Abstract
Rotavirus is the most common cause of diarrhea leading to hospitalization in young children. Rotavirus vaccines are available in Canada but have not been introduced in all provinces. In a controlled trial, 2 study sites (Prince Edward Island and the Capital District Health Authority (District 9, Nova Scotia) introduced universal rotavirus vaccine programs for infants at 2 and 4 months of age beginning 1 December 2010, using public health nurse or general practitioner-delivery models, respectively. A third site (Saint John, NB) served as the non-intervention control setting. Vaccine coverage, rotavirus hospitalizations, intussusception and all-cause diarrhea were monitored. A universal rotavirus vaccine program with >90% coverage was associated with reductions in rotavirus-associated hospitalizations (from a peak of 52.8 hospitalizations/100,000 population to 0 hospitalizations) in infants < 12 months and 1 to < 2 y of age 12 months after program implementation. No apparent reduction occurred in the site with vaccine coverage of < 40%, or in the non-intervention control site. No cases of intussusception were associated with vaccine receipt, and no increase in all-cause diarrhea was observed. A universal infant rotavirus vaccine program with high coverage was associated with reductions in rotavirus and no safety signals; no reduction was observed in settings with low vaccine coverage.
Introduction
The introduction of universal infant rotavirus vaccine programs in the US, Australia, Europe, and over 10 South American countries has resulted in dramatic reductions in rotavirus-associated diarrhea and dehydration leading to hospitalization, medical visits and, especially in lower income settings, death.Citation1 In 2009 the World Health Organization recommended the inclusion of rotavirus vaccine in the national immunization programs of all countries.Citation2
In Canada rotavirus illness is associated with an average of 11hospitalizations per 1,000 children less than 5 y of age/year and causes the majority of childhood gastroenteritis resulting in hospitalization.Citation3,4 Rotavirus vaccines became available in Canada in 2006 (RotaTeq®) and 2007 (Rotarix™).Citation5 The National Advisory Committee on Immunization recommended routine immunization of Canadian children in 2010,Citation3 as did the Canadian Immunization Committee in 2014.Citation6
In this study, initiated in 2010 before any province had initiated a routine rotavirus vaccine program, we sought to determine if a universal infant rotavirus vaccine program implemented in 2 provinces would be associated with reductions in the burden of rotavirus as measured by hospitalization in children under 12 months of age. Further, we sought to evaluate the effectiveness of 2 different program delivery models (public health nurse-administered or physician office-administered), in comparison to a non-intervention control jurisdiction where no vaccine was provided.
Results
Vaccine coverage
Vaccine coverage in in year one was 93.4% for one dose and 91.2% for 2 doses in Site 1; and in year 2 it was 93.1% and 90.3% for dose one and 2 respectively. In Site 2, vaccine coverage in year one was 33.1% for dose one and 33.6% for 2 doses; and in year 2 it was 38.3% and 33% for dose one and 2 respectively. Details of vaccine uptake over time for each dose are reported elsewhere.Citation7
Rotavirus-associated hospitalizations
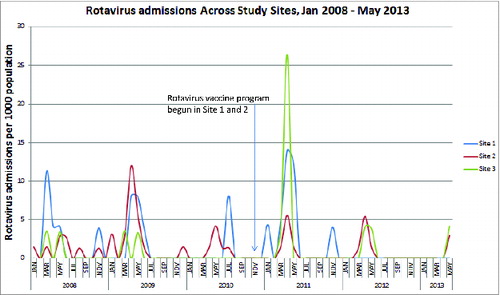
Rotavirus admissions in children less than 12 months of age per 100,000 children in that age group decreased in Site 1, with no further cases after November 2012 (, ). In children 12 months to less than 2 y of age there were no rotavirus admissions in 2012 or 2013 (). All children <12 months of age would have had an opportunity to be fully immunized by February 2012 and children < 2 y of age by February 2013; vaccine coverage was over 90%. In contrast, no trend in rotavirus hospitalizations/100,000/year in Site 2 (vaccine coverage < 40%) or the non-intervention comparator site was discernible. In Sites 2 and 3 rotavirus epidemics occurred each spring, with considerable year-to-year variation in attack rates.
Table 1. Rotavirus admissions in infants <12 months of age/100,000 population by year and by study site.
Table 2. Rotavirus admission in children one year of age to < 2 y of age/100,000 population by year and study site.
Adverse events following immunization
The frequency of intussusception did not increase after introduction of the rotavirus vaccine program. Among the 13 cases identified in Site 2 and 3 none had received rotavirus vaccine. No change in the incidence of all-cause diarrhea was observed (data not shown).
