita as threshold of willingness-to-pay.
Disclosure of Potential Conflicts of Interest
PKS Chan has participated in clinical trials supported by F. Hoffmann-La Roche and GlaxoSmithKline, received consultancy fees from F. Hoffmann-La Roche and GlaxoSmithKline, and served the Quadrivalent Influenza Vaccine Asia-Pacific Advisory Board of GlaxoSmithKline. Other authors have no conflicts of interest to disclose.
References
- Chan PK, Chan MC, Cheung JL, Lee N, Leung TF, Yeung ACM, Wong MCS, Ngai KLK, Nelson AS, Hui DSC. Influenza B lineage circulation and hospitalization rates in a subtropical city, hong kong, 2000-2010. Clin Infect Dis 2013; 56:677–84; PMID:23074315; http://dx.doi.org/10.1093/cid/cis885
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993-8; PMID:22226861; http://dx.doi.org/10.1016/j.vaccine.2011.12.098
- Lee BY, Bartsch SM, Willig AM. The economic value of a quadrivalent versus trivalent influenza vaccine. Vaccine 2013; 31:2477-9; PMID:23084849; http://dx.doi.org/10.1016/j.vaccine.2012.10.025
- You JHS, Wong WCW, Ip M, Lee NLS, Ho SC. Cost-effectiveness analysis of influenza and pneumococcal vaccination for Hong Kong elderly in long-term care facilities. J Epidemiol Commun Health 2009; 63:906-11; PMID:19608558; http://dx.doi.org/10.1136/jech.2008.081885
- Census and Statistics Department, The government of hong kong SAR. Assessed on 24 October 2013: http://www.censtatd.gov.hk/
- Chan D. Seasonal influenza vaccination coverage survey for the 2012/2013 season. Commun Dis Watch 2013; 10:74-7.
- Centers for Disease Control and Prevention. Interim within-season estimate of the effectiveness of trivalent inactivated influenza vaccine—marshfield, wisconsin, 2007–08 influenza season. Morb Mortal Wkly Rep 2008; 57:393-8; PMID:18418344
- Frey S, Vesikari T, Szymczakiewicz-Multanowska A, Lattanzi M, Izu A, Groth N, Holmes S. Clinical efficacy of cell culture-derived and egg-derived inactivated subunit influenza vaccines in healthy adults. Clin Infect Dis 2010; 51:997-1004; PMID:20868284; http://dx.doi.org/10.1086/656578
- Monto AS, Ohmit SE, Petrie JG, Johnson E, Truscon R, Teich E, Rotthoff J, Boulton M, Victor JC. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med 2009; 361:1260-7; PMID:19776407; http://dx.doi.org/10.1056/NEJMoa0808652
- Ohmit SE, Victor JC, Rotthoff JR, Teich ER, Truscon RK, Baum LL, Rangarajan B, Newton DW, Boulton ML, Monto AS. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med 2006; 355:2513-22; PMID:17167134; http://dx.doi.org/10.1056/NEJMoa061850
- Ohmit SE, Victor JC, Teich ER, Truscon RK, Rotthoff JR, Newton DW, Campbell SA, Boulton ML, Monto AS. Prevention of symptomatic seasonal influenza in 2005–2006 by inactivated and live attenuated vaccines. J Infect Dis 2008; 198:312-7; PMID:18522501; http://dx.doi.org/10.1086/589885
- Skowronski DM, Masaro C, Kwindt TL, Mak A, Petric LiY, Sebastian R, Chong M, Tam T, De Serres G. Estimating vaccine effectiveness against laboratory-confirmed influenza using a sentinel physician network: results from the 2005–2006 season of dual A and B vaccine mismatch in canada. Vaccine 2007; 25:2842-51; PMID:17081662; http://dx.doi.org/10.1016/j.vaccine.2006.10.002
- Talbot HK, Griffin MR, Chen Q, Zhu Y, Williams JV, Edwards KM. Effectiveness of seasonal vaccine in preventing confirmed influenza-associated hospitalizations in community dwelling older adults. J Infect Dis 2011; 203:500-8; PMID:21220776; http://dx.doi.org/10.1093/infdis/jiq076
- Treanor JJ, Talbot HK, Ohmit SE, Coleman LA, Thompson MG, Cheng PY, Petrie JG, Lofthus G, Meece JK, Williams JV, et al. Effectiveness of seasonal influenza vaccines in the united states during a season with circulation of all three vaccine strains. Clin Infect Dis 2012; 55:951-9; PMID:22843783; http://dx.doi.org/10.1093/cid/cis574
