?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Herpes zoster (HZ; shingles) is a common viral disease that affects the nerves and surrounding skin causing a painful dermatomal rash and leading to debilitating complications such as, mainly, post-herpetic neuralgia (PHN). Currently, there is no effective treatment for HZ and PHN. The objective of this study was to assess the cost-effectiveness of a HZ vaccination program in Germany. An existing Markov Model was adapted to the German healthcare setting to compare a vaccination policy to no vaccination on a lifetime time-horizon, considering 2 scenarios: vaccinating people starting at the age of 50 or at the age of 60 years, from the perspective of the statutory health insurance (SHI) and the societal perspective. According to the perspective, vaccinating 20% of the 60+ German population resulted in 162,713 to 186,732 HZ and 31,657 to 35,793 PHN cases avoided. Corresponding incremental cost-effectiveness ratios (ICER) were 39,306 €/QALY from the SHI perspective and 37,417 €/QALY from a societal perspective. Results for the 50+ German population ranged from 336,468 to 394,575 HZ and from 48,637 to 56,087 PHN cases avoided from the societal perspective. Corresponding ICER were 39,782 €/QALY from a SHI perspective and 32,848 €/QALY from a societal perspective. Sensitivity analyses showed that results are mainly impacted by discount rates, utility values and use of alternative epidemiological data.The model indicated that a HZ vaccination policy in Germany leads to significant public health benefits and could be a cost-effective intervention. The results were robust and consistent with local and international existing literature.
Abbreviations:
- ASHIP, Association of Statutory Health Insurance Physicians
- CEAC, Cost-effectiveness acceptability curves
- CMI, Cell-mediated immunity
- DSA, Deterministic sensitivity analysis
- EBM, German uniform assessment standard (Einheitlicher Bewertungsmaßstab)
- EMA, European Medicines Agency
- EQ-5D, EuroQoL
- G-DRG, German Diagnosis Related Groups
- GePaRD German Pharmacoepidemiological Research Database
- HZ, Herpes zoster
- ICER, Incremental cost-effectiveness ratio
- IQWIG, German Institute for Quality and Efficiency in Health Care
- mBPI-SF Modified short form brief pain inventory
- NNV, Number needed to vaccinate
- PHN, Post-herpetic neuralgia
- PSA, Probabilistic sensitivity analysis
- QALY, Quality-adjusted life year
- SHI, Statutory health insurance
- SPS, Shingles Prevention Study
- STIKO, German Standing Committee on Immunisation
- STPS, Short-Term Persistence Substudy
- US, United States
- VZV, Varizella zoster virus
- YO, Years old
- ZEST, Zostavax® Efficacy and Safety Trial
Introduction
Herpes zoster (HZ; shingles) is a common health problem causing significant pain and morbidity, especially in the population aged 50 and over.Citation1 HZ is the clinical manifestation of the reactivation of latent varicella zoster virus (VZV), a virus commonly acquired in childhood (chickenpox), primarily due to a decrease in cell-mediated immunity (CMI). One in 4Citation1 people are likely to get HZ in their life, and the risk increases with age, roughly doubling in every decade after the age of 50, due to a decrease in specific cellular immunity against the virus.Citation2 VZV reactivation affects the nerves and surrounding skin, explaining the main symptoms of HZ: rash and pain which usually last from about 2 weeks to one month.Citation3
The most common neurological complication of HZ is post-herpetic neuralgia (PHN), which can be defined as pain persisting or occurring at least one month,Citation4 3 monthsCitation3,5,6 or 6 monthsCitation7,8 after the rash onset depending on the definition used. While there is no international consensus on the definition of PHN, the most commonly accepted definition is that of pain persisting at least 3 months after HZ rash onset.Citation3,5,6 One in 5Citation9 HZ patients develops PHN and similarly to acute HZ, the risk becomes greater with age, reaching 50% among patients aged ≥ 80 y.Citation4,10
Even though the severity of HZ and PHN may differ among patients, HZ and PHN substantially impair quality of daily life due to the physical, occupational and emotional disabilities they can cause.Citation1,11–13 In addition to direct impact on patients’ physical and mental health, HZ and PHN also affect social functioning and engagement as well as the ability to work, with consequences for family, friends and society.Citation14
Zostavax®, the first zoster vaccine, received marketing approval by the European Medicines Agency (EMA) in 2006 for the prevention of HZ and PHN in individuals aged ≥ 50 y In a randomized, double-blind, placebo-controlled trial (Shingles Prevention Study, SPS) involving 38,546 immunocompetent adults aged ≥ 60 years, Zostavax® has been shown to significantly reduce both the incidence of HZ and the incidence of PHN.Citation5 In addition, the vaccine demonstrated an impact on the severity of HZ, as vaccinated patients who contracted HZ experienced a milder form of the disease, and the number of HZ cases with severe and long lasting pain was significantly reduced.Citation5 Similarly, in Zostavax® Efficacy and Safety Trial (ZEST), which comprised more than 22,000 subjects 50 to 59 y of age, Zostavax® significantly reduced the incidence of HZ.Citation15
Given the frequency of the disease, the serious impact of HZ on patients’ physical, occupational and social functioning, the lack of effective treatments as well as the common neurological complication PHN, prevention with vaccination represents a crucial innovation.Citation16,17 In the context of an aging German population, prevention of HZ and its complications is of even increasing significance as prevalence of HZ rises with age. HZ vaccination could not only relieve the burden of disease but also help maintain autonomy and social functioning, contributing to supporting active and healthy aging in the growing elderly German population. The objective of this cost-effectiveness analysis was to quantify the health benefits and economic impact of the implementation of a VZV vaccination in prevention of HZ and PHN in the German population aged ≥ 50 y.
Results
Base case analysis
As shown in , vaccinating 20% of the German SHI population aged ≥ 60 y would potentially prevent 162,713 HZ cases and 31,657 PHN cases compared to no vaccination policy (SHI perspective). From the societal perspective (total German population aged ≥ 60 years) 186,732 HZ and 35,793 PHN cases were avoided, respectively. QALYs gained were 12,891 from the SHI perspective and 14,558 from the societal perspective.
