Abstract
In the winter influenza epidemic season, patients with respiratory illnesses including respiratory syncytial virus (RSV) infections increase among young children. Therefore, we evaluated the effectiveness of influenza vaccine against influenza-like illness (ILI) using a technique to identify outbreaks of RSV infection and to distinguish those patients from ILI patients. The study subjects were 101 children aged 12 to 84 months attending nursery school. We classified the cases into 6 levels based on the definitions of ILI for outcomes. We established observation periods according to information obtained from regional surveillance and rapid diagnostic tests among children. Multivariate odds ratios (ORs) for each case classification were obtained using a logistic regression model for each observation period. For the entire observation period, ORs for cases with fever plus respiratory symptoms were reduced marginally significantly. For the local influenza epidemic period, only the OR for the most serious cases was significantly decreased (0.20 [95%CI: 0.04-0.94]). During the influenza outbreak among the nursery school children, multivariate ORs for fever plus respiratory symptoms decreased significantly (≥ 38.0°C plus ≥ one symptoms: 0.23 [0.06-0.91), ≥ 38.0°C plus ≥ 2 symptoms: 0.21 [0.05-0.85], ≥ 39.0°C plus ≥ one symptoms: 0.18 [0.04-0.93] and ≥ 39.0°C plus ≥ 2 symptoms: 0.16 [0.03-0.87]). These results suggest that confining observation to the peak influenza epidemic period and adoption of a strict case classification system can minimize outcome misclassification when evaluating the effectiveness of influenza vaccine against ILI, even if influenza and RSV cocirculate in the same season.
Introduction
Annual morbidity associated with influenza is highest among young children, for whom the rate of hospitalization has been estimated to be 1 per 1000 children aged under 5 years.Citation1 Therefore, many studies have investigated the efficacy of influenza vaccines among young children.Citation2-10 However, the results of these studies were not consistent because influenza epidemics vary a great deal depending on the time, place and population.Citation11 Accordingly, confining the subjects to true influenza patients is a key point for minimizing outcome misclassification.
In the winter, there are doubts about whether ILI patients have influenza because the infectious seasons of influenza and other respiratory viruses overlap, compounding the clinical difficulty in distinguishing these illnesses.
Respiratory syncytial virus (RSV) infection is a typical respiratory tract infection among young children in the winter,Citation12-14 although the number of patients with RSV infection is smaller than that of influenza patients. However, outbreaks often occur among communal populations in households, nursery schools and inpatient facilities.Citation15-18 Therefore, detection of an outbreak among a specific population is difficult using only information obtained from a regional surveillance system. When the effectiveness of influenza vaccine against ILI is evaluated in an epidemiological study, it is critically important to differentiate patients with RSV infection from ILI patients in the influenza epidemic period.
On the basis of virus isolation data, respiratory viruses from ILI patients had been shown a characteristic seasonal pattern where the peaks of the influenza virus and RSV were distinct from each other.Citation19 However, some overlap of endemicity have been clearly demonstrated by nucleic acid-based diagnostic methods in the influenza season.Citation20-22 Therefore, to correctly assess the effectiveness of influenza vaccine, it is critical to exclude RSV patients. It took some years to come to this conclusion because above key finding was reported during recent years.
The nursery school children studied here were a suitable cohort to verify the effectiveness of influenza vaccine because their chances for exposure to influenza viruses were relatively homogeneous. Additionally, they were always under observation by school nurses or their guardians, so there was a greater likelihood that their illness could be determined precisely.
Accordingly, we evaluated effectiveness of influenza vaccine against ILI in a season with cocirculating RSV among nursery school children using collected 2006-07 influenza season. In this study, we attempted to minimize outcome misclassification caused by ambiguous definition of the influenza epidemic period and case classification by using regional surveillance information and rapid diagnostic tests of the nursery school children.