Discussion
In this controlled study comparing 2 methods of implementing a universal infant rotavirus vaccine program, the jurisdiction with high vaccine uptake in a public health nurse-delivered program had an elimination of rotavirus admissions in children less than 2 y of age beginning in the second year of the program, which was sustained for an additional 17 months of observation. By contrast, no appreciable difference in rotavirus admissions in infants under 12 months or those 12 to 24 months was observed in the jurisdiction without a program, or in the jurisdiction with a family physician delivered program. These data suggest that a rotavirus vaccine program with high population coverage is effective. The government of Site 1 subsequently continued a universal rotavirus vaccine program for infants after the project ended, based on the effect of the program on childhood rotavirus illness.Citation8
In Site 2, the jurisdiction with a universal program provided by family physicians, no detectable change in rotavirus admissions occurred. It is important to consider other reasons than poor vaccine coverage (<40 %) in this site for the absence of a change in rotavirus hospitalizations. Rotavirus vaccine effectiveness is lower in a low income setting compared with a high income setting,Citation1,9 ranging for example from 85 to 88 % effectiveness in the US and Australia to 51–76% in Asia, Africa and South America.Citation1 This would not explain the unchanged epidemiology of rotavirus hospitalizations in the study site with the coverage of ∼40% however, as these jurisdictions have comparable socioeconomic status and universal access to health care. Differences in circulating rotavirus strain antigens compared to vaccine antigens could theoretically reduce vaccine effectiveness. However, in a Canadian study stool samples from pediatric hospitals were tested for G (VP7) and P (VP4) genotypes, and all were well matched to Rotarix™ and Rotateq™ vaccines.Citation10
The most likely reason that no appreciable reduction in rotavirus admissions occurred at Site 2 is that an insufficient percentage of vaccine-eligible children were immunized in order to observe direct protection in children less than 12 months of age, or indirect protection (herd immunity) in children 12 months to less than 2 y of age. In a previous assessment of immunization coverage in the province where Site 2 is situated, the overall immunization completeness rate was 49% at 12 months, 40% at 18 months and 58% at 24 months of age.Citation11 It is thus not unexpected that the coverage rate for rotavirus vaccines was under 40% in this effectiveness study. As a federal country, vaccines in Canada are delivered by provinces, so immunization schedules and immunization delivery programs vary.Citation12 There is little study of the role of program delivery in the success of vaccine programs.
Routine vaccination of infants in the US with pentavalent rotavirus vaccine (RV5) began in February 2006, and by January 2008 vaccine coverage was 57% among children less than one year of ageCitation13 and 80% in infants up to 2 y of age in 2008–2009. In 2006–2007 the total number of rotavirus hospitalizations did not change, but in 2007–2008 the season was delayed in onset and the peak number of hospitalizations diminishedCitation14; post-licensure estimates of rotavirus vaccine effectiveness are similar to those in the pre-licensure efficacy trials in developed countries.Citation1,15 The ability of a vaccination program to control disease in the population depends on several factors, including vaccine coverage and the infectivity, or reproductive number (R0) of the infectious agent (i.e., number of secondary cases among susceptible individuals for each case). Rotavirus is highly infectious with a low infectious dose; epidemiologic analyses from 15 countries show R0 ranging from 23.3 to 191.Citation16 Clearly higher vaccine coverage rates than 40%, the rate observed in Site 2 in this study, are necessary to alter population epidemiology with such an infectious agent, and this should be a priority in the implementation of rotavirus vaccine programs.
The World Health Organization Global Advisory Committee on Vaccine Safety has recommended a standard approach for intussusception surveillance for countries introducing rotavirus vaccine programsCitation17,18 which includes documenting the background rate of intussusception. No cases of intussusception were observed following rotavirus vaccine in this study. The background rate of intussusception in this study varied from 0 to ∼12/100,000 annually, much less than the background rate in the US of ∼34/100,000 in infants less than 12 months of age,Citation19 and no related cases of intussusception were observed. Given the small population (<15 ,000) in this study it is not surprising that this rare AEFI was not seen.
Our study has several limitations. The epidemiology of rotavirus is variable, and reductions in the incidence of disease can occur naturally. It has been previously noted that the size of outbreaks can vary each year.Citation20 However, the elimination of disease in the site with high coverage for 2.5 y suggests that vaccine rather than natural epidemiologic variation explains the elimination of illness. The duration of protection cannot be determined from this study, and ongoing post-marketing surveillance is necessary. Finally, data on vaccine delivery by physician prescription with private payment was not available.
