Abstract
We assessed the safety, reactogenicity and immunogenicity of a staphylococcal vaccine combining capsular polysaccharides types 5 and 8 (CPS5/8), conjugated to tetanus toxoid (TT), with mutated detoxified α-toxin (AT) and clumping factor A (ClfA). In this phase I, randomized, placebo-controlled, observer-blind trial (NCT01160172), 88 healthy 18- to 40-year-olds received CPS5-TT/CPS8-TT/AT/ClfA vaccine (5/5/10/10 μg or 10/10/30/30 μg dose, each with or without AS03B adjuvant) or saline, at months 0, 1, 6. Solicited and unsolicited adverse events (AEs) were recorded for 7 and 30 d post-vaccination, respectively; potential immune-mediated diseases (pIMDs) and serious AEs (SAEs) were recorded throughout the study. Humoral and antigen-specific CD4+/CD8+ T-cell immunity were assessed from Day (D) 0 to D540 post-vaccination. The most frequently reported solicited local and general AEs were pain (78.6%–100% of subjects), fatigue (36.4%–93.3% of subjects post-dose 1–2) and headache (20%–44.4% of subjects post-dose 3). Overall, 4 SAEs and 2 potential immune-mediated diseases (pIMDs) (none fatal or vaccine-related) were reported. For each antigen, pre-vaccination seropositivity rates were high (85.7%–100%) and geometric mean concentrations (GMCs) in vaccine recipients sharply increased from D0 to D14, then plateaued to study end. Exploratory group comparisons suggested higher GMCs with higher dosage, without AS03B effect. Vaccine-induced antibodies were functional (CPS5 opsonophagocytic assays, and AT/ClfA inhibition assays). AT- and ClfA-specific CD4+ T-cells with Th0/Th1 cytokine profile were induced at low levels (median <0.05%) by each formulation (intracellular cytokine staining). In conclusion, no safety concerns were identified and each vaccine formulation induced robust humoral immune responses after the first vaccine dose.
Abbreviations:
- AEs
- adverse events; AT
- α-toxin; ATP
- According–to-Protocol; CIs
- Confidence Intervals; ClfA
- clumping factor A; CPS5 and 8
- capsular polysaccharides types 5 and 8; GMC
- geometric mean concentration; GMT
- geometric mean titer; ICS
- intracellular cytokine staining; IFN
- interferon; IL
- interleukin; LNR
- laboratory normal range; MRSA
- methicillin-resistant S. aureus; OPA
- opsonophagocytic assay; pIMDs
- potential immune-mediated diseases: PVL
- Panton-Valentine leucocidin; rEPA
- recombinant exoprotein A from Pseudomonas aeruginosa; SAEs
- serious adverse events; TT
- tetanus toxoid
Introduction
Staphylococcus aureus, both a commensal and a pathogen to its human host, can cause a broad spectrum of diseases, from skin and soft tissues infections, joint and bone infections, to bacteremia, endocarditis, pneumonia or toxic shock syndrome.Citation1 Following increased use of broad-spectrum antibiotics, methicillin-resistant S. aureus (MRSA) clones emerged, representing up to 50% of strains identified in hospital settings.Citation2,3 Up to 60% of the general population is either persistently or intermittently colonized with S. aureus. However, although subjects who permanently carry S. aureus are at increased risk of staphylococcal bacteremia, they have a lower infection-related mortality rateCitation4 suggesting that some protective immunity to S. aureus develops during carriage. Therefore, vaccination against S aureus may contribute to the prevention of such infections but the challenges for developing a staphylococcal vaccine are enormous.Citation5
S. aureus virulence factors include the expression of capsular polysaccharides (CPS) and several cell-surface and secreted proteins implicated in different aspects of pathogenesis.Citation6-8 The capsule contributes to virulence by promoting the ability of S. aureus to resist opsonophagocytic killing.Citation9 S. aureus has also developed other immune evasion mechanisms.Citation10-17 In addition, it can survive under the form of small colony variants in mammalian cells, triggering subacute latent infections,Citation18 and is able to form persistent biofilm infections that prevent macrophage phagocytosishence triggering persistent infection.Citation19
Previous staphylococcal immunotherapy or vaccine candidates failed to show efficacy in humans.Citation20-26 The lack of efficacy against infection occurred despite of high vaccine-induced antibody levels.Citation23,24,26 Due to the complexity of S. aureus virulence mechanisms and previous failures in developing effective S. aureus vaccines relying on one single type of antigen, the development of vaccines targeting multiple virulence factors (e.g. toxins and adhesion proteins in addition to CPS) by induction of humoral but also T-cell-mediated immunity has been considered.Citation4,27-29
GSK Vaccines has developed a 4-component staphylococcal vaccine combining CPS types 5 and 8 (CPS5 and CPS8), conjugated to tetanus toxoid (TT) (CPS5-TT; CPS8-TT), with mutant forms of hemolysin-1 (α-toxin; AT) and ClfA. CPS5 and CPS8 are the most common CPS types identified among clinical isolates (up to 75% of isolates).Citation30 Previous clinical trials with other polysaccharide conjugate vaccines have shown that TT conjugates induced high antibody levels and a robust immune memory response.Citation31 AT, a heptameric pore-forming exotoxin produced by the majority of S. aureus pathogenic strains, is involved in cell lysis, opsonophagocytosis impairmentCitation32,33 and survival of bacteria inside phagocytic cells.Citation34,35 S. aureus ClfA is a major surface adhesion factor that binds to fibrinogen and has anti-phagocytic activity.Citation11,22,36 Although well-conserved across strains, genetic variants with strain-specific epitopes have been recently described.Citation37 In humans, antibodies to AT were shown to be lower in patients with S. aureus sepsis,Citation38 and to protect against recurrent skin infections.Citation39 The protective effect of AT and ClfA was demonstrated in different animal models of S. aureus pneumonia, bacteremia and skin infection.Citation33,40-42 The combination of AT and CPS conjugates enhanced protection in a rat model of osteomyelitis,Citation43 while passive immunization with both CPS5 and ClfA antibodies inhibited the emergence of unencapsulated strains and significantly reduced the appearance of small colony variants.Citation44-46 Of note, IL-17-mediated cellular immunity has been found to play a key role in the protective effect induced by immunization with ClfA against S. aureus infection.Citation47
The majority of invasive MRSA infections are reported in persons aged 50 y and above, and patients with underlying diseases such as chronic renal insufficiency or diabetes are at increased risk.Citation44,48 An Adjuvant System was included in the investigational vaccine, to test if the magnitude and persistence of immune responses can be enhanced and to eventually overcome immunosenescence or impaired immunity in certain target populations. AS03 Adjuvant System, previously used in influenza vaccines, was selected as it had been shown to induce robust antibody and T-cell immune response.Citation49-53
Here we report the first-time-in-human Phase I trial evaluating the safety, reactogenicity and immunogenicity of the 4 formulations of the staphylococcal 4-component vaccine differing in terms of antigen dose and presence of AS03B adjuvant.
Results
Study population
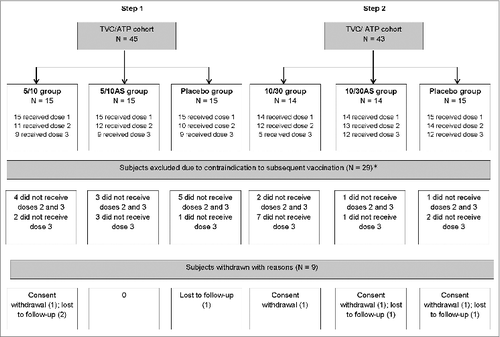
88 subjects were enrolled, received dose 1 and were included in the total vaccinated cohort and according-to-protocol cohort for immunogenicity. Due to strict criteria related to abnormal safety lab parameters for administration of the subsequent doses of the vaccines, 72 subjects received dose 2 and 56 dose 3, but all 88 subjects were kept in the study for safety follow-up (). Subjects vaccinated with the second or the third vaccine dose were evenly distributed across groups except for the 10/30 group for which only 5/15 subjects received the third vaccine dose. Nine subjects withdrew, none because of an adverse event (AE). The mean age of the study subjects ranged from 30.1 to 31.9 y and majority were of Caucasian heritage. The gender ratio was unevenly distributed (Table S1).
Figure 1. Participant flow diagram. Footnote to figure: 5/10 = 5 μg CPS5-TT; 5 μg CPS8-TT; 10 μgAT; 10 μg ClfA 5/10AS = 5 μg CPS5-TT; 5 μg CPS8-TT; 10 μg AT; 10 μg ClfA adjuvanted with AS03B 10/30 = 10 μg CPS5-TT; 10 μg CPS8-TT; 30 μg AT; 30 μg ClfA 10/30AS = 10 μg CPS5-TT; 10 μg CPS8-TT; 30 μg AT; 30 μg ClfA adjuvanted with AS03B TVC, total vaccinated cohort; ATP, according-to-protocol; N, number of subjects in a category. *Contraindications to subsequent vaccination: clinically relevant abnormal laboratory value (26), interval between dose 3 visit and visit before out of delay (>15 days) (2) and alcoholism (1). Laboratory values included: hematology (red blood cell count, white blood cell count, eosinophils count, neutrophils count, lymphocytes count, platelets count, reticulocytes index, hemoglobin), biochemistry (ALT, AST, creatinine, LDH, CPK, total bilirubin) and urinalysis (protein, glucose and blood).