Results
The subjects available for analysis were 101 children (45 children who were vaccinated twice, and 56 children who were not vaccinated). Ten children who were vaccinated once were excluded. The characteristics of vaccinees and nonvaccinees are compared in . Males were more frequent in the vaccinated group and the mean of age in months was higher in the unvaccinated group; however, there were no significant differences among these variables between vaccinees and nonvaccinees. Children who had asthma as an underlying illness, were vaccinated during the previous season, had a smoker in the family and had a vaccinated family were significantly more numerous in the vaccinated group. The children who slept longer and had more floor space per person were also more numerous in the vaccinated group. On the other hand, the number of family members was higher with marginal significance in the unvaccinated group.
Table 1. Baseline characteristics of vaccinees and nonvaccinees
The effectiveness of the vaccine for each outcome indicator during the entire observation period is shown in . In multivariate analysis, ORs for FaS, FaSS, FbS and FbSS were decreased, but not significance. For case definitions of ILI see Materials and Methods.
Table 2. Odds ratios of 2006–07 influenza vaccination for outcomes during the entire study period (weeks 1 through 15 of 2007).
The effectiveness of the vaccine during the outbreak of RSV infection among the nursery schoolchildren is shown in . There were no significant decreases in ORs for any of the outcome indicators in either univariate or multivariate analysis.
Table 3. Odds ratios of 2006–07 influenza vaccination for outcomes during RSV infection epidemic period at nursery school (weeks 1 through 5 of 2007).
The effectiveness of the vaccine during the local influenza epidemic period is shown in . The multivariate analysis revealed that vaccination was effective at preventing; FaSS and FbS with at least marginal significance (FaSS: 0.25 [0.06-1.01] and FbS: 0.22 [0.05-1.00]); however, the OR for FbSS, which was the most serious case classification among the outcome indicators, showed a significant decrease [0.20 (0.04-0.94]. Moreover, univariate and multivariate ORs for the outcome indicators were all less than 1. The point estimates for the defined levels of fever (38.0°C and 39.0°C), decreased gradually as the outcome classifications became more serious (Fa: 0.64, FaS: 0.31, FaSS: 0.25; or Fb: 0.53, FbS: 0.22, FbSS: 0.20). Additionally, when Fa and Fb, FaS and FbS, and FaSS and FbSS were compared, all point estimates decreased in the cases with higher fever levels.
Table 4 Odds ratios of 2006-07 influenza vaccination for outcomes during influenza epidemic period in Fukuoka prefecture (weeks 6 through 14 of 2007).
shows the effectiveness of the vaccine during the influenza outbreak among the nursery school children. The multivariate ORs for all outcome indicators were lower than the ORs in the other periods (–), and the ORs for FaS, FaSS, FbS and FbSS reached statistically significant levels (FaS: 0.23 [0.06-0.91]; FaSS: 0.21 [0.05-0.85]); FbS: 0.18 [0.04-0.93]; and FbSS: 0.16 [0.03-0.87]). For the local influenza epidemic period (), these point estimates for each defined level of fever (38.0°C and 39.0°C) also decreased gradually as the outcome classification level increased. All point estimates for outcome indicators decreased in the higher fever level. In multivariate analysis, it was found that the ORs for all outcome indicators decreased more than with univariate analysis. After adjustment for potential confounders, multivariate analysis showed a 68% ([1-0.16]/[1-0.50]) increase in the efficacy for preventing FbSS as compared to univariate analysis ().
Table 5 Odds ratios of 2006-07 influenza vaccination for each outcome during influenza outbreak period at the nursery school (weeks 10 through 14 of 2007).
Discussion
The entire period (weeks 1-15 of 2007: January 1-April 14) of this study is generally the epidemic season for influenza in Japan, therefore ILI patients increased among the study subjects from the beginning of the observation period. However, ORs for FaS, FaSS, FbS and FbSS were decreased, but not significantly throughout the entire period. This might have been because the presence of patients having respiratory infection due to non-influenza illnesses might have led to underestimation of the effectiveness of the vaccine.
According to the distribution of ILI patients in the nursery school (), there were patients who were RSV positive from the first week through the fifth week. At the same time, a small RSV epidemic was detected by using regional surveillance information during the period in Fukuoka prefecture. Therefore, it is possible that the outbreak of RSV infection might have overlapped the influenza epidemic in this period (weeks 1-5) among the study subjects. There was no significant reduction in the ORs of any outcome indicators in the RSV infection outbreak period (weeks 1-5: January 1-February 3) among the nursery school children, perhaps because ILI patients among the study subjects might not have had influenza in this period.