Materials and Methods
This was a controlled clinical study comparing implementation of universal infant rotavirus vaccine programs in 2 health jurisdictions, with a non-intervention comparator setting. The vaccine program implementation method (nurse-delivered vaccines in public health immunization clinics versus vaccines delivered by family physicians in their offices) was determined by the pre-existing public health immunization program in each jurisdiction, and was unblinded. A detailed description of the implementation process, educational interventions and key informant interviews in each setting is reported.Citation7,21 The trial is registered at Clinicaltrials.gov (NCT01273077).
Study setting
The three study sites were the entire province of Prince Edward Island which comprises one health system (Site 1), one health district in the province of Nova Scotia (Halifax Regional Municipality and the western part of Hants County) (Site 2), and the city of Saint John, which is in the province of New Brunswick (Site 3). Site 2 and 3 were chosen as they were the most populous of the multiple health districts in their provinces. The study sites are in the geographically adjacent Maritime Provinces of Canada and all include an urban setting and semi-urban and rural areas. Site 1 has a population of 140,204Citation22 and an annual live birth cohort of 1400.Citation8 Childhood immunizations are primarily provided by public health nurses in immunization clinics. Site 2 has a population of 390, 328Citation22 and a live birth cohort of 4209 (Atlee Perinatal Database, IWK Health Center, Halifax, NS personal communication). Preschool vaccinations are provided in physician offices. In both settings publically funded vaccines are routinely purchased by the provincial government Departments of Health and distributed to vaccine providers. At the time of the initiation of this study rotavirus vaccine was not funded by any provincial government in Canada. For the purposes of this study vaccine was provided for the 2 y study period to the Departments of Health for Sites 1 and 2, without cost. At Site 2 physicians request the requisite number of vaccines they need based on the number of children in their practice. Site 3 has a population of 70,063Citation22 and a birth cohort of 1757.
Participants and vaccine
Infants born on or after October 1, 2010, until September 30, 2012, were eligible to receive rotavirus vaccine (Rotarix™, GlaxoSmithKline Biologicals Inc..), along with their routinely scheduled infant immunizations at 2 and 4 months of age.Citation23
After the study period the province of Site 1 began funding of a universal infant rotavirus vaccine program, but the province of Site 2 did not. Any unused vaccine in doctors' offices in Site 2 after the study could still be used.
The protocol was approved by Research Ethics Boards at all participating sites prior to study initiation. If information was not available in the health record, parents were not approached for consent to request additional data. Cases for which clinical data was not accessible were excluded.
Study procedures
The method for vaccine program delivery in Sites 1 and 2 followed the usual procedures for implementing a new infant immunization program in those jurisdictions.Citation7 In Site 1, infant vaccine programs were delivered by public health nurses in immunization clinics. Referrals of all live born infants are sent from acute care hospitals to Public Health Nursing for follow-up home visits post-partum, at which time the reminder to schedule immunizations occurs. Infants received the rotavirus vaccine along with other routinely scheduled vaccines at 2 and 4 months of age: diphtheria (D), acellular pertussis (aP), tetanus (T), inactivated polio (P), and Haemophilus influenzae type B (Hib)(Pediacel®, Sanofi Pasteur Ltd.) vaccine, hepatitis B (Engerix®-B, GlaxoSmithKline Biologicals, Inc. or Recombivax HB®, Merck Canada Inc.) and pneumococcal conjugate vaccine (Prevnar®13, Pfizer Canada Inc.). Administration was recorded in an electronic provincial childhood immunization registry.
In Site 2, infant vaccines were delivered by family doctors along with vaccine given at the 2 and 4 month visits: (Quadracel®-Act-Hib®, Sanofi Pasteur and pneumococcal conjugate vaccine Prevnar®13, Pfizer Canada Inc..) A paper or electronic document (Reciprocal Notification Form) was completed by the physician and a copy returned by email, mail or fax to public health.
At Site 3, rotavirus vaccine was not provided by the study to any immunizers.
Outcome measures
The primary objective was to evaluate the effectiveness of these universal infant rotavirus vaccine programs, based on the frequency of hospital admissions for children less than one year of age with laboratory-confirmed rotavirus illness. Secondary objectives were to evaluate the frequency of rotavirus admissions in children 12 months to 2 y of age and the frequency of the serious adverse events (SAEs) intussusception and all-cause diarrhea in children less than 12 months of age. Other objectives of the Maritime Universal Rotavirus Vaccination Program (MURVP), reported elsewhere, were to assess the program implementation processes and coverage in Sites 1 and 27 and to describe the knowledge, attitudes and behaviors of health care providers and parents regarding rotavirus vaccine.