Safety and reactogenicity
As shown in , the most frequently reported solicited local AEs in the vaccine groups was pain (the majority was grade 1 after doses 1 and 2). Grade 3 pain was reported after dose 1 in 1 subject (5/10AS group) and after dose 3 in 2 subjects (1 in each of the high dose groups). Grade 3 redness was reported following 19 doses: 3 of 35, 4 of 36, 4 of 31 and 8 of 39 doses in the 5/10, 5/10AS, 10/30 and 10/30AS groups, respectively; 5 cases occurred after dose 1, 7 after dose 2 and 7 after dose 3. Grade 3 swelling was reported following 9 doses: 2 of 35, 3 of 31 and 4 of 39 doses in the 5/10, 10/30 and 10/30AS groups, respectively; 3 cases occurred after each vaccine dose. All Grade 3 solicited local AEs were reported in the vaccine groups and lasted for 1 to 3 d
Figure 2. The incidence of solicited local (A) and general (B and C) adverse events reported during the 7-day post-vaccination period (total vaccinated cohort) Footnote to figure: 5/10 = 5 μg CPS5-TT; 5 μg CPS8-TT; 10 μgAT; 10 μg ClfA 5/10AS = 5 μg CPS5-TT; 5 μg CPS8-TT; 10 μg AT; 10 μg ClfA adjuvanted with AS03B 10/30 = 10 μg CPS5-TT; 10 μg CPS8-TT; 30 μgAT, 30 μg ClfA 10/30AS = 10 μg CPS5-TT; 10 μg CPS8-TT; 30 μg AT; 30 μg ClfA adjuvanted with AS03B.
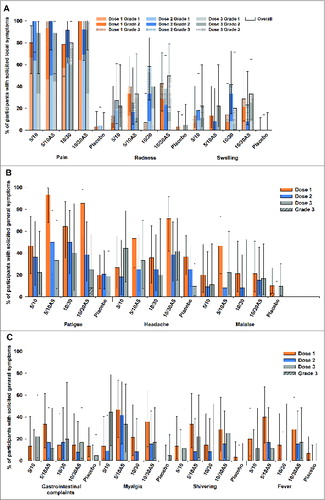
The most frequently reported solicited general AEs in the study groups were fatigue (up to 93.3% of subjects following dose 1; up to 50% of subjects following dose 2 and 3;) and headache (up to 71.4% of subjects following dose 1; up to 44.4% of subjects following dose 2 and 3). Reported fever was usually mild (<38.5°C), except for one event in group 5/10AS and one in group 10/30AS where grade 2 fever (>38.5°C–39.5°C) was recorded (). Grade 3 general symptoms were reported 2 times: malaise in the placebo group after dose 1 and fatigue in the 10/30AS group after dose 3.
The percentage of subjects reporting at least 1 unsolicited AE was comparable in the vaccine groups vs placebo (46/58 [79.3%] subjects vs 25/30 [83.3%] subjects after dose 1–2; 20/35 [57.1%] subjects vs 9/21 [42.9%] subjects after dose 3) (Table S2). None of the grade 3 unsolicited AEs was considered by the investigators as vaccine-related.
Four serious adverse events (SAEs) (appendicitis in group 5/10, cerebral concussion in group 5/10AS, hospitalization for surgical removal of recto-vaginal nodule in group 5/10AS and hospitalization for surgical removal of cholelithiasis in placebo group) were reported, all during the follow-up period after dose 3; none were fatal or considered by investigators as vaccine-related and all resolved by study end. One case of pIMD was diagnosed in the 10/30 vaccine group (a “psoriasis-like” cutaneous eruption diagnosed at 350 d after dose 1 and considered by the investigator as not related to vaccination) and 1 in the placebo group (ulcerative colitis diagnosed in a female at 182 d after dose 3).
Few Grade 3 laboratory abnormalities were reported: elevated creatine kinase following intense physical activity (6 subjects), decrease from baseline by more than 2 g/dL for hemoglobin (but the hemoglobin level was within normal range, 1 subject), the presence of protein (4 subjects) or glucose (1 subject) in the urine. Elevated creatine kinase of Grade 4 intensity due to intense physical activity was reported for 1 subject in the 5/10AS group 330 d after dose 2.
Immunogenicity
Antibody response
Pre-vaccination seropositivity rates for anti-CPS5, -CPS8, -AT and -ClfA tested by multiplex or ELISA (IgG) and by functional assays were high (78.6%–100%), except for the ClfA functional assay (0.0%–10.0%).
As shown in , geometric mean concentrations (GMCs) for anti-CPS5, -CPS8, -AT and -ClfA tested by multiplex or ELISA (IgG) sharply increased from pre-vaccination to Day-14 (4–47-fold in the low dose groups; 6–75-fold in the high dose groups). The second and third vaccine doses did not further enhance antibody concentrations. Exploratory group comparisons of GMCs at Day-14 post-dose 1 and post-dose 2, respectively, suggest a dose-response for CPS5, CPS8 and AT (P < 0.05) but not for ClfA (p = 0.2 and 0.8 for dose 1 and 2, respectively), with the high dose formulations eliciting higher GMCs than the low dose formulations. Exploratory group comparisons of GMCs did not show any differences between the adjuvanted and non-adjuvanted formulations (P >0 .05).
Figure 3. Geometric mean concentrations of anti-capsular polysaccharide types 5 (panel A) and 8 (panel B), anti-AT (panel C) and anti-ClfA antibodies (panel D) (according-to-protocol cohort for immunogenicity) Footnote to figure: 5/10 = 5 μg CPS5-TT; 5 μg CPS8-TT; 10 μg AT; 10 μg ClfA 5/10AS = 5 μg CPS5-TT; 5 μg CPS8-TT; 10 μg AT; 10 μg ClfA adjuvanted with AS03B 10/30 = 10 μg CPS5-TT; 10 μg CPS8-TT; 30 μg AT; 30 μg ClfA 10/30AS = 10 μg CPS5-TT; 10 μg CPS8-TT; 30 μg AT; 30 μg ClfA adjuvanted with AS03B 95% CI; 95% confidence interval; GMC, geometric mean concentration; AT; α-toxin; CPS5 and 8, capsular polysaccharides types 5 and 8; TT, tetanus toxoid; ClfA, clumping factor A; L.U, Luminex units; EL.U, ELISA units. PRE, pre-dose 1; PI(D7), 7 d post-dose 1; PtdIns(D14), 14 d post-dose 1; PI(D30), 30 d post-dose 1; PII(D37), 7 d post-dose 2; PII(D44), 14 d post-dose 2; PII(D60), 30 d post-dose 2; PII(D179), pre-dose 3; PIII(D187), 7 d post-dose 3; PIII(D194), 14 d post-dose 3; PIII(D210), 30 d post-dose 3; PIII(D360), 1 y post-dose 1 or 6 months post-dose 3; PIII(D540), 1.5 y post-dose 1 or 1 y post-dose 3 The cut-off values for these assays (dashed line) were 23.6 L.U/mL for CPS5, 26.5 L.U/mL for CPS8, 22.5 L.U/mL for AT and 6 EL.U/mL for ClfA. Blue arrows indicate days of vaccine or placebo administration. *Only 4 subjects were included in the analysis of ClfA antibody levels in the 10/30 group from PIII(D187) onwards. This explains the large 95% CI for these time points.
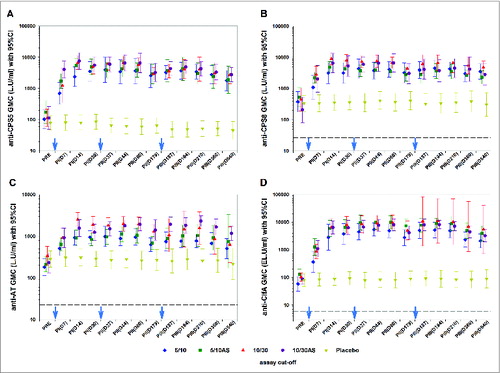
Results from CPS5 opsonophagocytic assay (OPA) and AT and ClfA inhibition assays were generally consistent with IgG data. An increase of geometric mean titers (GMTs) was generally observed as of Day-7 post-dose 1 for each of the 4 vaccine groups and GMTs remained above those in the placebo group up to the study end (Fig. S1). Exploratory group comparisons however, suggested a dose effect for both AT (post-dose 2; p = 0.035) and ClfA (post-dose 1; p = 0.007) with higher GMTs achieved in the higher dose formulations. Adjuvant effect was seen for ClfA (p = 0.043), with higher GMT post-dose 1 in the pooled adjuvanted groups compared to the non-adjuvanted groups.