Table 3. Model input parameters
Table 4. Cost input parameters
Table 2. Base case ICER (lifetime time horizon)
Table 1. Base case results related to the cost and effectiveness outcomes
Accordingly, vaccinating 20% of the German SHI population aged ≥ 50 y would potentially prevent 336,468 HZ cases and 48,637 PHN cases compared to no vaccination policy (SHI perspective). From the societal perspective (total German population aged ≥ 50 years) 394,575 HZ and 56,087 PHN cases were avoided, respectively (). QALYs resulted in 19,132 from the SHI perspective and 22,016 from the societal perspective.
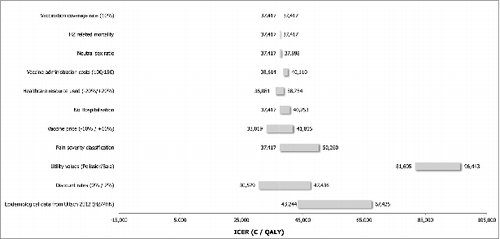
As shown in the incremental cost-effectiveness ratio (ICER) for German SHI population aged ≥ 60 y was 39,306 €/QALY gained compared to a no vaccination policy. From a societal perspective the ICER decreased to 37,417 €/QALY gained. From the SHI perspective the NNV was 24 to avoid one case of HZ and 114 to avoid one case of PHN. From the societal perspective, the NNV to avoid one case of HZ was 23, and it was 113 to avoid one case of PHN.
The ICER for the German SHI population aged ≥ 50 y was 39,782 €/QALY gained compared to a no vaccination policy. From a societal perspective the ICER decreased to 32,848 €/QALY gained (). From both perspectives, the NNV to avoid one case of HZ was 17, and it was 110 to avoid one case of PHN.
Sensitivity analyses
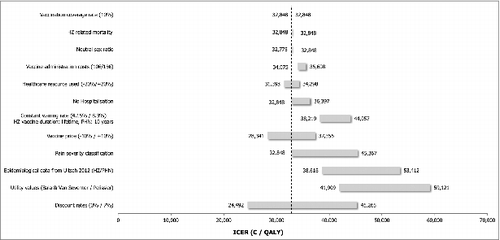
The tornado diagrams () illustrate the impact of independent parameters on the base case results for both populations. Variables with the most significant impact on the ICER were the discounting rates, the utility values associated with pain, the incidence of HZ and PHN, the pain severity classification, the waning of the vaccine efficacy as well as the vaccine price.
The discount rates showed a noticeable impact on the analysis results for both populations (50+ and 60+), with ICERs varying plus or minus €10,000 per QALY around the base case estimates.
Using alternative utility inputs extracted from Pellissier et al.,Citation18 Bala et al.Citation19 and van Seventer et al.,Citation20 resulted for both populations (50+ and 60+) in an unfavourable effect on the ICER for the vaccine, since this data does not take into account severe pain states as much as the base case inputs do.
The alternative epidemiological inputs for HZ and PHN extracted from Ultsch et al.Citation21 led in both populations (50+ and 60+) to higher ICERs, up to €70,456 (SHI perspective for population aged ≥ 60 years) and 67,425 respectively for the SHI and societal perspective for population ≥50 years.
Alternative efficacy waning and duration had significant impact on the results, too. Assuming a lifetime vaccine efficacy duration for HZ and a 10 y vaccine efficacy duration for PHN and a constant waning rate of 8.3% on the HZ vaccine efficacy, the ICERs increased for the population aged ≥50 y. When considering the same vaccine efficacy duration on HZ and PHN but with an alternative efficacy waning of 4.15%, the ICER from the SHI perspective for the population aged ≥50 y slightly decreased (€37,126) whereas it increased the ICER from a societal perspective (€38,219). For the population aged ≥60 y the ICERs decreased up to 25,638.
A variation of 10% of the vaccine price led to a variation of 11% (SHI perspective) to 14% (societal perspective) of the ICER, in the same direction.
HZ-related mortality, choosing a neutral sex ratio, increasing the vaccine administration cost and varying health care resource use (20% variation), had only a limited effect on the cost-effectiveness results for both populations.
A change from 20% to 10% coverage rate did not have any impact on the results due to model structure (static cohort model) and flat coverage rates assumed among age-groups.
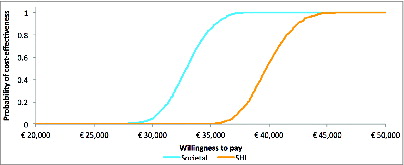
PSA were performed using Monte Carlo simulations on the distributions of the parameters that were found to have an impact on the results in the DSA. displays the 2 cost-effectiveness acceptability curves (CEACs) showing that the probability of the vaccine being cost-effective reached 0.80 for thresholds of about €34,500 and €41,500, respectively for the societal and SHI perspective, and reached 1 for a threshold of €45,000 for both perspectives. Overall, the PSA confirms the robustness of the cost-effectiveness results.
Figure 8. Evolution of vaccine efficacy over time DSA = Deterministic sensitivity analysis; yo = years old.
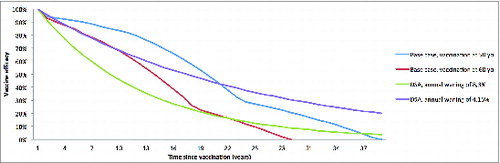
Figure 1. Sensitivity analysis overview: SHI perspective for 60+ population DSA = deterministic sensitivity analyses; HZ = herpes zoster; ICER = incremental cost-effectiveness ratio; PHN = post-herpetic neuralgia; SHI = statutory health insurance.
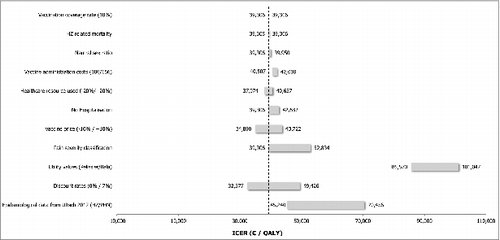
Figure 2. Sensitivity analysis overview: SHI perspective for 50+ population DSA = deterministic sensitivity analyses; HZ = herpes zoster; ICER = incremental cost-effectiveness ratio; PHN = post-herpetic neuralgia; SHI = statutory health insurance.
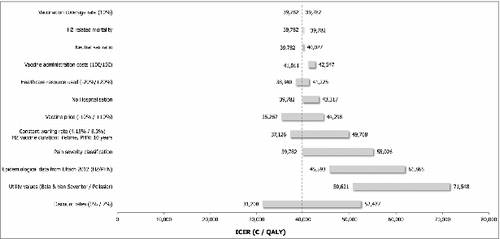
Figure 3. Sensitivity analysis overview: societal perspective for 60+ population DSA = deterministic sensitivity analyses; HZ = herpes zoster; ICER = incremental cost-effectiveness ratio; PHN = post-herpetic neuralgia.