The number of live births during the study was obtained from the Vital Statistics office in each jurisdiction. Ascertainment of children hospitalized for rotavirus gastroenteritis (primary effectiveness outcome), and the SAEs was conducted for the 2 y period prior to implementation of the vaccine program and for the subsequent 2.5 y (1 October 2010–30 May 2013).
At Site 2 prospective collection of rotavirus hospitalization data began in 2005, using the pediatric hospital-based national active surveillance system IMPACT (Immunization Monitoring Program ACTive),Citation24 and continued during and after study initiation. In Sites 1 and 3 retrospective data was collected on rotavirus hospitalizations and SAEs from 2008–2010 by laboratory and health record review using the IMPACT case definitions and prospectively from 2010 onwards. Unfortunately, there is no system for recording vaccines that are prescribed by a physician and paid for by the patient in Site 3 (or any of the sites). The following International Classification of Disease 10th Modification (ICD10) codes were used for initial identification of rotavirus cases and of all-cause diarrhea from medical records: viral and other intestinal infections (A08), diarrhea and gastroenteritis of infectious origin (A09), non-infectious gastroenteritis (K52.9), R11 (nausea and vomiting) and R15 (fecal incontinence).
For the primary objective, the case definition of rotavirus gastroenteritis hospitalization required that the child be less than one year of age, an inpatient at the study site hospital, with laboratory confirmed rotavirus infection by enzyme immunoassay, polymerase chain reaction (PCR) or other molecular tests, or an electron microscopic diagnosis in a stool specimen taken within 14 d after the onset of gastrointestinal symptoms (diarrhea with or without vomiting). The admission could be a direct admission, a transfer from another hospital for management of the infection, or the child could have acquired the infection during a hospital stay for another illness (i.e., hospital-acquired infection). Cases identified by autopsy must have had gastrointestinal symptoms before death. For the analysis of children under 2 y of age with rotavirus the case definition was the same except the child was 12 months to less than 24 months of age. The study did not intervene in diagnostic testing practice.
Adverse event surveillance was conducted for intussusception and/or severe diarrhea following vaccine receipt. The case identification of intussusception was based on searches of hospital records for the ICD10 codes: K56.1 (idiopathic), K91.3 (due to surgery), K38.8 (intussusception of the appendix), or the corresponding ICD-9 codes (560.0 and 543.9) and could have occurred in a hospital admission, emergency department visit or diagnostic radiology visit.
Intussusception cases that met Level 1 Brighton definitionCitation25 were included. Children with intussusception which was a direct complication of a surgical procedure (such as J-tube insertion) were excluded. Any case of diarrhea in a child less than 12 months of age that required hospitalization was considered severe diarrhea.
Statistical considerations and data analysis
The frequency of events was estimated using point estimates and exact binomial confidence intervals for the proportion of the target population (birth cohort eligible for vaccination) undergoing hospitalization due to rotavirus. Rates and proportions were summarized using binomial point estimates and confidence intervals. Continuous variables were summarized using point estimates of the mean, and associated intervals.
Vaccine coverage for each dose was defined as eligible children who received the vaccine as a percentage of the cumulative population in the birth cohort at that age.
No formal sample size calculation was performed for this surveillance study as the entire population in each jurisdiction was included.
Disclosure of Potential Conflicts of Interest
Drs. Halperin, Langley, and McNeil have served as consultants on ad hoc advisory boards organized by GlaxoSmithKline who provided funding for this study. Dr. Langley holds the Canadian Institutes of Health Research - GlaxoSmithKline Chair in Pediatric Vaccinology.
Note
MURVP Authors: Beth A. Halperin (Dalhousie University), Scott A. Halperin (Dalhousie University), Joanne M. Langley (Dalhousie University), Donna MacDougall (Saint Francis Xavier University), Donna MacKinnon-Cameron (Dalhousie University), Shelly A. McNeil (Dalhousie University), Anne Neatby (PEI Department of Health and Wellness), Pam Publicover-Brouwer (Dalhousie University), Carol Lynn Raithby (Capital Health, NS), Corinne Rowswell (PEI Department of Health and Wellness), Carolyn Sanford (PEI Department of Health and Wellness), Caryll Tawse (Capital Health, NS), Gaynor Watson-Creed (Capital Health, NS), Noella Whelan (Capital Health, NS), Mitchell Zelman (PEI Department of Health and Wellness).