Antigen-specific CD4+ and CD8+ T-cells
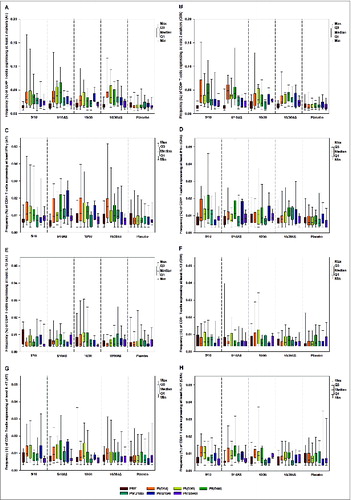
Low levels (median < 0.05%) of AT- and ClfA-specific CD4+ T-cells expressing at least 2 markers among IL-2, IFN-γ, IL-13, IL-17, TNF-α and CD40L were observed in all vaccine groups (). Functional characterization of the CD4+ T-cell response showed cells expressing at least IFN-γ (although at low levels) (). No CD4+ T-cells expressing IL-13 or IL-17 () and no CD8+ T-cells were detected after vaccination with any of the formulations tested, irrespective of antigen dose and presence/absence of the AS03B adjuvant.
Figure 4. Frequency (%) of S. aureus AT-specific (panels A, C, E and G) and ClfA-specific (panels B, D, F and H) CD4+ T-cells expressing at least 2 markers among IL-2, IFN-γ, IL-13, IL-17, TNF-α and CD40L (panels A and B), at least IFN-γ (CD4-Th1 profile) (panels C and D), IL-13 (panels E and F) and IL-17 (panels G and H) prior and after each vaccination (according-to-protocol cohort for immunogenicity) Footnote to figure: 5/10 = 5 μg CPS5-TT, 5 μg CPS8-TT, 10 μg AT, 10 μg ClfA 5/10AS = 5 μg CPS5-TT, 5 μg CPS8-TT, 10 μg AT, 10 μg ClfA adjuvanted with AS03B 10/30 = 10 μg CPS5-TT, 10 μg CPS8-TT, 30 μg AT, 30 μg ClfA 10/30AS = 10 μg CPS5-TT, 10 μg CPS8-TT, 30 μg AT, 30 μg ClfA adjuvanted with AS03B AT, α-toxin; ClfA, clumping factor A; PRE, pre-dose 1; PtdIns(D14), 14 d post-dose 1; PI(D30), 30 d post-dose 1; PII(D44), 14 d post-dose 2; PII(D179), pre-dose 3; PIII(D194), 14 d post-dose 3; PIII(D540), 1.5 y post-dose 1 or 1 y post-dose 3; Min/Max, Minimum/Maximum; Q1,Q3, First and third quartile.
A robust TT-specific CD4+ T-cell immune response was observed for all vaccine groups with a trend for higher CD4+ T-cell frequencies in the AS03B-adjuvanted vaccine groups (median after dose 1: 0.1%–0.6% for CD4+ T-cells expressing at least 2 markers and 0.05%−0.3% for CD4+ Th1 (Fig. S2). TT-specific CD8+ T-cells were detected, although at low levels (median <0.01%).
S. aureus carriage
No trend was observed in carriage status (Table S3). However, no firm conclusions can be made due to the low number of subjects included in the analysis.
Discussion
In this first-time-in-human study, no safety concerns were identified with the staphylococcal vaccine formulations tested. The most frequently reported Grade 3 solicited AEs were redness and swelling at injection site, with the highest frequencies observed in groups receiving the higher antigen dose formulation. The unsolicited Grade 3 AEs and the 4 SAEs reported during the trial were assessed by the investigator to be unrelated to the study vaccine administration and all resolved by the study end. Except for local solicited symptoms and fever, we did not discriminate between grade 1 and 2 AEs, which is a limitation in this study, as grade 1 symptoms are substantially different from grade 2.
This safety profile is similar to that reported in previous clinical trials with related antigens. StaphVAX™, containing S. aureus CPS5 and CPS8, each conjugated to rEPA, was shown to have a clinically acceptable safety profile when administered at up to 100 μg antigen per dose to more than 3,000 subjects.Citation23,24,54 Other vaccines conjugated to tetanus toxoid such as Haemophilus influenzae type B (Hib), meningococcal and pneumococcal polysaccharide conjugate vaccines, have been extensively administered in all age groups worldwide, including infants, and have been shown to have a clinically acceptable safety profile, with a low incidence of grade 3 solicited symptoms and SAEs.Citation55-57
A previous study conducted with an AS03A-adjuvanted pneumococcal and non-typeable H. influenzae (NTHi) tri-protein vaccine was stopped due to high reactogenicity and systemic adverse events.Citation53 The increased reactogenicity of AS03-adjuvanted vaccines compared to non-adjuvanted vaccinesCitation53,58,59 may be the consequence of the enhanced migration of monocytes and macrophages and the production of cytokines and chemokines at the injection site and in the draining lymph nodes, secondary to AS03 adjuvant administration.Citation60 Our study was a Phase I study enrolling a limited number of subjects, and thus no formal comparisons between vaccine groups were done to explore differences in reactogenicity profile between groups. However, we did not observe major differences in the frequency of grade 3 solicited AEs between adjuvanted and non-adjuvanted vaccine groups; nor did we observe increased reactogenicity with subsequent doses 2 or 3. In our study, we elected to use 2 relatively low antigen dosages for dose escalation, and a formulation of AS03 that contains half the dose of α-tocopherol (AS03B). No safety concerns were raised during safety evaluations prior to each subsequent dose escalation.
We observed robust antibody responses to each of the 4 staphylococcal antigens as of post-dose 1, with relatively stable antibody concentrations until study end. This observation and the high pre-vaccination seropositivity rates suggest a booster-like response, potentially explaining the lack of effect of doses 2 and 3. This was also observed in clinical trials with StaphVAX™, in subjects immunologically primed to S. aureus due to previous exposure.Citation54,61,62 In our study, the identified antibodies were functional, and persisted well above the preexisting vaccination levels up to one year after the last dose. Due to technical issues in the development of the OPA assay, we were not able to demonstrate the functionality of the CPS8 antibodies induced by our vaccine. Previously it was shown that staphylococcal polysaccharide conjugate vaccines induce opsonophagocytic antibodies against both CPS5 and CPS8.Citation54 The rapid increases in antibody concentrations following the first dose, with the fairly consistent trend for all antigens tested to plateau with little apparent difference by adjuvant effect, serve to lessen the impact of declining subject numbers receiving subsequent doses on the interpretation of overall immunogenicity. However, the decreased subjects number receiving dose 3 coupled with the increased variance (for anti-AT and anti-ClfA) in the high dose unadjuvanted group, limits our ability to fully assess the potential impact of the 6-month booster dose. Our data suggest that a single dose of our vaccine may suffice to induce long-lasting antibodies against each of the 4 antigens included in the vaccine.
The antibody response in our study was higher with the higher dose formulations. In other staphylococcal vaccine trials conducted in healthy subjects or end-stage renal disease patients and using vaccines with related antigens, antibody titers also increased with the antigen dose.Citation54,63,64 We evaluated 5 μg and 10 μg of CPS5 and CPS8, whereas StaphVAX™ contains 100 μg of each CPS type. In a tri-valent vaccine combining staphylococcal CPS types 5 and 8 conjugated to CRM197 and a mutant form of ClfA, 10, 30 or 100 μg of each CPS were previously evaluated and the dose of 30 μg was selected for further development.Citation64,65 The fold increases in the anti-CPS IgG response 1 month after the first dose in our study were at least as high as those observed previously with StaphVAX® (30–37-fold and fold7- for StaphVAX® vs 48-fold and 25-fold in the current study for types 5 and 8, respectively).Citation54 This is not unexpected as it was previously observed with other polysaccharide conjugate vaccines that a higher antigen content does not necessarily translate into a better antibody response.Citation66 The different carrier proteins can also play a role in the immunogenicity of conjugate vaccines.Citation31,67 We observed an antigen dose-effect for the AT (post-dose 2) and ClfA assays (post-dose 1), with higher functional antibody levels in the higher 30 μg dose formulation groups. In one trial testing a recombinant AT and a sub-unit of Panton-Valentine leukocidin (PVL) toxoids, Landrum et al. reported statistically significantly higher AT-specific antibody GMC levels in the group receiving 50 μg of AT compared with the other groups who received lower antigen doses.Citation63 In a tri-valent vaccine, 10, 60 and 200 μg of ClfA were evaluated and the 60 μg dose was selected for further clinical trial evaluation.Citation64,65 In the current study, we evaluated only 2 escalating doses for the antigen part of our vaccine and we can therefore not conclude on the optimal dosage for the AT and ClfA antigens in our vaccine; it should be noted however that the addition of a 2nd and 3rd dose did not further increase the antibody response.
