?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Japanese encephalitis virus (JEV), a leading cause of Japanese encephalitis (JE) in children and adults, is a major public health problem in Asian countries. This study reports a meta-analysis of the immunogenicity and safety of vaccines used to protect infants or children from JE. Three types of JE vaccine were examined, namely, Japanese encephalitis live-attenuated vaccine (JEV-L), Japanese encephalitis inactivated vaccine (Vero cell) (JEV-I(Vero)), and Japanese encephalitis inactivated vaccine (primary hamster kidney cell) (JEV-I(PHK)). These vaccines are used to induce fundamental immunity against JE; however, few studies have compared their immunogenicity and safety in infants and young children less than 2 years of age. Data were obtained by searching 5 databases: Web of Science, PubMed, China National Knowledge Infrastructure, the China Wanfang database, and the Cochrane database. Fifteen articles were identified and scored using the Jadad score for inclusion in the meta-analysis. Random effect models were used to calculate the pooled seroconversion rate and adverse reaction rate when tests for heterogeneity were significant. The results showed that the pooled seroconversion rate for JEV-I(PHK) (62.23%) was lower than that for JEV-I(Vero) (86.49%) and JEV-L (83.52%), and that the pooled adverse reaction rate for JEV-L (18.09%) was higher than that for JEV-I(PHK) (10.08%) and JEV-I(Vero) (12.49%). The pooled relative risk was then calculated to compare the seroconversion and adverse reaction rates. The results showed that JEV-I(Vero) and JEV-L were more suitable than JEV-I(PHK) for inducing fundamental immunity to JE in infants and children less than 2 years of age.
Abbreviations:
- JE, Japanese encephalitis
- JEV-I, Japanese encephalitis inactivated vaccine
- JEV-L, Japanese encephalitis live-attenuated vaccine
- JEV-I(Vero), Japanese encephalitis inactivated vaccine (Vero cell)
- JEV-I(PHK), Japanese encephalitis inactivated vaccine (Primary Hamster Kidney cell)
- JEV-PIV, Japanese encephalitis purified inactivated vaccine (Vero cell-derived SA14-14-2 strain)
- JE-SV, JE subunit vaccine
- RCT, randomized controlled trial
- RR, relative risk
Introduction
Japanese encephalitis (JE) is a blood-borne disease of the central nervous system. The causative agent is the JE virus (JEV), which was first identified in Japan in 1934. Like most flaviviruses, JEV is transmitted by mosquitoes.Citation1 Today, JE is still a major public health problem in most Asian regions, particularly southeast Asia, with more than 50,000 cases reported annually.Citation2People of all ages are susceptible to infection by JEV, but most cases occur in children under the age of 15 years.Citation3Severe cases have an acute onset, with a high fever, disturbance of consciousness, convulsions, spasms, and meningeal irritation, which may have sequelae.Citation4The identification of JEV as the causative agent led to the development of vaccines to prevent infection and epidemics.
A mouse brain-derived vaccine based on the Nakayama strain of JEV was first developed in Japan before the 1930s.Citation5 This vaccine is now produced in a number of countries, including Japan,Citation6 and India.Citation7 Prior to 2003, the commercial Nakayama vaccine was widely distributed for use against JEV (WHO 2003);Citation8 however, reports of systemic and neurological adverse effects raised concerns about the safety of the commercially available vaccine. Japan stopped using the Nakayama vaccine in May 2005 and replaced it with a vaccine candidate based on the Beijing-1 strain (derived from Vero cell cultures), which is considered to be a safer and more effective vaccine against JEV.Citation8 At present, China uses 3 commercial vaccines: a live-attenuated vaccine (JEV-L) and 2 inactivated vaccines based on primary hamster kidney cells (JEV-I(PHK))Citation9and Vero cells (JEV-I(Vero)).