External validity
For external validation, we compared our model with another German model (Ultsch et al.Citation22). When inserting identical parameters, the model leads to comparable ICER (SHI perspective: 39,306 €/QALY gained for 60+ and 39,782 €/QALY gained for 50+ population in our model vs. Thirty,212 €/QALY gained in Ultsch et al.Citation22 model; societal perspective: 37,417 €/QALY gained for 60+ and 32,848 €/QALY gained for 50+ population vs. Twenty-eight,146 €/QALY gainedCitation22) and therefore shows good external validity.
Discussion
The results of the present study show that HZ vaccination is able to provide significant public health benefits in Germany, both in terms of HZ and PHN cases avoided as well as QALYs gained. Indeed, it was found according to the societal perspective that vaccinating the population ≥ 60 y would prevent 186,732 HZ cases, 35,793 PHN cases and result in 14,558 QALYs gained. In comparison, a vaccination program targeting people ≥ 50 y would lead to prevent 394,575 HZ cases, 56,087 PHN cases and gain 22,016 QALYs. It has to be noted that, in the context of an aging German population and with the coming crisis of work resources, prevention of HZ and its complications is of even greater importance to sustain seniors employment in Germany. In terms of cost-effectiveness, it appeared that the cost-effectiveness ratio was more favorable to the population older than 50 y compared to the 60 y with respective ICER of 32,848 €/QALY gained and 37,417 €/QALY gained when considering a societal perspective. That can be explained by the importance of the indirect costs in the age group 50–60 years, which is mainly an active population. Alternatively, when considering the SHI perspective, the ICER of the cohort ≥ 60 y is equivalent to the ICER of the cohort ≥ 50 y (39,306 €/QALY gained vs. 39,782 €/QALY gained respectively).
Consideration of vaccine efficacy waning since the time of vaccination along with the age at vaccination are crucial in estimating the cost-effectiveness of HZ vaccination.Citation23 So far, previous publications of this model were using efficacy waning assumptions that were not age specific (limited duration of full vaccine efficacy followed by no efficacy or a waning rate on vaccine efficacy) in the absence of follow-up data.Citation24–26 A specific interest of the present analysis is that a statistical model developed by using data from SPS and STPSCitation27 has been used in our model to take into consideration real life waning in efficacy. Thus, for the first time, the waning in vaccine efficacy depends on the age at vaccination and time since vaccination.
It is worth noting that the results are in line with another cost-effectiveness analysis of HZ vaccination for the German healthcare setting that has been published by Ultsch et al.Citation22 In the latter, a scenario with administration of an HZ vaccine for individuals aged 60 y old was compared with no vaccination policy.Citation22 The Markov model used by Ultsch et al.Citation22 differed from ours regarding cycle length (1 month vs.. 3 months), cohort size (German population vs. 1 million), consideration of waning rate (waning rate dependent on time since and age at vaccination versus constant waning of 8.3% starting 10 y post vaccine) as well as pain split for health states HZ and PHN (yes vs. no). Additionally there were some differences in sources and values of input parameters, notably for HZ and PHN incidence (Hillebrand et al.Citation28 vs. Ultsch et al.Citation21) and utilities (Oster et al.Citation13 vs. Drolet et al.Citation29). Despite these discrepancies, analysis of Ultsch et al.Citation22 lead to ICER comparable to those of the present article. Furthermore, the results conform to the conclusions drawn from 3 recent literature reviewsCitation30-32 that reflect existing health economic evaluations of HZ vaccination among adults over 50 that have been published in Europe and North America.Citation8,25,33-35
Strengths of the present study are (i) the robustness of the model that was supported with both internal validation and independent expert review and confirmed by the external validation, (ii) the use of a waning function dependent to the age at vaccination and time since vaccination that decrease the uncertainty related to the duration of protection of the vaccine and (iii) the use of numerous local data sources that insure an accurate estimation of the cost–effectiveness of zoster vaccination and the impact of this vaccination policy in the German setting.
The present study has some limitations that have to be discussed. First, the cost-effectiveness analysis incorporated some international data when local data were missing. Due to transferring international data to the German health care setting, uncertainty surrounding those parameters arises. However, data were retrieved from countries with similar settings to Germany, and only in case no local data source could be identified. Second, cost data were based on the ASHIP data from the AOK Hesse (Ultsch et al.Citation21), which reflects a limited regional sample and therefore may not be representative for total Germany. Additionally, no cost data regarding different pain splits could be identified. Nevertheless, it is expected that these uncertainties surrounding the cost inputs were of minor impact on the results as demonstrated by the deterministic sensitivity analysis.
Overall, our model which incorporated for the first time the real-life time efficacy waning with age, produced robust results aligned with existing literatureCitation22,30,31 on HZ vaccination cost-effectiveness and is highly transferable to the German setting. This study bring useful evidence to document what could be the potential expected efficiency of new vaccination program, benefits difficult to estimate prior vaccination implementation. Indeed, health economic evidence provided by this study support the value of an HZ vaccination program for German population aged 50 and older, value even greater in a context where senior population represent a significant and increasing proportion of the overall population like in Germany. When combined with local data regarding disease burden, demographic evolution, healthcare system constraints and social aspects it should help designing the optimal way to define and implement local vaccination program in Germany.
Conclusion
Our analysis showed that a HZ vaccination program for adults aged ≥ 50 y and ≥ 60 y in Germany is able to bring substantial public health benefits and support a good cost-effective profile of HZ vaccination.
The results of our cost-effectiveness analysis were robust and comparable to results from another health economic evaluation in Germany.Citation22 Furthermore, the results were in line with the conclusions drawn from 2 recent literature reviewsCitation30–32
Methods
Model Structure
An existing Markov Model was adapted to the German healthcare setting.Citation26 The model utilised a Markov process to simulate the lifetime incidence and consequences of HZ among the current aged 50+ and 60+ German population. The population was analyzed as separate 5 y age cohorts (i.e. the 50–54 y old population was first analyzed over its lifetime, then the 55–59 y old population, and so on).