No increase in the antibody response against CPS5, CPS8 and AT was observed when formulations were adjuvanted with AS03B. This may be due to the high pre-vaccination seropositivity rates to CPS5, CPS8 and AT. Also, high levels of anti-ClfA IgG natural antibodies were detected by ELISA in nearly all subjects. In contrast, functional antibodies blocking ClfA binding to fibrinogen were detected in very few (less than 10%) subjects pre-vaccination, while post-vaccination, higher titers were observed in the adjuvanted groups compared to the unadjuvanted ones. High anti-ClfA antibody levels have also been observed during natural infections with S. aureus, but their presence did not correlate with protection against infectionCitation21 and it has been shown that contrarily to vaccine-induced antibodies, they do not block ClfA binding to fibrinogen.Citation68
Passive and active immunization strategies relying on antibodies targeting one single virulence factor to prevent S. aureus infection have failed to date, suggesting the need to consider multi-component vaccines and a possible role for T-cell-mediated immune response.Citation22 Th17 lymphocytes play an important role in the immunity against S. aureus skin or lung infections in mice via IL-17 production, which plays a key role in neutrophil recruitment and in the activation of keratinocytes and mucosal cells.Citation69,70 High incidences of S. aureus skin and soft tissue infections were observed in patients with impaired Th17-cell function, such as patients with Job (hyper-IgE) syndrome,Citation71-73HIV patients with low peripheral blood CD4+ T cell counts,Citation74 patients treated with prednisone,Citation20,73,74 or in subjects with atopic dermatitis.Citation75,76 In vivo mouse models of S. aureus cutaneous infection have demonstrated the role of γδ T-cells in IL-17-dependent production of pro-inflammatory cytokines and chemokines by keratinocytes and subsequent neutrophils recruitment and bacterial clearance.Citation77,78 Altogether, new approaches focusing on Th1 and Th17 T-cells may be important to consider when developing staphylococcal vaccines.Citation20
Several studies in humans have shown that AT stimulation may induce a combined Th1-Th17 immune response in peripheral blood mononuclear cells and isolated CD4+ T-cells.Citation79,80 Also ClfA was shown to induce IL-17-producing cells in mice.Citation47 Surprisingly, in our study, all 4 vaccine formulations, whether with or without AS03B adjuvant, induced only low levels of AT- and ClfA-specific CD4+ T-cells expressing at least 2 cytokine markers with a trend for IFN-γ expression; no CD4+ T-cells expressing IL-13 or IL-17 and no induction of CD8+ T-cells were detected. This overall weak CD4+ T-cell response observed in our study was unexpected given that T-cell epitope predictions realized on various populations (Japan, USA, Europe) with the focus on more prevalent alleles revealed that AT and to a lesser extent ClfA contained CD4+ T-cell epitopes. The T-cell assay showed robust TT-specific CD4+ T-cell responses induced by all vaccine formulations, with a clear adjuvant effect for the higher dose formulation. This suggests that the CD4+ T-cell response to ClfA and AT was impaired, but not the response to TT (used as a positive control). The absence of the Th17 response could potentially be explained by the overall weak T-cell response as well as the short-term (2 hours stimulation) T-cell read-out used for this study. Indeed, longer term T-cell cultures seem to be required to reveal antigen specific Th17 populations.Citation84,85 The lack of adjuvant effect is also unexpected, as previous clinical trials have shown that AS03A-adjuvanted vaccines induced stronger antigen-specific CD4+ T-cell responses with a Th1 (IFN-γ) and Th17 cytokine profile than those induced by non-adjuvanted vaccine.Citation52,53,81,82 Those trials used AS03A while our study used AS03B. However, we do not believe this could explain the lower CD4+ T-cell response in our trial as AS03B was also shown to be a strong inducer of CD4+T-cell response with other antigensCitation83 and it had marked effect upon stimulation in the TT ICS assay in our current study.
In the development of a vaccine capable of inducing an appropriate Th17 response, it is important to choose an antigen and adjuvant system that would activate the inflammasome.Citation84 IL-1β plays a key role in promoting IL-17 production,Citation85,86 as was demonstrated with pneumolysin.Citation87 Pneumolysin is one of the antigens included in the pneumococcal-NTHi vaccine adjuvanted with AS03A described above and for which a Th17 response was observed, suggesting the importance of the combination of both the antigen and adjuvant in eliciting an approriate T cell response.Citation53 IL-6, another cytokine that plays a key role in the differentiation of the Th17 cells,Citation88,89 is induced by AS01B-adjuvanted vaccines but to a much lower extent by AS03A-adjuvanted vaccines.Citation60 Therefore, other adjuvants such as AS01B which has been shown to induce a potent T-cell response with different types of antigens,Citation90-92 could enhance the Th1/Th17 immune response. Additionally, mucosal (intranasal) administration of the vaccine may be an alternative route to induce a Th17 response.Citation84 Last but not least, S. aureus is a commensal microorganism that survives despite the host immune system.Citation1 Commensalism has been shown to occur through TLR-2-dependent induction of immune regulatory mechanisms.Citation93 S. aureus is also able to promote T-cells and innate cells to produce IL-10.Citation89,94 Immuno-downregulation may have therefore contributed to the failure of the tested vaccine formulations to induce robust antigen-specific T-cell responses.Citation16
In conclusion, no safety concerns were identified with any of the staphylococcal vaccine formulations tested. Each of the vaccine formulations induced a robust antibody response post-dose 1 which then plateaued up to the study end. Vaccine-induced antibodies against CPS5, AT and ClfA were functional. Exploratory group comparisons suggested higher antibody levels in the groups receiving a formulation with higher antigen content and no adjuvant effect. Only low levels of AT and ClfA-specific CD4+ T-cells were detected upon administration of the 4 vaccine formulations.
Materials and Methods
Study design and subjects
This Phase I, randomized, observer-blind, placebo-controlled, dose-escalation study (NCT01160172) across 6 vaccine groups was conducted at the ImmuneHealth center in La Louvière, Belgium, between 2010 and 2012. Healthy women and men 18–40 y of age were enrolled in 2 steps. In Step 1, 45 subjects were randomized at first dose using an internet-based randomization system and a randomization ratio of 1:1:1 to receive the 5/10 vaccine formulations (CPS5-TT/CPS8-TT/AT/ClfA; 5/5/10/10 μg) either with or without AS03B (5/10 and 5/10AS groups), or placebo (saline). In Step 2, 43 additional subjects were randomized (randomization ratio: 1:1:1) to receive the 10/30 formulations (CPS5-TT/CPS8-TT/AT/ClfA; 10/10/30/30 μg) with or without AS03B (10/30 and 10/30AS groups), or placebo. Subjects received 3 doses of the study vaccines or placebo according to a 0–1–6 month schedule and were followed for 1 y after the last vaccine dose administration. Dose escalation from the 5/10 to 10/30 formulations was conditioned by a favorable safety evaluation of the 5/10 formulations. The second and third vaccine doses were also only administered on the condition that a favorable outcome was obtained from the available safety data.
This study was set up in an observer-blind manner, where the vaccine recipient and staff responsible for the evaluation of any study endpoint were unaware of which vaccine was administered. As the vaccines in this study were of different appearance, vaccine preparation and administration were done by unblinded authorised medical personnel who did not participate in any of the study clinical evaluation assays.
Female subjects were not pregnant or lactating. Subjects meeting the following criteria were excluded: use of any investigational or non-registered product within 30 d preceding the first dose of study vaccine; any clinically significant acute or chronic infection proven or suspected to be caused by S. aureus and requiring antibiotic treatment within the 6 months preceding the first vaccination; and previous administration of any investigational S. aureus vaccine or antibodies. Subjects with clinically relevant out-of-range values for hematology (hemoglobin, red blood cells, white blood cells and differential count, platelets and reticulocytes index), biochemistry (ALT, AST, creatinine, LDH, CPK, total bilirubin) or urinary (blood, proteins, glucose) tests at screening were also excluded. Contraindications to subsequent administration of the study vaccines included: hypersensitivity reaction, pregnancy, pIMDs, bleeding or coagulation disorders, or any clinically relevant abnormal hematological, biochemical or urinary laboratory value following vaccine administration. All out-of-range values were considered clinically relevant contra-indications, with the following exceptions: menstrual blood in urine; higher CPK levels without any other abnormal values and a plausible explanation (such as sport activity); WBC <10,800/mm3 (upper limit of laboratory normal range (LNR) was 10,000/mm3), WBC >10,800 cell/mm3 if WBC remain stable over time (i.e. maximum 10% change with respect to baseline); neutrophils >8,000 cell/mm3 if neutrophils remain stable over time (i.e., maximum 10% change with respect to baseline); hemoglobin or RBC above the LNR; RBC below the LNR and hemoglobin normal; reticulocyte index >5%, if the hemoglobin level is within the normal range; LDH or total bilirubin <LNR, and protein concentration in urine <30 mg/dL.