The aim of this study was to conduct a meta-analysis to compare the immunogenicity and safety of JEV-I (PHK), JEV-I (Vero), and JEV-L, and to quantitatively estimate their immunogenicity and safety in infants and children less than 2 years of age. In this study, JEV-I(PHK) is an inactivated vaccine cultivated from PHK cells using the Beijing-1 strain. JEV-L is a live-attenuated vaccine cultivated from PHK cells using S14-14-2 strain. JEV-I(Vero) was an inactivated vaccine cultivated from Vero cells using Beijing-1, S14-14-2 and Beijing-3 strain. Three strains mentioned above have 3 different serotypes that are JaGAr, Nakayama and Mie. Three serotypes have much cross reaction so that these 3 serotypes can be considered as one serotype. Therefore, we will pay more attention to the immunity and safety effects of vaccines cultivated from different cells, between live attenuated vaccine and inactivated vaccines rather than the immunity and safety effects of vaccines derived from different strains.Citation10 In this study, we considered the seroconversion rate as measurable variable for immunogenicity and the adverse rate for safety.
Results
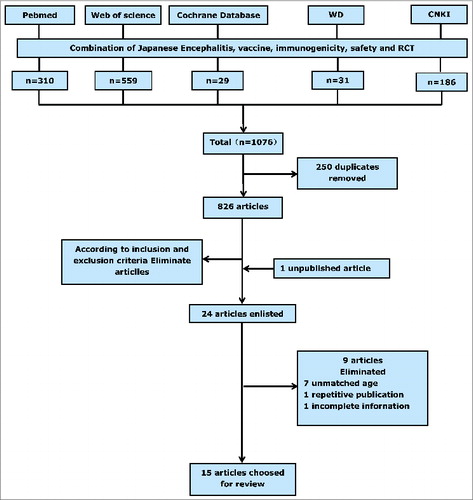
Fourteen papersCitation11-24 that met our inclusion criteria were identified by searching the China National Knowledge Infrastructure, the Wanfang database, PubMed, Web of Science, and the Cochrane database. Several experts that we contacted also drew our attention to one unpublished article,Citation25 and thus 15 articles were included in the meta-analysis. summarizes the screening process and lists the characteristics of the studies included. In all, we identified 8 trials that evaluated the JEV-I(PHK) vaccine,Citation11,14,15,18,21-23,25 5 trials that evaluated the JEV-I(Vero) vaccine,Citation18,19,22,24,25 and 11 trials that evaluated the JEV-L vaccine.Citation11-13,16-21,23,24 All participants were healthy infants or children between 8-months-oldand 2-years-old. Subjects vaccinate one dose for live-attenuated vaccine and are taken blood after 28 days. Subjects vaccinate 2 doses for inactivated vaccine and also are taken blood after 28 days. There is a 7–10 day interval between the 2 doses of inactivated vaccine. All trials apart from 2Citation14,15 were randomized controlled trials (RCT). We calculated the Jadad score for each paper and found that 33% papers scored >3 (range, 0 to 7), leading to them being categorized as “high quality” (). Begg's rank correlation testCitation26 did not identify publication bias among the studies used to pool the seroconversion rate or among the studies used to conduct pairwise comparisons between different vaccines; however, the studies used to pool adverse reaction rates for JEV-I(PHK) did show publication bias (Z = 2.20, p = 0.027).
Table 1. Characteristics of the 3 Japanese Encephalitis vaccines included in the analysis
Immunogenicity of the 3 vaccines
The pooled seroconversion rates for the JEV-I(PHK), JEV-I(Vero), and JEV-L vaccines were calculated by extracting the information from 8,Citation11,14,15,18,21-23,25 5Citation18,19,22,24,25 and 11Citation11-13,16-21,23,24 papers, respectively. Tests for heterogeneity were significant (chi-squared = 380.88, df = 7, P < 0.0001; chi-square = 92.60, df = 4, P < 0.0001; and chi-squared = 156.15, df = 11, P < 0.0001, respectively). Therefore, a random effects modelCitation27 was used for meta-analysis. The results showed that JEV-I(Vero) had the highest pooled seroconversion rate (86.49%), followed by JEV-L (83.52%), and JEV-I(PHK) (62.23%) ().