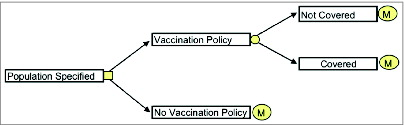
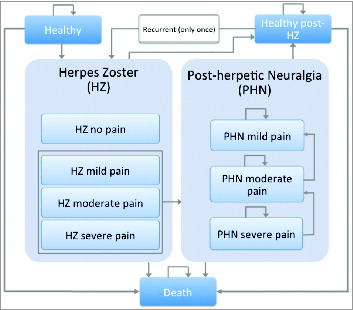
Patients stayed within a defined health state during one cycle (one month). Health states considered were healthy, HZ, PHN, healthy post-HZ and death as well as recurrent HZ and subsequent PHN. In the model, a recurrent HZ and subsequent PHN was considered to occur once in the remaining life time. HZ and PHN health states were further divided into health states by pain severity (no pain (only for HZ), mild, moderate, severe pain). The basic decision tree and the structure of the Markov model are represented in and .
The model ran through a lifetime time-horizon, meaning that monthly cycles ran until the entire cohort has died. Within each 1-month cycle, members of the cohort could remain in their current health state or make a transition into one of the allowable states. Transitions were governed by a matrix of probability values. With each successive monthly cycle, an increasing proportion of the cohort moved through the HZ and PHN states and eventually to death.
Model outcomes included cost per HZ case avoided, cost per PHN case avoided and cost per quality-adjusted life year (QALY) gained. Furthermore, we calculated number needed to vaccinate (NNV) to avoid one case of HZ/PHN.
Perspectives
Two perspectives were considered in the model: the statutory health insurance (SHI) perspective and the societal perspective.
Under the SHI perspective, only direct medical costs paid by SHI were covered, while the societal perspective also included co-payments arising from outpatient and inpatient care in addition to indirect costs corresponding to productivity losses.
While the societal perspective covered the entire 60+ population in Germany (n=21,778,791), the SHI perspective included only the part of 60+ population covered by the SHI in Germany (n=19,364,217) (a small part of the German population is covered by private insurances, and are therefore not included in the SHI perspective).Citation36
Similarly for the 50+ population, the societal perspective covered all 50+ in Germany (n=33,751,798), whereas the SHI perspective included only the part of 50+ population covered by the SHI in Germany (n=29,382,613).Citation36
Epidemiological data
Age-specific incidence rates of 1st episode of HZ were retrieved from a recent German study that analyzed data from 2005 to 2009 from the German Pharmacoepidemiological Research Database (GePaRD).Citation28 The authors conducted a retrospective cohort study analyzing data from about 7 million SHI insured individuals in Germany. Observed HZ incidence rates from the GePaRD ranged between 10.4 to 12.5 per 1,000 person-years.Citation28 Incidence rates from the GePaRD were reported separately for each year, therefore, for insertion in the model, a cumulative incidence was calculated for the period from 2005 to 2009. As shown in , the resulting annual HZ incidence rates varied between 0.66% for the age group 50–54 y and 1.43% for individuals aged ≥ 100 y.
Alternative data on HZ incidence rates from the Association of Statutory Health Insurance Physicians (ASHIP)Citation37 were used for sensitivity analyses.Citation37 The alternative annual HZ incidence rates varied between 0.66% for age group 50–54 y and 1.28% for individuals aged ≥ 100 yCitation.37
Recurrent rate of HZ episodes was set to zero in the model, as HZ cases which appeared recurrently more than one year later were considered as another incident case in the GePaRD incidence data.Citation28 Alternative values based on a matched cohort study in the USACitation38 were used for sensitivity analyses, resulting in 0.10% rate of HZ recurrence for age group 50–54 y and 0.27% for individuals aged ≥ 100 yCitation.38
The proportion of HZ patients that developed PHN was based on GePaRD data as well.Citation28 As for the HZ incidence rates, an average PHN cumulative incidence per 1,000 person-years was calculated for the years 2005 to 2009. The model using a 1-month cycle length is based on the 1-month definition of PHN to formulate its calculations. The 1-month PHN proportion was calibrated to ensure that, 3 months following rash onset in the model, the proportions of PHN cases matched those found in the 3-month PHN definition. This resulted in PHN incidence proportions of 12% for age group 50–54 y and 15.36% for individuals aged ≥ 100 y.
To investigate the robustness of the results with respect to the PHN incidence rates, alternative values based on PHN proportions and pain split data reported in Ultsch et al.Citation21 and either Drolet et al.Citation22 or Oxman et al.Citation5 were included as sensitivity analyses. Details regarding parameters used in the sensitivity analysis are provided in .
Gender split values were incorporated in the model where gender specific values were relevant. Data regarding gender split of HZ were identified in Ultsch et al.Citation37 based on the Association of Statutory Health Insurance Physicians (ASHIP) database of 2007/2008.Citation37 The authors reported a female proportion of 65% of HZ cases in the ASHIP sample, covering the AOK Hessen.Citation37 Gender split for PHN of 81% females was identified in Weinke et al.Citation39 who collected data through telephone interviews with 11,009 respondents.
The split between the different HZ and PHN pain states were obtained from the SPS pivotal trial study.Citation5 The latter provided information on pain severity levels using a questionnaire specifically developed to assess HZ and PHN associated pain (the Zoster Brief Pain Inventory, ZBPI).Citation5 Reported pain splits by age group are shown in . Further data for HZ and PHN pain split from published literature were considered in sensitivity analyses.Citation29,40
HZ and PHN duration information provided in the SPS studyCitation5 was used in base case analysis. The SPS study confirmed that most patients experience a 30-day duration of HZ, as reported in the literature, and a 9 month average duration of PHN episode, respectively.Citation5
Demographic data and mortality
Demographic data were gathered from data of the German Federal Statistical Office.Citation41 Corresponding general population mortality (background mortality) was based on mortality tables from the German Federal Statistical Office.Citation42
Mortality due to HZ was taken from Ultsch et al.Citation37 based on the ASHIP database. Mortality rate based on the ASHIP database ranged between 0.02 per 100,000 person-years for age group 50–54 and 3.86 per 100,000 person-years for individuals aged ≥ 90 y No mortality was deemed to be associated with PHN. This was confirmed by the lack of literature on the subject, as well as expert opinion. Therefore, mortality due to PHN was set to zero for the base case and sensitivity analyses.