The study was conducted according to the Declaration of Helsinki and Good Clinical Practice. The protocol and associated documents were reviewed and approved by an institutional review board. All subjects signed a written informed consent prior to any study procedures. The protocol summary was posted on ClinicalTrials.gov (NCT01160172) before study start. The full protocol is available at http://www.gsk-clinicalstudyregister.com/ (GSK study ID 113949).
Study vaccines
The investigational S. aureus vaccine contained 5 or 10 μg of each CPS5-TT and CPS8-TT; 10 or 30 μg of AT and 10 or 30 μg of ClfA. Mutant AT and ClfA forms were produced using recombinant Escherichia coli strains. The detoxified AT H35R mutant, included in the vaccine, lacks pore-forming activity, while the ClfA mutant (ClfA_P336Y338) lacks fibrinogen-binding activity. For simplicity, they are referred to as AT and ClfA throughout this paper. AS03B is an Adjuvant System containing α-tocopherol and squalene in an o/w emulsion (5.93 mg tocopherol). Adjuvanted doses were prepared by mixing the S. aureus antigens and AS03B (1:1) from separate mono-dose vials. Saline placebo was used as a negative control. A volume of 0.5 mL of the assigned study vaccine or placebo was administered intramuscularly into the deltoid within 4 hours after preparation.
Assessment of safety and reactogenicity
Solicited local and general AEs were recorded for 7 d after each vaccine dose and unsolicited AEs for 30 d after each dose. The intensity of all solicited AEs was graded on a scale of 0–3. pain: (1) any pain neither interfering with nor preventing normal every day activities, (2) painful when limb was moved and interfered with every day activities, (3) pain at rest, preventing normal every day activities; redness or swelling by diameter (1) >20 mm to ≤50 mm; (2) >50 to ≤100 mm; (3) >100mm; fever by temperature (1) ≥37.5°C to ≤38.5°C, (2) >38.5°C to ≤39.5°C, (3) >39.5°C. Other AEs that prevented normal activities were considered as Grade 3 (grade 1–2 AEs were recorded, but not analyzed). Data regarding pIMDs and SAEs were collected throughout the study period.
Safety laboratory parameters (blood: ALT, AST, CPK, creatinine, LDH, total bilirubin, hemoglobin, RBC, WBC, neutrophils, eosinophils, lymphocytes, neutrophils, basophils, monocytes, platelets, reticulocyte index) were assessed. The intensity of abnormal laboratory parameters was graded according to the Food and Drug Administration Toxicity Grading Scale for laboratory abnormalities, when available.Citation95
Based on clinical judgment, the investigators assessed the relationship between the study vaccine and the occurrence of each AE/SAE.
Assessment of immunogenicity
Serum samples for evaluation of anti-CPS5, anti-CPS8, anti-AT (luminex-based multiplex assay using purified native CPS5, CPS8, and recombinant AT as coating antigens) and anti-ClfA concentrations (ELISA using purified native ClfA as coating antigen) were collected prior to and at 7, 14 and 30 d after each vaccine dose, and 6 and 12 months after the last dose (i.e. Days 0, 7, 14, 30, 37, 44, 60, 179, 187, 194, 210, 360 and 540).
Anti-CPS5 opsonophagocytic activity titers were measured by an OPA. Lack of sensitivity, presumably linked to the complement used in the assay, prohibited the development of OPA for CPS8 before study conclusion and thus this assay was not performed. Functionality of anti-AT and anti-ClfA was measured by specific in vitro inhibition assays (hemolysis and fibrinogen-binding inhibition assay, respectively). In order to improve its sensitivity, the initial ClfA inhibition assay was adapted after the testing of post-primary samples. Samples were assessed using the initial test up to Day 210 for Step 1 (groups 5/10, 5/10AS and placebo) and up to Day 60 for Step 2 (groups 10/30, 10/30AS and placebo), after which a new test was used up to study end for both Steps.
Blood samples were collected for PBMC separation prior to and 14 d after each vaccine dose and 12 months after the last dose (i.e., Days 0, 14, 30, 44, 179, 194 and 540).The frequency of antigen-specific CD4+ and CD8+ T-cells elicited by AT, ClfA and TT, as characterized by cytokines secretion after short-term in vitro stimulation (IL-2, IFN-γ, TNF-α, IL-13, IL-17 and CD40L), was measured by flow cytometry using intracellular cytokine staining (ICS) as previously described.Citation81
Assessment of S. aureus carriage
Swabs for the evaluation of nose, throat, armpit and groin colonization with S. aureus were collected at screening and prior to and 30 d after each vaccine dose and 12 months after the last dose (i.e. Days 0, 30, 60, 180, 210 and 540). Cultures for S. aureus were performed at the clinical laboratory of Center Hospitalier Universitaire Tivoli, La Louvière, Belgium, using standard methods.
Statistical analysis
The safety analysis was performed on the Total Vaccinated Cohort (TVC) that included all vaccinated subjects. The incidence of AEs per study group was calculated with exact 95% Confidence Intervals (CIs).
The immunogenicity analysis was performed on the According–to-Protocol (ATP) cohort for immunogenicity. This cohort included all subjects who met the eligibility criteria, complied with the protocol procedures and for whom immunogenicity data was available for at least one post-vaccination time point and for at least one antibody. Seropositivity rates, GMCs (for anti-CPS5, -CPS8, -ClfA and -AT tested by multiplex or ELISA assays) and GMTs (for functional characterization of antibodies) with their 95%CIs were calculated for each study group. Antibody response to each vaccine antigen 2 weeks after each vaccine dose administration was compared between the study groups using a 2-way ANCOVA model on the log-transformed concentration/titer with the antigen content and adjuvant as fixed effect and the pre-vaccination log-transformed concentration as regressor. Interaction (antigen content, adjuvant) was considered as statistically significant if the P-value was <0.10. Main factors (antigen content, adjuvant) were considered as statistically significant if the P-value was <0.05. These comparisons were exploratory. No adjustment for multiplicity has been performed. The frequency (%) of antigen-specific CD4+/CD8+ T-cells was determined by study group using descriptive statistics. Change of carriage status from baseline to each post-vaccination time point was summarized by study group.
Disclosure of Potential Conflicts of Interest
P.M., P.L, SD, P.V.B. and D.B. are employees of the GSK group of companies; P.M., P.L. and D.B. own GSK restricted shares. J.L., L.L. and E.H. received funding via their institute from GlaxoSmithKline Biologicals SA to perform the clinical trial reported here. The authors do not declare any other conflict of interest.
Acknowledgments
We are indebted to all trial participants, and acknowledge the contributions of the clinicians, nurses and laboratory technicians. We would like to address special thanks to Laurence Nuret (Immunehealth) who played a major role in study conduct as study nurse, Dr. Catherine Potvliege (CHU Tivoli) for microbiology and safety testings. From GSK Vaccines, we would like to thank Ann Delforge, Julie Saliez, and Galina Gueneva for study coordination, Sophie Germain for lab support, Pierre Carteron for statistical support, Sofia Buonocore and Alessandra Ferraro for critical reading of the manuscript and helpful suggestions. We would also like to thank Adriana Rusu and Vasile Coman (XPE Pharma & Science on behalf of GSK Vaccines) for writing support and Wouter Houthoofd (XPE Pharma & Science on behalf of GSK Vaccines) for manuscript coordination.
Authors' Contributions
JL, LL and EH were involved as investigator in the study design, collection and interpretation of data. PM and PL were involved in the design, analyses and interpretation of the immunogenicity results. SD was biostatistician who was involved in the statistical design, analysis and interpretation of the data. PvB was involved in the design and interpretation of the study. DB was involved in all aspects of the study responsible for study design, conduct, analysis and interpretation of the study.
Trademark Statement
StaphVAX™ is a trademark of the Nabi Biopharmaceuticals.
supp.pdf
Download PDF (238.9 KB)Funding
P.M., P.L, SD, P.V.B. and D.B. are employees of the GSK group of companies; P.M., P.L. and D.B. own GSK restricted shares. J.L., L.L. and E.H. received funding via their institute from GlaxoSmithKline Biologicals SA to perform the clinical trial reported here. The authors do not declare any other conflict of interest.