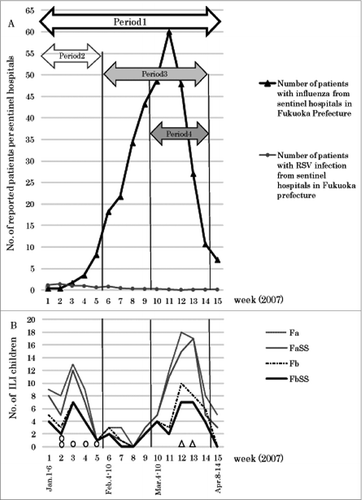
Figure 1. (A) Numbers of patients with influenza and RSV infections reported weekly from sentinel hospitals in Fukuoka Prefecture. (B) The cumulative total numbers of patients among the nursery school children classified by the case definitions (Fa: ≥38.0°C alone, FaSS: Fa plus ≥2 respiratory symptoms, Fb: ≥ 39.0°C alone FbSS: Fb plus ≥2 respiratory symptoms). Positive cases confirmed by rapid diagnostic tests for influenza virus and RSV among patients ≥37.5°C are shown as influenza virus:△ and RSV:∘. Four observational periods were defined as follows: period 1, from weeks 1 through 15 of 2007 (the entire observation period); period 2, from weeks 1 through 5 (the period of the outbreak of RSV infection among the nursery school children); period 3, from weeks 6 through 14 (the period of the influenza epidemic in Fukuoka Prefecture); and period 4, from weeks 10 through 14 (the period of the outbreak of influenza among the nursery school children).
In the multivariate analysis, only the OR for FbSS, which was the most severe outcome level, significantly decreased in the local influenza epidemic period (weeks 6-14: February 4-April 7). The following interpretations could explain this result. First, the observation period was limited to the local influenza epidemic period by the use of regional surveillance information. Therefore patients with RSV infection might have been congregated with the ILI patients. However, it is highly probable that this would have occurred in any case because this period was immediately after the outbreak of RSV infection (weeks 5–8) among the study subjects. RSV infection might be severe among infants aged < 1 year, but the fever and respiratory symptoms are usually mild.Citation23 Therefore, adoption of rigorous outcome classification levels could decrease the congregation of patients having RSV infection with ILI patients (). Consequently, the OR for FbSS, the most severe level, might have significantly decreased.
In the analysis during the influenza outbreak among the nursery school children (weeks 10-14: March 4-April 7), multivariate analysis revealed that vaccination was effective at preventing FaS, FaSS, FbS and FbSS. The effectiveness of the vaccine for FbSS, compared with result of the local influenza epidemic period, increased by 5% ([1-0.16])/([1-0.20]). The distribution of ILI patients with detected influenza virus among the study subjects was consistent with this observation period (weeks 8-15). Therefore this period appears to have been the influenza outbreak period among the study subjects. Consequently, the outcome misclassification could be minimized because ILI patients in this period were more likely to have true influenza. However, multivariate ORs for Fa and Fb, outcome classifications with only a fever, did not significantly decrease. These results indicated that a fever without respiratory symptoms might not be a symptom of influenza. On the other hand, even if the outcome was fever only, the point estimates in this period were lower than those in other observation periods. The reason for this may have been that the patients who had only a fever were likely to mix with true influenza patients during the peak influenza epidemic period. Moreover, the point estimates gradually decreased in the order Fa, FaS and FaSS or Fb, FbS and FbSS in the multivariate analysis, as with the results for the local influenza epidemic period. In addition, their 95%CIs gradually became narrower. These results indicated that following procedures such as determining the peak of the influenza epidemic, adopting a rigorous outcome classification system and adjusting potential confounders could minimize outcome misclassification.