Vaccine characteristics
Vaccine efficacy data came from 2 pivotal clinical trials, the ZEST and SPS study.Citation5,15 Using vaccine efficacy data from the 2 pivotal clinical trials–the ZEST and the SPS studiesCitation5,15–both direct and indirect effects of the vaccine were included in the model. The direct effect of the vaccine relates to the to the decrease in both the incidence of HZ and the proportion of PHN per HZ case. The indirect effect relates to the number of PHN cases avoided through the decrease in the number of HZ cases. The values inserted in the model for direct and indirect vaccine effects on HZ and PHN are shown in . Furthermore, the duration of PHN is reduced through vaccination, and this ultimately affects the pain severity experienced by the patients, as they spend a shorter period of time in each painful PHN health state.Citation5
Vaccine efficacy on PHN duration was accounted for in the model by adjusting the age-specific transition probabilities for the vaccinated individuals. On one hand, vaccine efficacy on HZ was implemented as a relative risk reduction and applied to the probability of contracting HZ when healthy. On the other hand, efficacy on PHN was reflected in the lower risk of developing PHN when having HZ and in higher probabilities of recovery when having PHN.
Clinical data on Zostavax® efficacy and persistence over time have shown that vaccine efficacy is age-specific and is maintained up to 10 yCitation43 However, vaccine efficacy wanes over time, and this waning of vaccine efficacy over time is correlated to patients’ age at vaccination. The current model takes into account these 2 aspects in order to reflect real-life evolution of vaccine efficacy over time, using a combination of 2 Poisson regression models.Citation27 These durability models were developed to reflect efficacy waning over time using data from ZEST, SPS and STPS. The average result of models A and B (equations below) was used to derive the waning rates by age groups (age at time of vaccination) entered in the cost-effectiveness model.
The equation of regression model A is the following:Eq. 1
Eq. 1
The equations of regression model B are the following:
If age < 60 years old:Eq. 2
Eq. 2
If age > 60 years old:Eq. 3
Eq. 3
Due to the lack of statistically significant data for PHN, the regression could only be done for efficacy on HZ incidence. As a consequence, the vaccine protection on the occurrence of PHN and its duration was conservatively assumed to last 10 y in the cost-effectiveness model.
This illustration of vaccine efficacy was tested in sensitivity analyses using alternative conservative scenarios considering a fixed annual waning rate with variable vaccine duration as well as scenarios with variable waning rate and variable vaccine duration, as done in a previous adaptation of this model.Citation18,24,25,31 Difference in the profiles of vaccine efficacy waning over time is presented in .
HZ coverage rates are not available in Germany and could hardly be compared to coverage observed in other countries such as UK or the US as vaccination policies differ. Possible local benchmark to consider could be influenza or pneumococcal vaccination rates, but again these vary (between 10–30% for pneumoCitation22 to 40–60% for influenza.Citation44,45 To stay conservative and consistent with previous publication from Ultsch et al.,Citation22 a vaccine coverage rate of 20% was therefore assumed for all age-groups.
Utility values
Considering the model structure, utility values were collected for all levels of HZ and PHN pain. In the absence of local disease specific data, international studies were screened to identify the more reliable sources for disease-specific utilities.Citation33,46,47 Therefore, data from a US-study analyzing the relationship of pain and quality of life in 385 individuals aged ≥ 65 y with pain caused by PHNCitation13 were used. Data in this survey was collected by a 15-items questionnaire, which covered pain intensity, pain interference and health-related quality of life. Quality of life was measured via the EuroQoL (EQ-5D) survey, which consists of 5 items related to mobility, self-care, usual activities, pain/discomfort and anxiety/depression.Citation13 Utility values according to the level of pain were retrieved from an observational survey, including 84 European patients with PHN.Citation20 Data in this study were collected with a questionnaire, which included among others, pain severity, measured by the modified short form brief pain inventory (mBPI-SF). Quality of life was assessed via the EuroQoL (EQ-5D) survey as well.Citation20
The disease specific utilities were used to calculate the proportion of the baseline utility (in this case, HZ with no pain) that needs to be subtracted to obtain the utility value for a given health state. These decrements were then applied to the age-specific utility for the cohort.
The upper input parameters for burden of disease data, mortality, vaccination efficacy and utility values are summarized in . In the following, the cost parameters inserted in the Markov Model are described.
Costs
To consider inflation, the health care consumer price index was identified from the German Federal Statistical Office.Citation48 Cost parameters are shown in .
Vaccination costs included costs of vaccination, administration costs and vaccination co-payment. For the base case analysis, it was assumed that a recommendation for a HZ vaccination would be given by the German Standing Committee on Immunisation (STIKO) in which case the SHI is likely to reimburse total costs for the vaccination within the recommended population. In case of no recommendation, each federal state of Germany can make their own state-specific recommendation resulting in a co-payment for patients.
Vaccine unit price for Zostavax® was set to €147 to reflect official retail price listed in the German “Lauer Taxe” and the 2013 mandatory rebate. Vaccine administration cost was set to €6, according to the vaccination agreement among the Saxony Association of Statutory Health Insurance Physicians and several SHI companies.Citation49 Thus, total cost per HZ vaccination amounted to €153.
Treatment costs were differentiated to outpatient visit costs and inpatient costs for hospitalization. Outpatient visit costs were identified in Ultsch et al.Citation21 It was assumed that all HZ costs appear in the first month with the HZ event, due to the mean duration of HZ of one month. For PHN the annual costs were divided by the average duration of 9 months, therefore the annual costs of €160.23 resulted in a monthly cost of €17.80. An outpatient physician visit rate of 99.9% for HZ and 100% for PHN patients was applied in the Markov Model.Citation21
Ultsch et al.Citation21 assessed hospitalization costs, using database insurance records reporting inpatient diagnosis and length of stay in hospital. The mean annual hospitalization costs per user were calculated by means of German Diagnosis Related Groups (G-DRG), amounting to €3,080.85 and €3,890.62 respectively for HZ and PHN patients.Citation21 Hospitalization rate was reported to be 3.2% for HZ patients and 14.6% for PHN patients aged ≥ 50 yCitation21.