Table 2. Pooled analysis of seroconversion rates and adverse reaction rates
We next performed a post-hoc multiple comparison of seroconversion rates among the 3 vaccines. Studies by Guo et al.,Citation18 Yin et al.Citation22 and Zhu et al.Citation25 were used to compare the seroconversion rate between JEV-I(PHK) and JEV-I(Vero). The test for heterogeneity was again significant (chi-square = 41.11, df = 2, P < 0.0001), so a random effects model was used to calculate the pooled RR. The result (1.36, 95% CI: 1.21–1.53) showed that the seroconversion rate for JEV-I(Vero) was 1.36 times higher than that for JEV-I(PHK) (). Studies conducted by Zhang et al.,Citation23 Bai et al.,Citation11 Yang et al.Citation21 and Guo et al.Citation18 were used to compare the seroconversion rate between JEV-I(PHK) and JEV-L. In this case, the test for heterogeneity was not significant (chi-squared = 0.28, df = 3, p = 0.963); therefore, a fixed effects model was used to calculate the pooled RR. The result (1.38, 95% CI: 1.23–1.56) showed that the seroconversion rate for JEV-L was 1.38 times higher than that for JEV-I(PHK) (). Studies conducted by Zhang et al.,Citation24 Li et al.Citation19 and Guo et al.Citation18 were used to compare the seroconversion rate between JEV-I(Vero) and JEV-L. The test for heterogeneity was significant (chi-square = 37.34, df = 2, P < 0.0001), therefore a random effects model was used to calculate the pooled RR. The result (1.00, 95% CI: 0.90–1.12) showed that there was no difference in the seroconversion rate between JEV-I(Vero) and JEV-L ().
Table 3. Comparison of seroconversion and adverse events among JEV-I(Vero), JEV-I(PHK), and JEV-L
Safety of the 3 vaccines
The pooled adverse reaction rates for the JEV-I(PHK), JEV-I(Vero), and JEV-L vaccines were calculated by extracting the information from 5,Citation18,21-23,25 5,Citation11,18,19,22,24,25 and 6Citation17,19-21,23,24 papers, respectively. Tests for heterogeneity were significant (chi-squared = 42.29, df = 4, P < 0.0001; chi-square = 30.64, df = 4, P < 0.0001; and chi-squared = 1,407.70, df = 5 P < 0.0001, respectively). Therefore, a random effects model was used to perform meta-analysis. The results showed that JEV-PHK had the lowest pooled adverse reaction rate (10.08%), followed by JEV-I (Vero) (12.49%) and JEV-L (18.09%) ().
Post-hoc multiple comparisons of pooled adverse reaction rates for the 3 vaccines were then conducted. Studies by Guo et al.,Citation18 Yin et al.Citation22 and Zhu et al.Citation25 were used to compare the adverse reaction rate between JEV-I(PHK) and JEV-I(Vero). The test for heterogeneity was significant (chi-square = 20.09, df = 2, P < 0.0001), so a random effects model was used to calculate the pooled RR. The result (2.32, 95% CI: 1.43–3.76) showed that there was a difference in adverse reaction rate between JEV-I(PHK) and JEV-I(Vero). The rate for JEV-I(Vero) was 2.32 times higher than that for JEV-I(PHK) (). Studies conducted by Zhang et al.,Citation23 Yang et al.Citation21 and Guo et al.Citation18 were used to compare adverse reaction rates between JEV-I(PHK) and JEV-L. The test for heterogeneity was significant (chi-squared = 19.71, df = 3, P < 0.0001), so a random effects model was used to calculate the pooled RR. The result (1.21, 95% CI: 0.67–2.17) showed there was no difference in the adverse reaction rate between JEV-I(PHK) and JEV-L (). Studies conducted by Zhang et al.,Citation24 Li et al.Citation19 and Guo et al.Citation18 were used to compare adverse reaction rates between JEV-I(Vero) and JEV-L. The test for heterogeneity was not significant (chi-square = 3.26, df = 2, p = 0.196), so a fixed effects model was used to calculate the pooled RR. The result (74, 95% CI: 0.49–1.12) showed that there was no difference in the adverse reaction rate between JEV-I(Vero) and JEV-L ().