The number of patients affected by RSV, which causes lower respiratory tract infection, was comparatively small, even in the RSV epidemic period. However, numerous outbreaks of RSV infection are reported among communal populations such as families, hospitalized children and elderly people in nursing homes. Thus, it is possible that RSV infection is masked by influenza infection in many patients. Therefore, it might be difficult to identify outbreaks of RSV infection among certain populations by using only regional surveillance information.
In this study, we used information about not only influenza but also RSV infection obtained from regional surveillance to identify the outbreak of influenza among the study subjects. Additionally, pathogen detection was performed using rapid diagnostic tests for some of the subjects. Therefore, we were able to detect the outbreak of RSV infection in the nursery school. Consequently, the main observation could be limited to the peak influenza epidemic period. Furthermore, adoption of the rigorous case classification system made it possible to minimize outcome misclassification of patients with RSV infection as influenza patients, even if the influenza epidemic overlapped the circulation of RSV.
In our study, we found that the effectiveness of the vaccine was higher than that reported in a previous study that evaluated inactivated influenza vaccine among young children.Citation24 In the previous study, since the subjects were recruited from several different areas of Japan, the definition of the peak epidemic period of influenza might not have been optimal. Considering our results, if the observations of the previous study were limited to the optimal peak epidemic period of influenza among the study subjects, the effectiveness of the vaccine might have been found to be higher. However, on a critical review and re-analysis of 15 meta-data, parenteral inactivated influenza vaccine efficacy or effectiveness against children remains scarce.Citation25 Therefore, standard setting about various points as follows may be required.
Influenza epidemics follow different patterns depending on the time, place, and population.Citation11 According to this principle, analysis in the same epidemic season, in the same area and among a homogeneous population for exposure to influenza could be the key to evaluating the effectiveness influenza vaccine correctly. Moreover, the following procedures are essential to minimize the effect of outcome misclassification in field trials of influenza vaccine effectiveness.Citation26-28 First, all study subjects should be followed with equal intensity. Second, the influenza epidemic should be relatively large. Third, the circulating influenza viruses should antigenically match the vaccine strains. In this study, parents or guardians collected information on the children's body temperatures and symptoms each week during the entire follow-up period using a questionnaire, so all subjects were followed with almost equal intensity. In addition, all the subjects were recruited from a single nursery school, so their exposure to influenza might have been homogeneous. There was a relatively large epidemic of influenza that exceeded 60 patients per sentinel hospital in the peak epidemic period in Fukuoka prefecture during the 2006-07 influenza season. Furthermore type A H3N2 and type B were mainly cocirculatiing during this season, and these strains matched the vaccine strains.
These results suggested that confining observation to the peak influenza epidemic period and adoption of rigorous case definitions were both essential techniques to minimize outcome misclassification for analysis of the effectiveness of influenza vaccine against ILI under these advantageous conditions in for a field trial of influenza vaccine.
We detected high effectiveness of the influenza vaccine among nursery school children during the epidemic season when influenza cocirculated with RSV infection, which is difficult to distinguish from influenza. We succeeded in minimizing outcome misclassification by using special techniques to identify the peak of the influenza epidemic and by adopting a rigorous case classification system. These methods can generally be applied to evaluation of the efficacy of the influenza vaccine for ILI.
When ILI is used as the study outcome, it is less specific for influenza than laboratory confirmation. Nevertheless, as an outcome indicator it is more useful in actual field trials. Laboratory confirmation is normally expensive, especially in developing countries. In this study, we used both information obtained from regional surveillance and the distribution of patients in the nursery school. Detection of pathogens was conducted by using rapid diagnostic tests for influenza and RSV among some patients. Furthermore, adoption of a rigorous case classification system enabled us to differentiate patients with RSV infection from those with ILI patients during the influenza epidemic. Consequently, the effectiveness of the vaccine during the influenza outbreak in the nursery school approximated that among patients with true influenza.