The mean annual medication costs per user for HZ patients aged ≥ 50 years, were estimated to be €55.72 and for those with PHN €219.79.Citation21 The costs included consideration of prescribed medication in relation to a German dermatological guideline.Citation50 Medication groups considered were virostatics, specific immunoglobulin, topical analgesics, non-opioids, opioids, antidepressants and anticonvulsants.Citation21 Drug prices were calculated on basis of their national central pharmaceutical number and the uniform pharmacy retail price or the drug-related reference price.Citation21
For the societal perspective, patients co-payments were considered to be €11.63 for HZ patients aged ≥ 50 y and €38.04 for PHN patients.Citation21 These co-payments correspond to co-finance services or treatments out of pocket. The utilization rates amounted to 96% for HZ patients and 93% for PHN patients.Citation21
According to the German guideline on HZ and HZ pain, the common diagnostic method was carried out with an inspection of whether vesicles are visible.Citation51 In early stages, diagnosis of HZ can be difficult, and therefore a detection using a diagnostic test is recommended,Citation51 which is performed in 22% of patients according to a Spanish study.Citation52 Unit costs for diagnostic tests were found in the German uniform assessment standard (Einheitlicher Bewertungsmaßstab, EBM) with EBM-number 32664, which covers detection of antibodies for further diseases and amounts to €19.20.Citation53
Employment
Employment rates were taken from the German Federal Statistical Office.Citation54 It was assumed that employment rates do not differ between the HZ and PHN populations. According to Ultsch et al.,Citation21 average sick leave times were 15.10 d and 62.50 d for HZ and PHN, respectively. For wages, the average hourly wage rate in Germany, provided from the German Federal Statistical Office, was inserted in the Markov Model.Citation54 Additionally, the 70% reimbursement of wages from the insurance provider, which become first applicable after 6 months of absence, was considered.
Productivity costs were calculated by multiplying the reported productivity losses incurred by an employed individual with HZ or PHN by the specific employment rates associated with each age group in the study population, adjusted using the effective employment rate of the general German population.
Discounting
Costs and effects were discounted by a rate of 3% in the base case analysis consistently with suggestions of the German Institute for Quality and Efficiency in Health Care (IQWiG).Citation55
Analyses
Base case analyses were run for the German population aged ≥ 50 and aged ≥ 60 y.
The scenario of an implemented HZ-vaccination policy was compared to the current situation of no HZ-vaccination. Analyses were performed considering a lifetime time-horizon, and according to both the societal and SHI perspective described previously.
Furthermore, several sensitivity analyses including one-way deterministic sensitivity analyses (DSA) and a probabilistic sensitivity analysis (PSA) were conducted. DSA were performed to assess the uncertainty around the parameters used in the model. DSA were run on epidemiological inputs, discount rates, management care of HZ (no hospitalization), vaccine price, vaccine coverage, vaccine duration with fixed waning 8.3% and vaccine duration with variable waning, vaccine administration cost, vaccine co-payment, utilities, utility calculation method (QALYs substractive) and resource use costs ( and ).
PSA were performed using Monte Carlo simulations on the distributions of the parameters that were found to have an impact on the results in the deterministic sensitivity analyses. The following parameters were included: HZ/PHN vaccine efficacy, HZ/PHN outpatient care costs, HZ/PHN utility, HZ/PHN incidence. A total of 1,000 simulations have been performed, drawing a random value at each iteration from each distribution.
Validation
Both internal and external validations were realized to verify that the developed model produces reliable results.
Internal validation of the model consisted of comparing the results of a simulation of a cohort replicating SPS trial characteristics with the clinical data observed in the SPS study (Oxman et al.Citation5). The model showed good internal validity with results exactly matching the trial results. Thus, it is reasonable to believe that the model generates a valid estimation of the clinical benefits of the vaccine.
The model structure was validated by an expert health economist and an European expert panel meeting.
For external validation, the model was compared with another German cost-effectiveness model of HZ vaccination (Ultsch et al.Citation22).
Disclosure of Potential Conflicts of Interest
EP, NL and MU are employees of Sanofi Pasteur MSD, a provider of a herpes zoster vaccine approved in the European Union. The authors have no other conflicts of interest to declare.
Acknowledgments
The authors thank Isabelle Bertrand and Hélène Bricout, employees of Sanofi Pasteur MSD (Lyon, France), for their critical review.
Funding
This study was funded by Sanofi Pasteur MSD.
References
- Miller E, Marshall R, Vurdien J. Epidemiology, outcome and control of varicella-zoster infection. Rev Med Microbiol 1993; 4:222–30; http://dx.doi.org/10.1097/00013542-199310000-00006
- Stankus SJ, Dlugopolski M, Packer D. Management of herpes zoster (shingles) and postherpetic neuralgia. Am Fam Phys 2000; 61:2437-48; PMID:10794584
- Opstelten W, Mauritz JW, de Wit NJ, van Wijck AJ, Stalman WA, van Essen GA. Herpes zoster and postherpetic neuralgia: incidence and risk indicators using a general practice research database. Fam Prac 2002; 19:471-5; PMID:12356697; http://dx.doi.org/10.1093/fampra/19.5.471
- Hope-Simpson RE. Postherpetic neuralgia. The Jo R Coll Gen Prac 1975; 25:571-5; PMID:1195231
- Oxman M, Levin M, Johnson G, Schmader K, Straus S, Gelb L, Arbeit R, Simberkoff M, Gershon A, Davis L. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271-84; PMID:15930418; http://dx.doi.org/10.1056/NEJMoa051016
- Scott FT, Leedham-Green ME, Barrett-Muir WY, Hawrami K, Gallagher WJ, Johnson R, Breuer J. A study of shingles and the development of postherpetic neuralgia in East London. J Med Virol 2003; 70:S24-S30; PMID:12627483; http://dx.doi.org/10.1002/jmv.10316
- Thyregod HG, Rowbotham MC, Peters M, Possehn J, Berro M, Petersen KL. Natural history of pain following herpes zoster. Pain 2007; 128:148-56; PMID:17070998; http://dx.doi.org/10.1016/j.pain.2006.09.021