Sensitivity analysis
A sensitivity analysis was conducted for the seroconversion rates of the 3 vaccines using the Jack-knife method.Citation28 Studies were assigned zero weight to determine whether their inclusion in the meta-analysis affected the overall results obviously. We found that excluding each of the studies from the analysis one-by-one had no significant effect on the overall result. The pooled seroconversion rates for JEV-I(PHK), JEV-I(Vero), and JEV-L were 62.23%, 86.49%, and 83.52%, respectively, and the change ranges were 55.71–65.42%, 82.90–90.17%, and 83.46–87.13%, respectively. Sensitivity analysis of the adverse reaction rates for JEV-I(PHK), JEV-L also showed no obvious change. The pooled adverse reaction rates for JEV-I (PHK), JEV-I (Vero), were 10.08%, 12.49%, respectively, and the change ranges were 6.24–11.68%, 10.24–14.81%, respectively. Pooled adverse reactions rate of JEV-L was 18.09%, When the article by FeroldiCitation17 was excluded, the pooled adverse reaction rate was 8.34%. The adverse reaction rate reported by Feroldi was 66.5%, which is much higher than that reported by other Chinese studies. But several recently published studies described a high level of AEs such as Gatchalian et al.,Citation29 Kaltenbock et al.,Citation30 Schuller et al.Citation31 Therefore, Feroldi's high AE is not the only case. In fact, different researchers obtain AEs by different methods including telephone interview, live interview, filling in the AE card and non-solicited AE report, which causes the large deviations of experimental results. As a consequence, we cannot consider Feroldi's results unreasonable, and these large deviations of AEs rates may become a limitation of our study.
Discussion
The present study performed a meta-analysis of the effectiveness and safety of 3 JEV vaccines widely used in Asia-Pacific to protect infants and children against JE. The use of JEV-I(PHK) and JEV-L (which is also derived from PHK) has been questioned because some scholars believe that vaccines produced by PHK cells contain heterogeneous cellular matrix proteins that sensitize individuals against the vaccine.Citation32 Although JEV-L was pre-approved by the WHO,Citation33 JEV-L and JEV-I(PHK) are rarely used in countries apart from China. During the last 10 years, JEV-I(Vero) has become a popular candidate for JE vaccine studies; however, the study subjects are often adults. Few studies have examined JEV-I in infants or children outside China. The subjects included in the present study were infants and children under 2 years old that are at high risk of contracting JEV. Here, we examined the immunogenicity and safety of these 3 vaccines for use in infants and children with the aim of providing guidance regarding future clinical practice.
Meta-analysis is a statistical method of systematic review, which aims to examine data from a series of studies and pool the different independent effects to identify an overall effect; thus meta-analysis is widely used in the field of clinical vaccine trials studies.Citation34 The outcome of a meta-analysis is mainly used to evaluate/compare vaccine efficacy, immunogenicity, adverse reactions, adjuvant effects, costs-benefits, and delivery mechanisms; however, few meta-analyses of JE B vaccines have been undertaken. After searching multiple databases, we identified only one meta-analysis of a JE B vaccine,Citation9 in which the author pooled the effectiveness, immunogenicity, and adverse reaction rates of 2 available and 3 pre-licensed vaccines. However, the present study focused on vaccines widely used to induce fundamental immunity against JEV in infants and young children (the population most at risk of JEV infection in China and neighboring countries).