Limitations
The sample size was small in this study. However, the point estimates for all outcomes during the period of the influenza epidemic were consistently less than 1, with a narrow 95% CI. Therefore, we considered that the reliability of results was maintained. The effects of potential confounders were taken into consideration in multivariate analysis in this study. Variables associated with potential confounders besides the age in months and gender among the young children were included in the model for adjustment. However, the possibility of residual confounders cannot be denied. In addition, the disease investigation among study subjects was conducted by their parents and guardians. This enabled us to follow all the study subjects with equal intensity, but disease misclassification might have occurred. However, this is a nondifferential misclassification. If there were such a misclassification, it would be underestimated in the results.
Materials and methods
Study subjects
The study subjects were 111 children (45 who were vaccinated twice, 10 who were vaccinated once, and 56 who had not been vaccinated) aged 12 to 84 months, recruited from Shin Yoshitomi nursery school, Koge-machi, Fukuoka Prefecture. In Japan, vaccination of seasonal inactivated influenza vaccine has been recommended 2 doses from children aged between 6 months to 12 years due to the relatively weak immune response toward the vaccine. Among 111 subjects, 10 children fail to have 2-dose vaccination. For the critical analysis of the influenza vaccine efficacy, we focused on the 2-dose population. Thus, one-dose subjects (10 children), were excluded from the final sample. Finally, the analysis subjects comprised 101 children. The twice-vaccinated subjects had received commercially obtained 2006-07 trivalent inactivated influenza vaccine with an interval of at least 2 weeks between the 2 vaccinations in each clinic during November 4 to December 24, 2006. Each vaccination was given at the then recommended dose (0.2 mL for children aged 12 months to <72 months and 0.3 mL for age ≥72 months). The vaccine contained A/ New Caledonia/ 20/ 99 (H1N1), A/ Hiroshima/ 52/ 2005 (H3N2) and B/ Malaysia/ 2506/ 2004, 30 μg of hemagglutinin per 1 mL from each strain. Informed consent was obtained from the parent or guardian of each subject. The study was conducted with the approval of the Ethics Committee of the Graduate School of Medicine, Osaka City University.
Information collection
At the time of enrollment, the following information was collected as continuous data by means of a self-administered questionnaire given to each child's parent or guardian: gender, age in months, body weight, hours of sleep per day, number of family members, number of siblings and floor area of residence. As categorical data, underlying disease, the history of influenza vaccination in the preceding season, history of medical examination for cold-like symptoms during the previous 6 months, history of hospitalization, influenza vaccination of family members (in the 2006-07 season) and smoking by family members was ascertained.
As a follow-up survey, information about the following was collected by means of a weekly self-administered questionnaire from each child's parent or guardian: fever, cough, rhinorrhea, sore throat, joint pain and chills. The follow-up period was January 1 to April 14, 2007 (15 weeks). This information was submitted every week to the nursery school by the children's parents or guardians.
The epidemic in Fukuoka prefecture and the outbreaks in the nursery school of influenza and RSV infection.
Influenza epidemics normally occur from early January to mid-April in Japan. ILI patients were found at the nursery school from the first week (Jan. 1–6) that the observation started. At the time of starting observation, a small outbreak of RSV infection was confirmed at the same time as an influenza epidemic by the regional surveillance system in Fukuoka Prefecture. Therefore, for viral surveillance of ILI patients at the nursery school, when a subject developed temperature of ≥37.5°C, the school nurse collected nasal discharge from the subject.
Pathogen detection from collected samples was conducted using rapid diagnostic tests for influenza virus, RSV and adenovirus at the Osaka Prefectural Institute of Public Health. In Fukuoka prefecture, the number of patients with RSV infection reported weekly by the sentinel hospitals () reached a peak in the second week of 2007 (1.4 patients) and then gradually declined until week 15. On the other hand, the number of influenza patients exceeded 10 patients weekly as reported by the sentinel hospitals from the sixth week. The peak number was found in week 11 (60.1 patients). Thereafter, the number of influenza patients decreased rapidly, becoming less than 10 patients per sentinel hospital in week 15. The weekly occurrence of ILI patients at the nursery school is illustrated in . There were 2 peaks of the occurrence of ILI patients, in week 3 and week 13, approximately corresponding to a small peak of RSV infection and the peak of the influenza, respectively, in Fukuoka prefecture. Additionally, in the nursery school children, RSV was detected in 5 patients from the second through the fifth weeks and influenza virus was detected in 2 patients in weeks 12 and 13 by rapid diagnostic tests ().