- Rothberg MB, Virapongse A, Smith KJ. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Clin Infect Dis 2007; 44:1280-8; PMID:17443464; http://dx.doi.org/10.1086/514342
- Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain 2002; 18:350-4; PMID:12441828; http://dx.doi.org/10.1097/00002508-200211000-00002
- De Moragas JM, Kierland PR. The outcome of patients with herpes zoster. AMA Arch Derm 1957; 75:193-6; PMID:13393787; http://dx.doi.org/10.1001/archderm.1957.01550140037006
- Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Internal Med 1995; 155:1605-9; PMID:7618983; http://dx.doi.org/10.1001/archinte.1995.00430150071008
- Katz J, Cooper EM, Walther RR, Sweeney EW, Dworkin RH. Acute pain in herpes zoster and its impact on health-related quality of life. Clin Infect Dis 2004; 39:342-8; PMID:15307000; http://dx.doi.org/10.1086/421942
- Oster G, Harding G, Dukes E, Edelsberg J, Cleary PD. Pain, medication use, and health-related quality of life in older persons with postherpetic neuralgia: results from a population-based survey. J Pain 2005; 6:356-63; PMID:15943957; http://dx.doi.org/10.1016/j.jpain.2005.01.359
- Lukas K, Edte A, Bertrand I. The impact of herpes zoster and post-herpetic neuralgia on quality of life: patient-reported outcomes in six European countries. J Public Bealth 2012; 20:441-51; PMID:22822293; http://dx.doi.org/10.1007/s10389-011-0481-8
- Schmader KE, Levin MJ, Gnann JW, Jr., McNeil SA, Vesikari T, Betts RF, Keay S, Stek JE, Bundick ND, Su SC, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis 2012; 54:922-8; PMID:22291101; http://dx.doi.org/10.1093/cid/cir970
- Chen N, Li Q, Yang J, Zhou M, Zhou D, He L. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database System Rev 2014; 2:Cd006866; PMID:24500927
- Christo PJ, Hobelmann G, Maine DN. Post-herpetic neuralgia in older adults: evidence-based approaches to clinical management. Drugs Aging 2007; 24:1-19; PMID:17233544; http://dx.doi.org/10.2165/00002512-200724010-00001
- Pellissier JM, Brisson M, Levin MJ. Evaluation of the cost-effectiveness in the United States of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Vaccine 2007; 25:8326-37; PMID:17980938; http://dx.doi.org/10.1016/j.vaccine.2007.09.066
- Bala MV, Wood LL, Zarkin GA, Norton EC, Gafni A, O'Brien B. Valuing outcomes in health care: a comparison of willingness to pay and quality-adjusted life-years. J Clin Epidemiol 1998; 51:667-76; PMID:9743315; http://dx.doi.org/10.1016/S0895-4356(98)00036-5
- van Seventer R, Sadosky A, Lucero M, Dukes E. A cross-sectional survey of health state impairment and treatment patterns in patients with postherpetic neuralgia. Age Ageing 2006; 35:132-7; PMID:16431855; http://dx.doi.org/10.1093/ageing/afj048
- Ultsch B, Köster I, Reinhold T, Siedler A, Krause G, Icks A, Schubert I, Wichmann O. Epidemiology and cost of herpes zoster and postherpetic neuralgia in Germany. Eur J Health Econ 2013; 14:1015-26; http://dx.doi.org/10.1007/s10198-012-0452-1
- Ultsch B, Weidemann F, Reinhold T, Siedler A, Krause G, Wichmann O. Health economic evaluation of vaccination strategies for the prevention of herpes zoster and postherpetic neuralgia in Germany. BMC Health Ser Res 2013; 13:359; PMID:24070414; http://dx.doi.org/10.1186/1472-6963-13-359
- Bilcke J, Ogunjimi B, Hulstaert F, Van Damme P, Hens N, Beutels P. Estimating the age-specific duration of herpes zoster vaccine protection: a matter of model choice? Vaccine 2012; 30:2795-800; PMID:21964056; http://dx.doi.org/10.1016/j.vaccine.2011.09.079
- Annemans L, Bresse X, Gobbo C, Papageorgiou M. Health economic evaluation of a vaccine for the prevention of herpes zoster (shingles) and post-herpetic neuralgia in adults in Belgium. J Med Econom 2010; 13:537-51; PMID:20707768; http://dx.doi.org/10.3111/13696998.2010.502854
- Bresse X, Annemans L, Preaud E, Bloch K, Duru G, Gauthier A. Vaccination against herpes zoster and postherpetic neuralgia in France: a cost-effectiveness analysis. Expert Rev Pharmacoeconom Outcomes Res 2013; 13:393-406; PMID:23537397; http://dx.doi.org/10.1586/erp.13.19
- Moore L, Remy V, Martin M, Beillat M, McGuire A. A health economic model for evaluating a vaccine for the prevention of herpes zoster and post-herpetic neuralgia in the UK. Cost Eff Resour Alloc 2010; 8:7; PMID:20433704; http://dx.doi.org/10.1186/1478-7547-8-7
- Li X, Zhang JH, Betts R, Morrison V, Xu R, Itzler R, Acosta C, Dasbach E, Pellissier J, Johnson G, et al. Modeling the Durability of ZOSTAVAX® Vaccine Efficacy in People ≥60 Years of Age. Vaccine [ under review]
- Hillebrand K. Preliminary data from the BIPS report. 2013.
- Drolet M, Brisson M, Levin MJ, Schmader KE, Oxman MN, Johnson RW, Camden S, Mansi JA. A prospective study of the herpes zoster severity of illness. Clin J Pain 2010a; 26:656-66.
- Kawai K, Preaud E, Baron-Papillon F, Largeron N, Acosta C. Cost-effectiveness of vaccination against herpes zoster and postherpetic neuralgia: A Critical Review. Vaccine 2014; 26:1645-53; http://dx.doi.org/10.1016/j.vaccine.2014.01.058
- Szucs TD, Pfeil AM. A systematic review of the cost effectiveness of herpes zoster vaccination. PharmacoEconom 2013; 31:125-36; PMID:23335045; http://dx.doi.org/10.1007/s40273-012-0020-7
- de Boer P, Wilschut J, Postma M. Cost-effectiveness of vaccination against herpes zoster: a review. Hum Vaccines Immunother 2014; 10:2048-61; PMID:25424815; http://dx.doi.org/10.4161/hv.28670
- van Hoek AJ, Gay N, Melegaro A, Opstelten W, Edmunds WJ. Estimating the cost-effectiveness of vaccination against herpes zoster in England and Wales. Vaccine 2009; 27:1454-67; PMID:19135492; http://dx.doi.org/10.1016/j.vaccine.2008.12.024
- de Boer PT, Pouwels KB, Cox JM, Hak E, Wilschut JC, Postma MJ. Cost-effectiveness of vaccination of the elderly against herpes zoster in The Netherlands. Vaccine 2013; 31:1276-83; PMID:23306360; http://dx.doi.org/10.1016/j.vaccine.2012.12.067
- Brisson M, Pellissier JM, Camden S, Quach C, De Wals P. The potential cost-effectiveness of vaccination against herpes zoster and post-herpetic neuralgia. Human vaccines 2008; 4:238-45; PMID:18382137; http://dx.doi.org/10.4161/hv.4.3.5686
- The Information System of the Federal Health Monitoring. [Members and co-insured familiy members in the SHI on 1st of June each year (number)]. Mitglieder und mitversicherte Familienangehörige der gesetzlichen Krankenversicherung am 1.7. eines Jahres (Anzahl). 2013.