The results showed that the pooled seroconversion rate for JEV-I(PHK) was much lower than that for JEV-I(Vero) and JEV-L. In general, humans generate stronger cellular, humoral, and mucosal immune responses against live-attenuated vaccines because the virus maintains its immunogenic characteristics (although the pathogenic genes are attenuated through gene mutation). However, chemically inactivated vaccines induce stronger humoral immune responses and thus it is easy to see why JEV-L's has a higher seroconversion rate than JEV-I(PHK). However, we found that JEV-I(Vero) showed a seroconversion rate similar to that of JEV-L. We used a study conducted by Guo et al. Error! Hyperlink reference not valid. to compare the seroconversion rates of JEV-L and JEV-I(Vero) and found that the seroconversion rate of JEV-I(Vero) was higher than that of JEV-L. This result is different from that reported by Zhang et al, Error! Hyperlink reference not valid. who found that the seroconversion rate of JEV-L was higher than that of JEV-I(Vero). However, Li et al.Citation19 reported that the seroconversion rate of JEV-I (Vero) was similar to that of JEV-L. The sample sizes in these 3 studies were 285, 193, and 61, respectively. Based on a single study, the results were unstable, this study pooled several researches and the sample size increased to 639. The results showed that there was no significant difference between JEV-I(Vero) and JEV-L in terms of seroconversion rate. Therefore, more data are needed to effectively compare these 2 vaccines. The pooled adverse reaction rate for JEV-L was much higher than that for JEV-I(Vero) and JEV-I(PHK); however, this conclusion may be biased because the sensitivity analysis showed that the data generated by FeroldiCitation17 had a major influence on the adverse reaction rates for the live-attenuated vaccine. After excluding Feroldi's data, the adverse reaction rate for JEV-L was only 8.34% (95% CI: 5.38–11.18%), which is no different from that of JEV-I(Vero) and JEV-I(PHK). From the conservation perspective, although the pooled adverse reaction rate was 18.09% (higher than that of the other 2 vaccines), it is still relatively low. As a consequence, all 3 vaccines show an acceptable safety profile.
Taken together, the results show that although the safety profile of JEV-I(PHK) is quite good, its immunogenicity is not; however, both JEV-I(Vero) and JEV-L had an acceptable safety profile and good immunogenicity. The disadvantage of JEV-I(Vero) is that 2 doses are required whereas the live-attenuated vaccine requires only one, which means that live-attenuated vaccines are more cost-effective. However, there are potential safety issues with live-attenuated vaccines due to the risk of viral gene mutation. Therefore, avoiding epidemic seasons is an important premise when using live-attenuated vaccines.
In conclusion, JEV-I(Vero) and JEV-L appear to be good choices for inducing fundamental immunity of infants and children. When determining the vaccines to be used, both cost and epidemic factors (such as the season) need to be considered.
A limitation of this study is that In this study, when we compared vaccines using post-hoc multiple comparison method and obtained the pooled RR value, the studies were required to include both specific vaccines for comparison. Because of the small amount of studies used for post-hoc multiple comparison, the results of publication bias may have little practical significance. In this study, 11 studies adopted plague reduction neutralization testCitation13-19,22-25 and other 4 studiesCitation11,12,20,21 did not mention the methods for antibody measurement. Then, another limitation is that this study is not based on the same laboratory methods and criteria of evaluating results, which would lead the heterogeneity of seroconversion rate. In addition, serocoversion rate was used as the only variable to evaluate the immunogenicity, which would lead partial results of immunogenicity characteristics. In fact, This study only include 3 RCT researches conducted outside China, which may affect the generalizability of our findings. A search of different bibliographic databases led to the inclusion of 13 RCT studies; others were excluded due to different study subjects or lack of detailed data. Few other countries use these 3 JEV vaccines for fundamental immunity. JEV-MB was once a commonly used JEV in Asia-Pacific area;Citation35 however, JEV-MB is banned in Japan due to its poor safety profile since 2005,Citation8 Japan, Australia, and the USA have developed new JE vaccines, including a JE subunit vaccine (JE-SV) which takes advantage of JE virus's superficial component (antigen) without nucleic acid for inducing antibody, and a JE purified inactivated vaccine (Vero cell-derived SA14-14-2 strain; JEV-PIV).Citation35 But few studies have examined these new JE vaccines for fundamental immunity of infant and young children between 8-months-old and 2-years-old and, therefore, they were not included in the present analysis; this is the major reason for the lack of non-China studies included in the meta-analysis. Because the included studies have limited region range, the conclusion of this study may not reflect the situation of other regions.
Materials and Methods
Literature search
The search strategy used involved “exploding” the terms “Japanese encephalitis,” “Immunogenicity,” “Vaccine,” “Safety” and “RCT” in the CNKI, WD, PubMed, Web Science, and Cochrane Databases. No restrictions were placed on language or year of publication. To ensure that the search was as complete as possible, 2 leading researchers on JE vaccination were asked to provide details of unpublished or ongoing studies.