Analysis
We classified the cases into 6 levels of ILI for outcomes: (1) Fa: fever ≥ 38.0°C alone, (2) FaS: Fa plus ≥ one respiratory symptoms (rhinorrhea, cough and/or sore throat), (3) FaSS: Fa plus ≥ 2 respiratory symptoms, (4) Fb: fever ≥ 39.0°C alone, (5) FbS: Fb plus ≥ one respiratory symptoms, (6) FbSS: Fb plus ≥ 2 respiratory symptoms.
Four observation periods were set up (). (1) The entire observation period (weeks 1-15 of 2007: January 1-April 14), (2) The RSV infection outbreak period among the nursery school children: the period during which there were ≥5 FaSS patients and RSV was detected from these patients by using a rapid diagnostic test at the nursery school (weeks 1-5: January 1-February 3), (3) the local influenza epidemic period during which ≥10 influenza patients were reported weekly by the sentinel hospitals in Fukuoka Prefecture (weeks 6-14: February 4-April 7), and (4) the influenza outbreak period among the nursery school children, during which there were ≥5 FaSS patients and influenza virus was detected in these patients by using a rapid diagnostic test at the nursery school (weeks 10-14: March 4-April 7). Furthermore, the total cumulative number of FaSS patients was assessed each week.
To compare the characteristics of vaccinees and nonvaccinees, the chi-square test or Fisher's exact test, and the Wilcoxon rank-sum test were employed. Logistic regression models were used to calculate the odds ratios (OR) and 95% confidence interval (CI) of vaccination for outcome indicators 1 to 6. In multivariate analysis, age in months and gender were put into the model as variables, and other variables that were different between vaccinees and nonvaccinees with P values of less than 0.1 were added to the model. All reported P values were 2-sided values of 5%. All data analyses were carried out using SAS Version 9.3 (SAS Institute, Inc, Cary, North Carolina).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
I would like to express my gratitude to Dr. M. Kim Barrymore for her professional and critical reading. Finally, special thanks to the late Ms. Sayori Sonoda, who made a great contribution to this study.
Funding
This study was supported by a research grant for emerging and re-emerging infectious diseases from the Ministry of Health, Labor and Welfare of Japan (Grant H17-SHIKO-010).
References
- Poehling KA, Edwards KM, Weinberg GA, Szilagyi P, Staat MA, Iwane MK, Bridges CB, Grijalva CG, Zhu Y, Bernstein DI, et al. The underrecognized burden of influenza in young children. N Engl J Med 2006; 355:31-40; PMID:16822994; http://dx.doi.org/10.1056/NEJMoa054869
- Fu C, He Q, Li Z, Xu J, Li Y, Lu J, Li K, Yang Q, Dong Z, Liu X, et al. Seasonal influenza vaccine effectiveness among children, 2010-2012. Influenza Other Respi Viruses 2013; 7(6):1168-74; PMID:23981250
- Shen S, Campitelli MA, Calzavara A, Guttmann A, Kwong JC. Seasonal influenza vaccine effectiveness in pre- and full-term children aged 6-23 months over multiple seasons. Vaccine 2013; 31:2974-8; PMID:23688524; http://dx.doi.org/10.1016/j.vaccine.2013.05.011
- Staat MA, Griffin MR, Donauer S, Edwards KM, Szilagyi PG, Weinberg GA, Hall CB, Prill MM, Chaves SS, Bridges CB, et al. Vaccine effectiveness for laboratory-confirmed influenza in children 6-59 months of age, 2005-2007. Vaccine 2011; 29:9005-11; PMID:21945256; http://dx.doi.org/10.1016/j.vaccine.2011.09.037
- Eisenberg KW, Szilagyi PG, Fairbrother G, Griffin MR, Staat M, Shone LP, Weinberg GA, Hall CB, Poehling KA, Edwards KM, et al. Vaccine effectiveness against laboratory-confirmed influenza in children 6 to 59 months of age during the 2003-2004 and 2004-2005 influenza seasons. Pediatrics 2008; 122:911-9; PMID:18977968; http://dx.doi.org/10.1542/peds.2007-3304