References
- Lowy FD. Staphylococcus aureus infections. N Engl J Med 1998; 339:520-32; PMID:9709046; http://dx.doi.org/10.1056/NEJM199808203390806
- Grundmann H, Aires-de-Sousa M, Boyce J, Tiemersma E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet 2006; 368:874-85; PMID:16950365; http://dx.doi.org/10.1016/S0140-6736(06)68853-3
- WHO. Antimicrobial resistance. Fact sheet N°194. Updated May 2013. Available at: http://www.who.int/mediacentre/factsheets/fs194/en/
- Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JA, van Keulen PH, Vandenbroucke-Grauls CM, Meester MH, Verbrugh HA. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 2004; 364:703-5; PMID:15325835; http://dx.doi.org/10.1016/S0140-6736(04)16897-9
- Bagnoli F, Bertholet S, Grandi G. Inferring reasons for the failure of Staphylococcus aureus vaccines in clinical trials. Front Cell Infect Microbiol 2012; 2:16; PMID:22919608; http://dx.doi.org/10.3389/fcimb.2012.00016
- Thakker M, Park JS, Carey V, Lee JC. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect Immun 1998; 66:5183-9; PMID:9784520
- Foster TJ, Geoghegan JA, Ganesh VK, Hook M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 2014; 12:49-62; PMID:24336184; http://dx.doi.org/10.1038/nrmicro3161
- Novick RP. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 2003; 48:1429-49; PMID:12791129; http://dx.doi.org/10.1046/j.1365-2958.2003.03526.x
- O'Riordan K, Lee JC. Staphylococcus aureus capsular polysaccharides. Clin Microbiol Rev 2004; 17:218-34; PMID:14726462; http://dx.doi.org/10.1128/CMR.17.1.218-234.2004
- Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol 2005; 3:948-58; PMID:16322743; http://dx.doi.org/10.1038/nrmicro1289
- Hair PS, Ward MD, Semmes OJ, Foster TJ, Cunnion KM. Staphylococcus aureus clumping factor A binds to complement regulator factor I and increases factor I cleavage of C3b. J Infect Dis 2008; 198:125-33; PMID:18544012; http://dx.doi.org/10.1086/588825
- McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol 2001; 55:77-104; PMID:11544350; http://dx.doi.org/10.1146/annurev.micro.55.1.77
- Postma B, Kleibeuker W, Poppelier MJ, Boonstra M, Van Kessel KP, Van Strijp JA, de Haas CJ. Residues 10-18 within the C5a receptor N terminus compose a binding domain for chemotaxis inhibitory protein of Staphylococcus aureus. J Biol Chem 2005; 280:2020-7; PMID:15542591; http://dx.doi.org/10.1074/jbc.M412230200
- Silverman GJ, Goodyear CS. Confounding B-cell defences: lessons from a staphylococcal superantigen. Nat Rev Immunol 2006; 6:465-75; PMID:16724100; http://dx.doi.org/10.1038/nri1853
- Peterson PK, Verhoef J, Sabath LD, Quie PG. Effect of protein A on staphylococcal opsonization. Infect Immun 1977; 15:760-4; PMID:870431
- Wang J, Roderiquez G, Norcross MA. Control of adaptive immune responses by Staphylococcus aureus through IL-10, PD-L1, and TLR2. Sci Rep 2012; 2:606; PMID:22930672
- Kim HK, Thammavongsa V, Schneewind O, Missiakas D. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr Opin Microbiol 2012; 15:92-9; PMID:22088393; http://dx.doi.org/10.1016/j.mib.2011.10.012
- Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol 2006; 4:295-305; PMID:16541137; http://dx.doi.org/10.1038/nrmicro1384
- Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, Engebretsen IL, Bayles KW, Horswill AR, Kielian T. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol 2011; 186:6585-96; PMID:21525381; http://dx.doi.org/10.4049/jimmunol.1002794
- Proctor RA. Is there a future for a Staphylococcus aureus vaccine? Vaccine 2012; 30:2921-7; PMID:22115633; http://dx.doi.org/10.1016/j.vaccine.2011.11.006
- Schaffer AC, Lee JC. Staphylococcal vaccines and immunotherapies. Infect Dis Clin North Am 2009; 23:153-71; PMID:19135920; http://dx.doi.org/10.1016/j.idc.2008.10.005
- Daum RS, Spellberg B. Progress toward a Staphylococcus aureus vaccine. Clin Infect Dis 2012; 54:560-7; PMID:22186773; http://dx.doi.org/10.1093/cid/cir828
- Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, Yeoh H, Law D, Robbins JB, Schneerson R, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med 2002; 346:491-6; PMID:11844850; http://dx.doi.org/10.1056/NEJMoa011297
- Fattom A, Matalon A, Buerkert J, Taylor K, Damaso S, Boutriau D. Efficacy profile of a bivalent Staphylococcus aureus glycoconjugate vaccine in adults on hemodialysis: phase III randomized study. Hum Vaccin Immunother 2014; e34414.
- Creech CB, 2nd Johnson BG, Alsentzer AR, Hohenboken M, Edwards KM, Talbot TR, 3rd. Vaccination as infection control: a pilot study to determine the impact of Staphylococcus aureus vaccination on nasal carriage. Vaccine 2009; 28:256-60; PMID:19799842; http://dx.doi.org/10.1016/j.vaccine.2009.09.088
- Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, Corey GR, Carmeli Y, Betts R, Hartzel JS, et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 2013; 309:1368-78; PMID:23549582; http://dx.doi.org/10.1001/jama.2013.3010
- Spellberg B, Daum R. A new view on development of a Staphylococcus aureus vaccine: insights from mice and men. Hum Vaccin 2010; 6:857-9; PMID:20930569; http://dx.doi.org/10.4161/hv.6.10.12469
- Anderson AS, Miller AA, Donald RG, Scully IL, Nanra JS, Cooper D, Jansen KU. Development of a multicomponent Staphylococcus aureus vaccine designed to counter multiple bacterial virulence factors. Hum Vaccin Immunother 2012; 8:1585-94; PMID:22922765; http://dx.doi.org/10.4161/hv.21872
- Wacker M, Wang L, Kowarik M, Dowd M, Lipowsky G, Faridmoayer A, Shields K, Park S, Alaimo C, Kelley KA, et al. Prevention of Staphylococcus aureus infections by glycoprotein vaccines synthesized in Escherichia coli. J Infect Dis 2014; 209:1551-61; PMID:24308931; http://dx.doi.org/10.1093/infdis/jit800
- Watts A, Ke D, Wang Q, Pillay A, Nicholson-Weller A, Lee JC. Staphylococcus aureus strains that express serotype 5 or serotype 8 capsular polysaccharides differ in virulence. Infect Immun 2005; 73:3502-11; PMID:15908379; http://dx.doi.org/10.1128/IAI.73.6.3502-3511.2005
- Richmond P, Borrow R, Goldblatt D, Findlow J, Martin S, Morris R, Cartwright K, Miller E. Ability of 3 different meningococcal C conjugate vaccines to induce immunologic memory after a single dose in UK toddlers. J Infect Dis 2001; 183:160-3; PMID:11078484; http://dx.doi.org/10.1086/317646
- Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev 1991; 55:733-51; PMID:1779933
- Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med 2008; 205:287-94; PMID:18268041; http://dx.doi.org/10.1084/jem.20072208
- Jarry TM, Memmi G, Cheung AL. The expression of alpha-haemolysin is required for Staphylococcus aureus phagosomal escape after internalization in CFT-1 cells. Cell Microbiol 2008; 10:1801-14; PMID:18466345; http://dx.doi.org/10.1111/j.1462-5822.2008.01166.x
- Kubica M, Guzik K, Koziel J, Zarebski M, Richter W, Gajkowska B, Golda A, Maciag-Gudowska A, Brix K, Shaw L, et al. A potential new pathway for Staphylococcus aureus dissemination: the silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLoS One 2008; 3:e1409; PMID:18183290; http://dx.doi.org/10.1371/journal.pone.0001409
- Loughman A, Fitzgerald JR, Brennan MP, Higgins J, Downer R, Cox D, Foster TJ. Roles for fibrinogen, immunoglobulin and complement in platelet activation promoted by Staphylococcus aureus clumping factor A. Mol Microbiol 2005; 57:804-18; PMID:16045623; http://dx.doi.org/10.1111/j.1365-2958.2005.04731.x
- Brady RA, Mocca CP, Burns DL. Immunogenicity analysis of Staphylococcus aureus clumping factor A genetic variants. Clin Vaccine Immunol 2013; 20:1338-40; PMID:23803901; http://dx.doi.org/10.1128/CVI.00275-13