Inclusion and exclusion criteria
Studies were included if (1) they used JEV-L and/or JEV-I(Vero) and/or JEV-I(PHK) to induce fundamental immunity; (2) the study subjects comprised healthy infants and children under 2 years old; (3) the studies were RCT or partial observational studies; and (4) papers provided the seroconversion and/or adverse reaction rates. Studies were excluded if they (1) involved experimentation on animals; (2) studied vaccine technologies such as genetic studies or vaccine purification studies; (3) studied the effects of combined vaccines; and (4) studied special populations, such as children with HIV/AIDS children. All identified studies were reviewed independently for eligibility by 2 authors.
Validity assessment
“Quality” was defined as the confidence that the study design, conduct, analysis, and presentation limited the biased comparisons of the intervention(s) under consideration, which was assessed using the Jadad score.Citation36 The Jadad score asks yes/no questions regarding randomization, double-blinding, dropout and withdrawal rates, random number generation, and allocation concealment. _ENREF_11A pre-piloted validity assessment formCitation37 was used to categorize trials as “high quality” (scoring more than 3 out of a maximum 7) or “low quality” (scoring 2 or less out of a maximum 7). These assignments were made before the start of the study.
Data extraction
Two authors independently extracted data from all included studies, including outcome measures, inclusion and exclusion criteria, study design and population, laboratory examination methods, vaccine type and dose, study location, and the adverse reaction rate. Any disagreements or discrepancies were resolved by discussion or by consulting a third reviewer.
Statistical analysis
Stata, Version 12 (Stata Corporation, College Station, Austin, TX) was used for analysis. Begg's test was used to examine whether there was any publication bias._ENREF_29Citation38Heterogeneity was assessed using a chi-square-based Q statistic. A p-value of ≤ 0.05 was considered statistically significant. If there was significant heterogeneity, outcome data from the trials were analyzed using a random effects modelCitation39 to estimate the pooled RR. In the absence of heterogeneity, meta-analysis was performed using a fixed effects model.Citation40 In post-hoc multiple comparison of 3 types of vaccines, we used Bonfferoni method to adjust α value which was 0.0167, according to following formula:
Where n is comparing times.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Important National Science & Technology Specific Projects (Grant Number 2011ZX 1004–902).
References
- Wang YL, Zhun QX, Lin GW, Yu YY, Chen ZL. Immunogenicity of live attenuated Japanese encephalitis vaccine in Xian Ju. Mod Pre Med 2008; 154
- Kaltenbock A, Dubischar-Kastner K, Schuller E, Datla M, Klade CS, Kishore TSA. Immunogenicity and safety of IXIARO (IC51) in a Phase II study in healthy Indian children between 1 and 3 years of age. Vaccine 2010; 834–9; PMID:19857447; http://dx.doi.org/10.1016/j.vaccine.2009.10.024
- Wang XX, Li YX, Yin ZD, Li JH, Ning GJ, Liang XF. Development on epidemiological charateristics and residual sequelae of Japanese encephalitis. Chinese J Vaccin Immun 2008:176–9
- Zhang YP, Feng ZJ, CK.Lee. Japanese encephalitis prevention strategies in China. HenJ Prevent Med 2006:193–5
- Jing C. Review of advances in Japanese encephalitis virus vaccines. J Path Biol 2010:375–7
- Miyazaki C, Okada K, Ozaki T, Hirose M, Iribe K, Yokote H, Ishikawa Y, Togashi T, Ueda K. Phase III clinical trials comparing the immunogenicity and safety of the vero cell-derived Japanese encephalitis vaccine Encevac with those of mouse brain-derived vaccine by using the Beijing-1 strain. Clin Vaccine Immunol 2014; 21:188–95; PMID:24334689; http://dx.doi.org/10.1128/CVI.00377-13
- Gowal D, Tahlan AK. Evaluation of effectiveness of mouse brain inactivated Japanese encephalitis vaccine produced in India. Indian J Med Res 1995; 102:267–71; PMID:8557319
- Schioler KL, Samuel M, Wai KL. Vaccines for preventing Japanese encephalitis. Cochr Data Sys Rev 2007; PMID:17636750
- Yin ZD, Luo HM, Li YX. Meta-analysis on serological effect of the primary vaccination for Japanese encephalitis of the literatures. Chinese J Vaccin Immun 2009; 15:501–6; PMID:20518324
- Japanese encephalitis vaccine. Weekly Epidemiological Record 2006:331–340.