- Allison MA, Daley MF, Crane LA, Barrow J, Beaty BL, Allred N, Berman S, Kempe A. Influenza vaccine effectiveness in healthy 6- to 21-month-old children during the 2003-2004 season. J Pediatr 2006; 149:755-62; PMID:17137887; http://dx.doi.org/10.1016/j.jpeds.2006.06.036
- Fujieda M, Maeda A, Kondo K, Kaji M, Hirota Y. Inactivated influenza vaccine effectiveness in children under 6 years of age during the 2002-2003 season. Vaccine 2006; 24:957-63; PMID:16171903; http://dx.doi.org/10.1016/j.vaccine.2005.08.083
- Shuler CM, Iwamoto M, Bridges CB, Marin M, Neeman R, Gargiullo P, Yoder TA, Keyserling HL, Terebuh PD. Vaccine effectiveness against medically attended, laboratory-confirmed influenza among children aged 6 to 59 months, 2003-2004. Pediatrics 2007; 119:E587-E95; PMID:17332179; http://dx.doi.org/10.1542/peds.2006-1878
- Hurwitz ES, Haber M, Chang A, Shope T, Teo ST, Giesick JS, Ginsberg MM, Cox NJ. Studies of the 1996-1997 inactivated influenza vaccine among children attending day care: immunologic response, protection against infection, and clinical effectiveness. J Infect Dis 2000; 182:1218-21; PMID:10979921; http://dx.doi.org/10.1086/315820
- Vesikari T, Fleming DM, Aristegui JF, Vertruyen A, Ashkenazi S, Rappaport R, Skinner J, Saville MK, Gruber WC, Forrest BD. Safety, efficacy, and effectiveness of cold-adapted influenza vaccine-trivalent against community-acquired, culture-confirmed influenza in young children attending day care. Pediatrics 2006; 118:2298-312; PMID:17142512; http://dx.doi.org/10.1542/peds.2006-0725
- Monto AS. Occurrence of respiratory virus: time, place and person. Pediatr Infect Dis J 2004; 23:S58-64; PMID:14730271; http://dx.doi.org/10.1097/01.inf.0000108193.91607.34
- Anderson LJ, Parker RA, Strikas RL. Association between respiratory syncytial virus outbreaks and lower respiratory tract deaths of infants and young children. J Infect Dis 1990; 161:640-6; PMID:2319164; http://dx.doi.org/10.1093/infdis/161.4.640
- Sugaya N, Mitamura K, Nirasawa M, Takahashi K. The impact of winter epidemics of influenza and respiratory syncytial virus on paediatric admissions to an urban general hospital. J Med Virol 2000; 60:102-6; PMID:10568771; http://dx.doi.org/10.1002/(SICI)1096-9071(200001)60:1%3c102::AID-JMV17%3e3.0.CO;2-D
- Kaneko M, Watanabe J, Kuwahara M, Ueno E, Hida M, Kinoshita A, Sone T. Impact of respiratory syncytial virus infection as a cause of lower respiratory tract infection in children younger than 3 years of age in Japan. J Infect 2002; 44:240-3; PMID:12099731; http://dx.doi.org/10.1053/jinf.2002.0981
- Hall CB, Geiman JM, Biggar R, Kotok DI, Hogan PM, Douglas GR. Respiratory syncytial virus infections within families. N Engl J Med 1976; 294:414-9; PMID:173995; http://dx.doi.org/10.1056/NEJM197602192940803
- Aymard M, Chomel JJ, Allard JP, Thouvenot D, Honegger D, Floret D, Boissel JP, Collet JP, Dürr F, Gillet J, et al. Epidemiology of viral infections and evaluation of the potential benefit of OM-85 BV on the virologic status of children attending day-care centers. Respiration 1994; 61 Suppl 1:24-31; PMID:7800968; http://dx.doi.org/10.1159/000196377
- Harrington RD, Hooton TM, Hackman RC, Storch GA, Osborne B, Gleaves CA, Benson A, Meyers JD. An outbreak of respiratory syncytial virus in a bone marrow transplant center. J Infect Dis 1992; 165:987-93; PMID:1583345; http://dx.doi.org/10.1093/infdis/165.6.987
- Kohno H. [Observation over three seasons of respiratory syncytial virus infection in a geriatric health service facility]. Nihon Ronen Igakkai Zasshi 2012; 49:608-11. Japanese; PMID:23459652; http://dx.doi.org/10.3143/geriatrics.49.608
- Mizuta K, Abiko C, Aoki Y, Ikeda T, Matsuzaki Y, Itagaki T, Katsushima F, Katsushima Y, Noda M, Kimura H, Ahiko T. Seasonal patterns of respiratory syncytial virus, influenza A virus, human metapneumovirus, and parainfluenza virus type 3 infections on the basis of virus isolation data between 2004 and 2011 in Yamagata, Japan. Jpn J Infect Dis 2013; 66:140–5.