- Lee N, Chan PK, Lui GC, Wong BC, Sin WW, Choi KW, Wong RYK, Lee ELY, Yeung ACM, Ngai KLK, et al. Complications and outcomes of pandemic 2009 influenza A (H1N1) virus infection in hospitalized adults: how do they differ from those in seasonal influenza? J Infect Dis 2011; 203:1739-47; PMID:21606532; http://dx.doi.org/10.1093/infdis/jir187
- Kwong KL, Lung D, Wong SN, Que TL, Kwong NS. Influenza-related hospitalisations in children. J Paediatr Child Health 2009; 45:660-4; PMID:19845841; http://dx.doi.org/10.1111/j.1440-1754.2009.01591.x
- Chowell G, Miller MA, Viboud C. Seasonal influenza in the united states, france, and australia: transmission and prospects for control. Epidemiol Infect 2008; 136:852-64; PMID:17634159
- Weinberg GA, Szilagyi PG. Vaccine epidemiology: efficacy, effectiveness, and the translational research roadmap. J Infect Dis 2010; 201:167-10; PMID:20402594; http://dx.doi.org/10.1086/652404
- Cowling BJ, Chan KH, Feng S, Chan EL, Lo JY, Peiris JS, Chiu SS. The effectiveness of influenza vaccination in preventing hospitalizations in children in hong kong, 2009-2013. Vaccine 2014; 15;32:5278-84; PMID:25092636; http://dx.doi.org/10.1016/j.vaccine.2014.07.084
- Sander B, Hayden FG, Gyldmark M, Garrison LPJ. Post-exposure influenza prophylaxis with oseltamivir: cost effectiveness and cost utility in families in the UK. Pharmacoeconomics 2006; 24:373-86; PMID:16605283; http://dx.doi.org/10.2165/00019053-200624040-00007
- Talbird SE, Brogan AJ, Winiarski AP, Sander B. Cost-effectiveness of treating influenza-like illness with oseltamivir in the united states. Am J Health-Syst Pharm 2009; 66:469-480; PMID:19233995; http://dx.doi.org/10.2146/ajhp080296
- Rothberg MB, Rose DN. Vaccination versus treatment of influenza in working adults: a cost-effective analysis. Am J Med 2005; 118:68-77; PMID:15639212; http://dx.doi.org/10.1016/j.amjmed.2004.03.044
- Mauskopf JA, Cates SC, Griffin AD, Neighbors DM, Lamb SC, Rutherford C. Cost effectiveness of zanamivir for the treatment of influenza in a high risk population in australia. Pharmacoeconomics 2000; 17:11-620; PMID:10977398; http://dx.doi.org/10.2165/00019053-200017060-00007
- Luce BR, Nichol KL, Belshe RB, Frick KD, Li SX, Boscoe A, Rousculp MD, Mahadevia PJ. Cost-effectiveness of live attenuated influenza vaccine versus inactivated influenza vaccine among children ages 24–59 months in the united states. Vaccine 2008; 26:2841-8; PMID:18462851; http://dx.doi.org/10.1016/j.vaccine.2008.03.046
- Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care 2000; 38:583-637; PMID:10843310; http://dx.doi.org/10.1097/00005650-200006000-00004
- Sackett DL, Torrance GW. The utility of different health states as perceived by the general public. J Chronic Dis 1978; 31:697-704; PMID:730825; http://dx.doi.org/10.1016/0021-9681(78)90072-3
- Petrie JG, Ohmit SE, Hohnson E, Cross RT, Monto AS. Efficacy studies of influenza vaccines: effect of end points used and characteristics of vaccine failures. J Infect Dis 2011; 203:1309-15; PMID:21378375; http://dx.doi.org/10.1093/infdis/jir015
- Chiu SS, Chan KH, So LY, Chen R, Chan ELY, Peiris JSM. The population based socioeconomic burden of pediatric influenza-associated hospitalization in Hong Kong. Vaccine 2012; 30:1895-1900; PMID:22222872; http://dx.doi.org/10.1016/j.vaccine.2011.12.027
- Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Medical Care 1998; 36:778-92; PMID:9630120; http://dx.doi.org/10.1097/00005650-199806000-00002
- Fees and Charges, Hospital Authority, Hong Kong. Assessed on 7 October 2013: http://www.ha.org.hk
- Cost-effectiveness thresholds. World health organization. Assessed on 3 December 2013: http://www.who.int/choice/costs/CER_thresholds/en/index.html