- http://www.who.int/immunization/Japanese_encephalitis_Chinese.pdf
- Bai ZY, Zhao GF, Liu J. The antibody response to the basic immunization of two forms of Japanese B encephalitis vaccine. Chinese J Vaccin Immun 2000:16–7
- Chen HY, Zhang YF, Ma FB, Zhang JL, Diao LD, Zhang GF, Sun JS, Bai ZY. Observation of immune efficacy of the be attenuated Japanese encephalitis vaccine. Chinese J Vaccin Immun 1999:215–6
- Chokephaibulkit K, Sirivichayakul C, Thisyakorn U, Sabchareon A, Pancharoen C, Bouckenooghe A, Gailhardou S, Boaz M, Feroldi E. Safety and immunogenicity of a single administration of live-attenuated Japanese encephalitis vaccine in previously primed 2- to 5-year-olds and naive 12- to 24-month-olds: multicenter randomized controlled trial. Pediatr Infect Dis J 2010; 29:1111–7; PMID:20856164; http://dx.doi.org/10.1097/INF.0b013e3181f68e9c
- Dai H, Chen HP, Hua RZ, Guo Xl, Zhang QD, Guo SH, Zhang YF, Dong CM, Wang MR, Diao LD, et al. Three-dose, inactivated Japanese encephalitis vaccine immunogenicity and safety trial. China Pub Health 1994:195–6; (in Chinese)
- Dai H, Zhang YF, Diao LD, Feng BX, Xu AL, Guo JT. Immunogenicity of inactivated Japanese encephalitis vaccine (JEV-I(PHK)) in children China public health. 1997; 61 (in Chinese); PMID:9252251
- Feroldi E, Pancharoen C, Kosalaraksa P, Chokephaibulkit K, Boaz M, Meric C, Hutagalung Y, Bouckenooghe A. Primary immunization of infants and toddlers in Thailand with Japanese encephalitis chimeric virus vaccine in comparison with SA14-14-2: A randomized study of immunogenicity and safety. Pediatr Infect Dis J 2014; 33:643–9; PMID:24717964; http://dx.doi.org/10.1097/INF.0000000000000276
- Feroldi E, Pancharoen C, Kosalaraksa P, Watanaveeradej V, Phirangkul K, Capeding MR, Boaz M, Gailhardou S, Bouckenooghe A. Single-dose, live-attenuated Japanese encephalitis vaccine in children aged 12-18 months: randomized, controlled phase 3 immunogenicity and safety trial. Hum Vaccin Immunother 2012; 8:929–37; PMID:22777096; http://dx.doi.org/10.4161/hv.20071
- Guo SH, Yin JM, Chen HP, Zhen L, Qian CH, Wang WQ, Dong CM, Ze WY. The immune effect and systemic reaction of the freeze-derived inactivated Japanese encephalitis vaccine made from vero cells in an epidemic area. Chinese J Vaccin Immun 2002:3–6
- Li WJ, Wang SQ, Wang ZY, Zhang EX, Xiao QY. Efficacy and safety evaluation of two kinds of Japanese encephalitis vaccine. Pract Prevent Med 2007:699–701
- Qu SW. Immunogenicity compare between double and three-dose Inactivated Japanese encephalitis vaccine Chinese. J Vaccin Immun 2000; 27 (in Chinese)
- Yang TL. Safety and immunogenicity of two types of Japanese encephalitis vaccine. Henan J Prevent Med 2005:328–9 (in Chinese)
- Yin JM, Qian CH, Xu GY, Sun GY, Zhang J, Gu H, Diao LD, Chen HP, Ze WY. Adverse reaction and immunological efficacy of primary immunization with different doses of inactivated freeze-derived vero cell Japanese encephalitis vaccine. Chinese J Vaccin Immun 2002:7–9
- Zhang HR, Wang LY, Chen LJ, Gao Jie, Xiao GQ, Huang Hui, Fan CY, Wang YM. Analysis on the immune effects and safety of the live attenuated and the inactivated Japanese encephalitis vaccines. Chinese J Vaccin Immun 2002:10–2
- Zhang ZH, Kang JQ, Liu JR. Observation of JE live attenuated vaccine and inactivated vaccine effects and human vaccination reaction. Ning Med J 2012:632–3
- ZHU FC. Inactivated Japanese encephalitis vaccine made from Vero cells: randomized, controlled phase II/III clinical trial 2008
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–101; PMID:7786990; http://dx.doi.org/10.2307/2533446
- DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: An update. Contemp Clin Trials 2007; 105–114; PMID:16807131; http://dx.doi.org/10.1016/j.cct.2006.04.004
- Lin HL. Jackknife Empirical Likelihood for the Variance in the Linear Regression Model. 2013.