- Adhikari RP, Ajao AO, Aman MJ, Karauzum H, Sarwar J, Lydecker AD, Johnson JK, Nguyen C, Chen WH, Roghmann MC. Lower antibody levels to Staphylococcus aureus exotoxins are associated with sepsis in hospitalized adults with invasive S. aureus infections. J Infect Dis 2012; 206:915-23; PMID:22807524; http://dx.doi.org/10.1093/infdis/jis462
- Fritz SA, Tiemann KM, Hogan PG, Epplin EK, Rodriguez M, Al-Zubeidi DN, Bubeck Wardenburg J, Hunstad DA. A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin Infect Dis 2013; 56:1554-61; PMID:23446627; http://dx.doi.org/10.1093/cid/cit123
- Adhikari RP, Karauzum H, Sarwar J, Abaandou L, Mahmoudieh M, Boroun AR, Vu H, Nguyen T, Devi VS, Shulenin S, et al. Novel structurally designed vaccine for S. aureus alpha-hemolysin: protection against bacteremia and pneumonia. PLoS One 2012; 7:e38567; PMID:22701668; http://dx.doi.org/10.1371/journal.pone.0038567
- Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, DeLeo FR. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis 2010; 202:1050-8; PMID:20726702; http://dx.doi.org/10.1086/656043
- Mocca CP, Brady RA, Burns DL. Role of Antibodies in Protection Elicited by Active Vaccination with Genetically Inactivated Alpha Hemolysin in a Mouse Model of Staphylococcus aureus Skin and Soft Tissue Infections. Clin Vaccine Immunol 2014; 21:622-7; PMID:24574539; http://dx.doi.org/10.1128/CVI.00051-14
- Lattar SM, Noto Llana M, Denoel P, Germain S, Buzzola FR, Lee JC, Sordelli DO. Protein antigens increase the protective efficacy of a capsule-based vaccine against Staphylococcus aureus in a rat model of osteomyelitis. Infect Immun 2014; 82:83-91; PMID:24126523; http://dx.doi.org/10.1128/IAI.01050-13
- Tuchscherr LP, Buzzola FR, Alvarez LP, Lee JC, Sordelli DO. Antibodies to capsular polysaccharide and clumping factor A prevent mastitis and the emergence of unencapsulated and small-colony variants of Staphylococcus aureus in mice. Infect Immun 2008; 76:5738-44; PMID:18809660; http://dx.doi.org/10.1128/IAI.00874-08
- Gong R, Hu C, Xu H, Guo A, Chen H, Zhang G, Shi L. Evaluation of clumping factor A binding region A in a subunit vaccine against Staphylococcus aureus-induced mastitis in mice. Clin Vaccine Immunol 2010; 17:1746-52; PMID:20826613; http://dx.doi.org/10.1128/CVI.00162-10
- Josefsson E, Higgins J, Foster TJ, Tarkowski A. Fibrinogen binding sites P336 and Y338 of clumping factor A are crucial for Staphylococcus aureus virulence. PLoS One 2008; 3:e2206; http://dx.doi.org/10.1371/journal.pone.0002206
- Narita K, Hu DL, Mori F, Wakabayashi K, Iwakura Y, Nakane A. Role of interleukin-17A in cell-mediated protection against Staphylococcus aureus infection in mice immunized with the fibrinogen-binding domain of clumping factor A. Infect Immun 2010; 78:4234-42; PMID:20679443; http://dx.doi.org/10.1128/IAI.00447-10
- Dantes R, Mu Y, Belflower R, Aragon D, Dumyati G, Harrison LH, Lessa FC, Lynfield R, Nadle J, Petit S, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med 2013; 173:1970-8; PMID:24043270
- Leroux-Roels I, Borkowski A, Vanwolleghem T, Drame M, Clement F, Hons E, Devaster JM, Leroux-Roels G. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet 2007; 370:580-9; PMID:17707753; http://dx.doi.org/10.1016/S0140-6736(07)61297-5
- Leroux-Roels I, Bernhard R, Gerard P, Drame M, Hanon E, Leroux-Roels G. Broad Clade 2 cross-reactive immunity induced by an adjuvanted clade 1 rH5N1 pandemic influenza vaccine. PLoS One 2008; 3:e1665; PMID:18301743; http://dx.doi.org/10.1371/journal.pone.0001665
- Morel S, Didierlaurent A, Bourguignon P, Delhaye S, Baras B, Jacob V, Planty C, Elouahabi A, Harvengt P, Carlsen H, et al. Adjuvant System AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 2011; 29:2461-73; PMID:21256188; http://dx.doi.org/10.1016/j.vaccine.2011.01.011
- Roman F, Clement F, Dewe W, Walravens K, Maes C, Willekens J, De Boever F, Hanon E, Leroux-Roels G. Effect on cellular and humoral immune responses of the AS03 adjuvant system in an A/H1N1/2009 influenza virus vaccine administered to adults during two randomized controlled trials. Clin Vaccine Immunol 2011; 18:835-43; http://dx.doi.org/10.1128/CVI.00480-10
- Berglund J, Vink P, Tavares Da Silva F, Lestrate P, Boutriau D. Safety, immunogenicity, and antibody persistence following an investigational Streptococcus pneumoniae and Haemophilus influenzae triple-protein vaccine in a phase 1 randomized controlled study in healthy adults. Clin Vaccine Immunol 2014; 21:56-65; PMID:24173029; http://dx.doi.org/10.1128/CVI.00430-13
- Fattom AI, Horwith G, Fuller S, Propst M, Naso R. Development of StaphVAX, a polysaccharide conjugate vaccine against S. aureus infection: from the lab bench to phase III clinical trials. Vaccine 2004; 22:880-7; PMID:15040941; http://dx.doi.org/10.1016/j.vaccine.2003.11.034
- Decker MD, Edwards KM, Bradley R, Palmer P. Comparative trial in infants of four conjugate Haemophilus influenzae type b vaccines. J Pediatr 1992; 120:184-9; PMID:1735812; http://dx.doi.org/10.1016/S0022-3476(05)80424-X
- Croxtall JD, Dhillon S. Meningococcal quadrivalent (serogroups A, C, W135 and Y) tetanus toxoid conjugate vaccine (Nimenrix). Drugs 2012; 72:2407-30; PMID:23231026; http://dx.doi.org/10.2165/11209580-000000000-00000
- Prymula R, Schuerman L. 10-valent pneumococcal nontypeable Haemophilus influenzae PD conjugate vaccine: Synflorix. Expert Rev Vaccines 2009; 8:1479-500; PMID:19863240; http://dx.doi.org/10.1586/erv.09.113
- Langley JM, Frenette L, Ferguson L, Riff D, Sheldon E, Risi G, Johnson C, Li P, Kenney R, Innis B, et al. Safety and cross-reactive immunogenicity of candidate AS03-adjuvanted prepandemic H5N1 influenza vaccines: a randomized controlled phase 1/2 trial in adults. J Infect Dis 2010; 201:1644-53; PMID:20423222; http://dx.doi.org/10.1086/652701
- McElhaney JE, Beran J, Devaster JM, Esen M, Launay O, Leroux-Roels G, Ruiz-Palacios GM, van Essen GA, Caplanusi A, Claeys C, et al. AS03-adjuvanted versus non-adjuvanted inactivated trivalent influenza vaccine against seasonal influenza in elderly people: a phase 3 randomised trial. Lancet Infect Dis 2013; 13:485-96; PMID:23518156; http://dx.doi.org/10.1016/S1473-3099(13)70046-X
- Leroux-Roels G, Marchant A, Levy J, Van Damme P, Schwarz T, Horsmans Y, Kremsner P, Gabor J, Esen M, Carletti I, et al. Learnings from clinical assessment of innate responses induced by several adjuvants combined with HBsAg model antigen. Keystone Symp Immunol Mech Vaccin, Ottawa, Canada, 13 to 18 Dec 2012, P1045.
- Fattom A, Schneerson R, Watson DC, Karakawa WW, Fitzgerald D, Pastan I, Li X, Shiloach J, Bryla DA, Robbins JB. Laboratory and clinical evaluation of conjugate vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides bound to Pseudomonas aeruginosa recombinant exoprotein A. Infect Immun 1993; 61:1023-32; PMID:8432585
- Welch PG, Fattom A, Moore J, Jr., Schneerson R, Shiloach J, Bryla DA, Li X, Robbins JB. Safety and immunogenicity of Staphylococcus aureus type 5 capsular polysaccharide-Pseudomonas aeruginosa recombinant exoprotein A conjugate vaccine in patients on hemodialysis. J Am Soc Nephrol 1996; 7:247-53; PMID:8785394
- Landrum M, Lalani T, Niknian M, Maguire J, Hospenthal D, Fattom A, Taylor K, Fraser J, Wilkins K, Ellis M, et al. A Randomized, Multi-Center Trial to Evaluate the Safety and Immunogenicity of Staphylococcus aureus Toxoids, rAT and rLukS-PV, in Healthy Volunteers. IDSA Oct 21 2011, P718.
- Richmond P, Nissen M, Marshall H, Shakib S, Hodsman P, Jiang Q, Rill D, Baber J, Eiden J, Jansen KU, et al. A randomised, placebo controlled phase 1 first-in-human study of a novel 3-antigen Staphylococcus aureus vaccine in healthy adults, poster LB2769. Proceedings of the 21st European Congress of Clinical Microbiology and Infectious diseases, 07 – 10 May 2011, Milan, Italy.