- de-Paris F, Beck C, Machado AB, Paiva RM, da Silva Menezes D, de Souza Nunes L, Kuchenbecker R, Barth AL. Optimization of one-step duplex real-time RT-PCR for detection of influenza and respiratory syncytial virus in nasopharyngeal aspirates. J Virol Methods 2012; 186:189-92; PMID:22796284; http://dx.doi.org/10.1016/j.jviromet.2012.07.008
- Karadag-Oncel E, Ciblak MA, Ozsurekci Y, Badur S, Ceyhan M. Viral etiology of influenza-like illnesses during the influenza season between December 2011 and April 2012. J Med Virol 2014; 86: 865-71; PMID:24105787; http://dx.doi.org/10.1002/jmv.23747
- Zimmerman RK, Rinaldo CR, Nowalk MP, Gk B, Thompson MG, Moehling KK, Bullotta A, Wisniewski S. Influenza and other respiratory virus infections in outpatients with medically attended acute respiratory infection during the 2011-12 influenza season. Influenza Other Respir Viruses 2014; 8: 397-405; PMID:24852890; http://dx.doi.org/10.1111/irv.12247
- Borchers AT, Chang C, Gershwin ME, Gershwin LJ. Respiratory syncytial virus–a comprehensive review. Clin Rev Allergy Immunol 2013; 45:331-79; PMID:23575961; http://dx.doi.org/10.1007/s12016-013-8368-9
- Ochiai H, Fujieda M, Ohfuji S, Fukushima W, Kondo K, Maeda A, Nakano T, Kamiya H, Hirota Y; Influenza Vaccine Epidemiology Study Group. Inactivated influenza vaccine effectiveness against influenza-like illness among young children in Japan—with special reference to minimizing outcome misclassification. Vaccine 2009; 27:7031-5; PMID:19786133; http://dx.doi.org/10.1016/j.vaccine.2009.09.067
- Manzoli L, Ioannidis JP, Flacco M, De Vito C, Villari P. Effectiveness and harms of seasonal and pandemic influenza vaccines in children, adults and elderly: a critical review and re-analysis of 15 meta-analyses. Hum Vaccin Immunother 2012; 8: 851-62; PMID:22777099; http://dx.doi.org/10.4161/hv.19917
- Hirota Y, Kaji M. [Principles and methods of influenza epidemiology: with special reference to field evaluation of vaccine efficacy]. Kansenshogaku Zasshi 1994; 68:1293-305. Japanese; PMID:7829897; http://dx.doi.org/10.11150/kansenshogakuzasshi1970.68.1293
- Hirota Y, Kaji M, Ide S, Kajiwara J, Kataoka K, Goto S, Oka T. Antibody efficacy as a keen index to evaluate influenza vaccine effectiveness. Vaccine 1997; 15:962-7; PMID:9261942; http://dx.doi.org/10.1016/S0264-410X(96)00302-7
- Ozasa K. The effect of misclassification on evaluating the effectiveness of influenza vaccines. Vaccine 2008; 26:6462-5; PMID:18573297; http://dx.doi.org/10.1016/j.vaccine.2008.06.039