- Gatchalian S, Yao Y, Zhou B, Zhang L, Yoksan S, Kelly K, Neuzil KM, Yaïch M, Jacobson J. Comparison of the immunogenicity and safety of measles vaccine administered alone or with live, attenuated Japanese encephalitis SA 14-14-2 vaccine in Philippine infants. Vaccine 2008; 2234–2241; PMID:18394765; http://dx.doi.org/10.1016/j.vaccine.2008.02.042
- Kaltenböck A, Dubischar-Kastner K, Eder G, Jilg W, Klade C, Kollaritsch H, Paulke-Korinek M, Sonnenburg FV, Spruth M, Tauber E, Wiedermann U, Schuller E. Safety and immunogenicity of concomitant vaccination with the cell-culture based Japanese Encephalitis vaccine IC51 and the hepatitis A vaccine HAVRIX→1440 in healthy subjects: A single-blind, randomized, controlled Phase 3 study. Vaccine 2009; 4483–4489; PMID:19486955; http://dx.doi.org/10.1016/j.vaccine.2009.05.034
- Schuller E, Klade CS, Wölfl G, Kaltenböck A, Dewasthaly S, Tauber E. Comparison of a single, high-dose vaccination regimen to the standard regimen for the investigational Japanese encephalitis vaccine, IC51: A randomized, observer-blind, controlled Phase 3 study. Vaccine 2009; 2188–2193; PMID:19200452; http://dx.doi.org/10.1016/j.vaccine.2008.12.062
- Xu CL, Tang QY. Detection of residual cell matrix in Primary Hamster Kidney cell inactivated Japanese encephalitis vaccine. Chinese J Biol 2001:48–50
- Newly accessible Japanese encephalitis vaccine will make saving children easier in developing countries. http://www.who.int/mediacentre/news/releases/2013/japanese_encephalitis_20131009/en/ 2013.
- Zhao N, Yu SZ. Meta-analysis: A new quantitative approach to research synthesis. Chinese J Prevent Cont Chr Non-Commun Dis 1993:277–80
- Solomon T. New vaccines for Japanese encephalitis. Lancet Neurol 2008; 7:116–8; PMID:18207104; http://dx.doi.org/10.1016/S1474-4422(08)70004-8
- Palys KE, Berger VW. A note on the Jadad score as an efficient tool for measuring trial quality. J Gastrointest Surg 2013; 17:1170–1; PMID:23233271; http://dx.doi.org/10.1007/s11605-012-2106-0
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P, Klassen TP. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998; 352:609–13; PMID:9746022; http://dx.doi.org/10.1016/S0140-6736(98)01085-X
- Wang D, Mou ZY, Zhai JX, Zong HX, Zhao XD. Study on STATA software in investigating publication bias in Meta-analysis. Modern Prevent Med 2008:2819–22
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–88; PMID:3802833; http://dx.doi.org/10.1016/0197-2456(86)90046-2
- Demets DL. Methods for combining randomized clinical trials: Strengths and limitations. Stat Med 1987; 6:341–50; PMID:3616287; http://dx.doi.org/10.1002/sim.4780060325