- Frenck R, Buddy Creech C, Sheldon E, Seiden DJ, Kankam M, Baber J, Zito E, Hubler R, Eiden J, Severs JM, et al. Safety, tolerability, and immunogenicity of a novel 4-antigen Staphylococcus aureus vaccine (sa4ag) in healthy adults: results of a randomized, placebo-controlled first-in-human phase 1/2 study, ePoster 134. 24th European Congress of Clinical Microbiology and Infectious diseases, 10 – 13 May 2014, Barcelona, Spain.
- Campbell JD, Edelman R, King JC, Jr., Papa T, Ryall R, Rennels MB. Safety, reactogenicity, and immunogenicity of a tetravalent meningococcal polysaccharide-diphtheria toxoid conjugate vaccine given to healthy adults. J Infect Dis 2002; 186:1848-51; PMID:12447774; http://dx.doi.org/10.1086/345763
- Knuf M, Kowalzik F, Kieninger D. Comparative effects of carrier proteins on vaccine-induced immune response. Vaccine 2011; 29:4881-90; PMID:21549783; http://dx.doi.org/10.1016/j.vaccine.2011.04.053
- Hawkins J, Kodali S, Matsuka YV, McNeil LK, Mininni T, Scully IL, Vernachio JH, Severina E, Girgenti D, Jansen KU, et al. A recombinant clumping factor A-containing vaccine induces functional antibodies to Staphylococcus aureus that are not observed after natural exposure. Clin Vaccine Immunol 2012; 19:1641-50; PMID:22896688; http://dx.doi.org/10.1128/CVI.00354-12
- Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, Fu Y, French SW, Edwards JE, Jr., Spellberg B. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog 2009; 5:e1000703; PMID:20041174; http://dx.doi.org/10.1371/journal.ppat.1000703
- Minegishi Y, Karasuyama H. Defects in Jak-STAT-mediated cytokine signals cause hyper-IgE syndrome: lessons from a primary immunodeficiency. Int Immunol 2009; 21:105-12; PMID:19088064; http://dx.doi.org/10.1093/intimm/dxn134
- Minegishi Y, Saito M. Molecular mechanisms of the immunological abnormalities in hyper-IgE syndrome. Ann N Y Acad Sci 2011; 1246:34-40; PMID:22236428; http://dx.doi.org/10.1111/j.1749-6632.2011.06280.x
- Minegishi Y, Saito M, Nagasawa M, Takada H, Hara T, Tsuchiya S, Agematsu K, Yamada M, Kawamura N, Ariga T, et al. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med 2009; 206:1291-301; PMID:19487419; http://dx.doi.org/10.1084/jem.20082767
- de Beaucoudrey L, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M, Feinberg J, von Bernuth H, Samarina A, Janniere L, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med 2008; 205:1543-50; PMID:18591412; http://dx.doi.org/10.1084/jem.20080321
- Senthilkumar A, Kumar S, Sheagren JN. Increased incidence of Staphylococcus aureus bacteremia in hospitalized patients with acquired immunodeficiency syndrome. Clin Infect Dis 2001; 33:1412-6; PMID:11565083; http://dx.doi.org/10.1086/322656
- Cho SH, Strickland I, Tomkinson A, Fehringer AP, Gelfand EW, Leung DY. Preferential binding of Staphylococcus aureus to skin sites of Th2-mediated inflammation in a murine model. J Invest Dermatol 2001; 116:658-63; PMID:11348452; http://dx.doi.org/10.1046/j.0022-202x.2001.01331.x
- Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med 2002; 347:1151-60; PMID:12374875; http://dx.doi.org/10.1056/NEJMoa021481
- Harper EG, Guo C, Rizzo H, Lillis JV, Kurtz SE, Skorcheva I, Purdy D, Fitch E, Iordanov M, Blauvelt A. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol 2009; 129:2175-83; PMID:19295614; http://dx.doi.org/10.1038/jid.2009.65
- Hedrick MN, Lonsdorf AS, Shirakawa AK, Richard Lee CC, Liao F, Singh SP, Zhang HH, Grinberg A, Love PE, Hwang ST, et al. CCR6 is required for IL-23-induced psoriasis-like inflammation in mice. J Clin Invest 2009; 119:2317-29
- Niebuhr M, Gathmann M, Scharonow H, Mamerow D, Mommert S, Balaji H, Werfel T. Staphylococcal alpha-toxin is a strong inducer of interleukin-17 in humans. Infect Immun 2011; 79:1615-22; PMID:21245272; http://dx.doi.org/10.1128/IAI.00958-10
- Breuer K, Wittmann M, Kempe K, Kapp A, Mai U, Dittrich-Breiholz O, Kracht M, Mrabet-Dahbi S, Werfel T. Alpha-toxin is produced by skin colonizing Staphylococcus aureus and induces a T helper type 1 response in atopic dermatitis. Clin Exp Allergy 2005; 35:1088-95; PMID:16120092; http://dx.doi.org/10.1111/j.1365-2222.2005.02295.x
- Moris P, van der Most R, Leroux-Roels I, Clement F, Drame M, Hanon E, Leroux-Roels GG, Van Mechelen M. H5N1 influenza vaccine formulated with AS03 A induces strong cross-reactive and polyfunctional CD4 T-cell responses. J Clin Immunol 2011; 31:443-54; PMID:21174144; http://dx.doi.org/10.1007/s10875-010-9490-6
- Van Damme P, Kafeja F, Bambure V, Hanon E, Moris P, Roman F, Gillard P. Long-term persistence of humoral and cellular immune responses induced by an AS03A-adjuvanted H1N1 2009 influenza vaccine: an open-label, randomized study in adults aged 18-60 years and older. Hum Vaccin Immunother 2013; 9:1512-22; PMID:23571166; http://dx.doi.org/10.4161/hv.24504
- Rumke HC, Richardus JH, Rombo L, Pauksens K, Plassmann G, Durand C, Devaster JM, Dewe W, Oostvogels L. Selection of an adjuvant for seasonal influenza vaccine in elderly people: modelling immunogenicity from a randomized trial. BMC Infect Dis 2013; 13:348; PMID:23890405; http://dx.doi.org/10.1186/1471-2334-13-348
- Miller LS, Cho JS. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol 2011; 11:505-18; PMID:21720387; http://dx.doi.org/10.1038/nri3010
- Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol 2010; 10:479-89; PMID:20559326
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol 2009; 27:485-517; PMID:19132915; http://dx.doi.org/10.1146/annurev.immunol.021908.132710
- McNeela EA, Burke A, Neill DR, Baxter C, Fernandes VE, Ferreira D, Smeaton S, El-Rachkidy R, McLoughlin RM, Mori A, et al. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathog 2010; 6:e1001191
- Olliver M, Hiew J, Mellroth P, Henriques-Normark B, Bergman P. Human monocytes promote Th1 and Th17 responses to Streptococcus pneumoniae. Infect Immun 2011; 79:4210-7; PMID:21788380; http://dx.doi.org/10.1128/IAI.05286-11
- Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature 2012; 484:514-8; PMID:22466287; http://dx.doi.org/10.1038/nature10957
- Chlibek R, Bayas JM, Collins H, de la Pinta ML, Ledent E, Mols JF, Heineman TC. Safety and immunogenicity of an AS01-adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults >=50 years of age. J Infect Dis 2013; 208:1953-61; PMID:23904292; http://dx.doi.org/10.1093/infdis/jit365
- Montoya J, Solon JA, Cunanan SR, Acosta L, Bollaerts A, Moris P, Janssens M, Jongert E, Demoitie MA, Mettens P, et al. A randomized, controlled dose-finding Phase II study of the M72/AS01 candidate tuberculosis vaccine in healthy PPD-positive adults. J Clin Immunol 2013; 33:1360-75; PMID:24142232
- Vandepapeliere P, Horsmans Y, Moris P, Van Mechelen M, Janssens M, Koutsoukos M, Van Belle P, Clement F, Hanon E, Wettendorff M, et al. Vaccine adjuvant systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccine 2008; 26:1375-86; PMID:18272264; http://dx.doi.org/10.1016/j.vaccine.2007.12.038
- Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 2011; 332:974-7; PMID:21512004; http://dx.doi.org/10.1126/science.1206095
- Schreiner J, Kretschmer D, Klenk J, Otto M, Buhring HJ, Stevanovic S, Wang JM, Beer-Hammer S, Peschel A, Autenrieth SE. Staphylococcus aureus phenol-soluble modulin peptides modulate dendritic cell functions and increase in vitro priming of regulatory T cells. J Immunol 2013; 190:3417-26; PMID:23460735; http://dx.doi.org/10.4049/jimmunol.1202563
- Food and Drug A. Guidance for Industry. Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. Available at: http://wwwfdagov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091977pdf2007