- Ultsch B, Siedler A, Rieck T, Reinhold T, Krause G, Wichmann O. Herpes zoster in Germany: quantifying the burden of disease. BMC Infect Dis 2011; 11:173; PMID:21679419; http://dx.doi.org/10.1186/1471-2334-11-173
- Tseng HF, Chi M, Smith N, Marcy SM, Sy LS, Jacobsen SJ. Herpes zoster vaccine and the incidence of recurrent herpes zoster in an immunocompetent elderly population. J Infect Dis 2012; 206:190-6; PMID:22669900; http://dx.doi.org/10.1093/infdis/jis334
- Weinke T, Edte A, Schmitt S, Lukas K. Impact of herpes zoster and post-herpetic neuralgia on patients' quality of life: a patient-reported outcomes survey. J Public Health 2010; 18:367-74; PMID:21124645; http://dx.doi.org/10.1007/s10389-010-0323-0
- Drolet M, Brisson M, Schmader K, Levin M, Johnson R, Oxman M, Patrick D, Camden S, Mansi JA. Predictors of postherpetic neuralgia among patients with herpes zoster: a prospective study. J Pain 2010b; 11:1211-21; http://dx.doi.org/10.1016/j.jpain.2010.02.020
- German Federal Statistical Office. [A1 Total population. Population of 31. December 2011 by year of age and birth]. Bevölkerung insgesamt. A1 Bevölkerung am 31.12.2011 nach Alters- und Geburtsjahren. 2013.
- German Federal Statistical Office. [Population and Emplyoment, Mortality Table Germany 2009/2011]. Bevölkerung und Erwerbstätigkeit, Sterbetafel Deutschland 2009/2011. 2013.
- European Medicines Agency. 06/05/2014 Zostavax -EMEA/H/C/000674 -IG/0435. Annex I Summary of Product Characteristics. 2014.
- Blank PR, Schwenkglenks M, Szucs TD. Influenza vaccination coverage rates in five European countries during season 2006/07 and trends over six consecutive seasons. BMC Public Health 2008; 8:272; PMID:18673545; http://dx.doi.org/10.1186/1471-2458-8-272
- Mereckiene J, Cotter S, D'Ancona F, Giambi C, Nicoll A, Levy-Bruhl D, Lopalco PL, Weber JT, Johansen K, Dematte L, et al. Differences in national influenza vaccination policies across the European Union, Norway and Iceland 2008-2009. Euro surveillance: European communicable disease bulletin 2010; 15.
- Brisson M. Estimating the number needed to vaccinate to prevent herpes zoster-related disease, health care resource use and mortality. Can J Public Health 2008; 99:383-6; PMID:19009921
- Edmunds WJ, Brisson M, Rose JD. The epidemiology of herpes zoster and potential cost-effectiveness of vaccination in England and Wales. Vaccine 2001; 19:3076-90; PMID:11312002; http://dx.doi.org/10.1016/S0264-410X(01)00044-5
- German Federal Statistical Office. [Gesamtwirtschaft und Umwelt -Verbraucherpreisindizes]. In: Destatis, ed., 2013.
- Saxony Association of Statutory Health Insurance Physicians. [Appendix 1 for Vaccination Agreement - obligat services]. Anhang 1 der Impfvereinbarung - Pflichtleistungen. 2013
- Gross G, Schofer H, Wassilew S, Friese K, Timm A, Guthoff R, Pau HW, Malin JP, Wutzler P, Doerr HW. Herpes zoster guideline of the German Dermatology Society (DDG). J Clin Virol 2003; 26:277-89; discussion 91-3; PMID:12637076; http://dx.doi.org/10.1016/S1386-6532(03)00005-2
- AWMF, Arbeitsgemeinschaft der wissenschaftlichen Medizinischen Fachgesellschaften. [Zoster and Zoster pain]. Zoster und Zosterschmerzen. 2005.
- Cebrian-Cuenca AM, Diez-Domingo J, San-Martin-Rodriguez M, Puig-Barbera J, Navarro-Perez J. Epidemiology and cost of herpes zoster and postherpetic neuralgia among patients treated in primary care centres in the Valencian community of Spain. BMC Infect Dis 2011; 11:302; PMID:22044665; http://dx.doi.org/10.1186/1471-2334-11-302
- National Association of Statutory Health Insurance Physicians and the regional Associations of Statutory Health Insurance Physicians. [Doctors' Fee Scale within the Statutory Health Insurance Scheme]. Einheitlicher Bewertungsmaßstab. http://www.kbv.de/html/arztgruppen_ebm.php, 2013
- German Federal Statistical Office. [Statistical yearbook. Germany and international]. Statistisches Jahrbuch. Deutschland und Internationales. 2012.
- Institute for Quality and Efficiency in Health Care. [General methods for evaluation of relation between benefit and cost]. Allgemeine Methoden zur Bewertung von Verhältnissen zwischen Nutzen und Kosten. 2009; Version 1.0 vom 12.10.2009:36.
- Gonzalez Chiappe S, Sarazin M, Turbelin C, Lasserre A, Pelat C, Bonmarin I, Chosidow O, Blanchon T, Hanslik T. Herpes zoster: Burden of disease in France. Vaccine 2010; 28:7933-8; PMID:20946861; http://dx.doi.org/10.1016/j.vaccine.2010.09.074
- Merck Sharp & Dohme Corp. ZOSTAVAX - Highlights of prescribing information. Available at: http://www.merck.com/product/usa/pi_circulars/z/zostavax/zostavax_pi2.pdf. 2014
- Drolet M, Levin MJ, Schmader KE, Johnson R, Oxman MN, Patrick D, Fournier SO, Mansi JA, Brisson M. Employment related productivity loss associated with herpes zoster and postherpetic neuralgia: a 6-month prospective study. Vaccine 2012; 30:2047-50; PMID:22285632; http://dx.doi.org/10.1016/j.vaccine.2012.01.045