Acknowledgments
The authors thank Jennifer Kalil, Christine Wang, Donna McKinnon-Cameron, Li Li, Carol-Lynn Raithby, Noella Whelan, Deanne Burnett, Katherine Gaudreau, Shirley Urquhart, Heather Morrison, Lamont Sweet, Marc Nicholson, PEI Public Health Nursing and District 9 family physicians for their contributions to this study.
Funding
The study was funded by an investigator-initiated grant from GlaxoSmithKline, Canada. The funder played no role in study design, implementation, analysis or preparation of manuscripts.
References
- Tate JE, Patel MM, Steele AD, Gentsch JR, Payne DC, Cortese MM, Nakagomi O, Cunliffe NA, Jiang B, Neuzil KM, et al. Global impact of rotavirus vaccines. Expert Rev Vaccines 2010; 9(4):395-407; PMID:20370550; http://dx.doi.org/10.1586/erv.10.17
- Strategic Advisory Group of Vaccine Experts World Health Organization. Meeting of the immunization Strategic Advisory Group of Experts, April 2009 – conclusions and recommendations. Wkly Epidemiol Rec. 2009;84(23):220-36.
- National Advisory Committee on Immunization. Updated statement of the use of rotavirus vaccines. Can Commun Dis Rep. 2010;36(ACS-4):1-36.
- Buigues RP, Duval B, Rochette L, Boulianne N, Douville-Fradet M, Dery P, De Serres G. Hospitalizations for diarrhea in Quebec children from 1985 to 1998: Estimates of rotavirus-associated diarrhea. Can J Infect Dis 2002; 13(4):239-44; PMID:18159396
- National Advisory Committee on Immunization. Statement on the recommended use of pentavalent human-bovine reassortant rotavirus vaccine. Can Commun Dis Rep. 2008;34(ACS-1):33.
- Canadian Immunization Committee. Recommendations for Rotavirus Immunization Programs Ottawa, ON: Public Health Agency of Canada; 2014 [cited 2014 01 March]. 24 January 2014: Available from: http://publications.gc.ca/collections/collection_2014/aspc-phac/HP40-95-2014-eng.pdf.
- Zelman M, Sanford C, Neatby A, Halperin BA, MacDougall D, Rowswell C, Langley JM, Halperin SA; Maritime Universal Rotavirus Vaccination Program (MURVP). Implementation of a universal rotavirus vaccination program: Comparison of two delivery systems. BMC Public Health 2014; 14(1):908; PMID:25182067; http://dx.doi.org/10.1186/1471-2458-14-908
- Department of Health and Wellness. Prince Edward Island Reproductive Care Program Perinatal Database Report 2011 Charlottetown, PEI: Government of Prince Edward Island; 2013 [28 April 2014]. Available from: http://www.gov.pe.ca/photos/original/dhw_rcp_rpt2011.pdf.
- Patel MM, Parashar UD, Santosham M, Richardson V. The rotavirus experience in Mexico: Discovery to control. Clini Infect Dis 2013; 56(4):548-51; PMID:23271788; http://dx.doi.org/10.1093/cid/cis939
- McDermid A, Le Saux N, Grudeski E, Bettinger JA, Manguiat K, Halperin SA, Macdonald L, Déry P, Embree J, Vaudry W, et al. Molecular characterization of rotavirus isolates from select Canadian pediatric hospitals. BMC infectious diseases 2012; 12:306; PMID:23153184; http://dx.doi.org/10.1186/1471-2334-12-306
- Dummer TJ, Cui Y, Strang R, Parker L. Immunization completeness of children under two years of age in Nova Scotia, Canada. Can J Public Health 2012; 103(5):e363-7; PMID:23617989
- Ismail SJ, Langley JM, Harris TM, Warshawsky BF, Desai S, FarhangMehr M. Canada's National Advisory Committee on Immunization (NACI): Evidence-based decision-making on vaccines and immunization. Vaccine 2010; 28 Suppl 1:A58-63; PMID:20412999; http://dx.doi.org/10.1016/j.vaccine.2010.02.035
- Cortese MM, Tate JE, Simonsen L, Edelman L, Parashar UD. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatr Infect Dis J 2010; 29(6):489-94; PMID:20354464
- Centers for Disease Control and Prevention (CDC). Delayed onset and diminished magnitude of rotavirus activity--United States, November 2007–May 2008. MMWR Morbidity and mortality weekly report. 2008;57(25):697-700.
- Rha B, Tate JE, Payne DC, Cortese MM, Lopman BA, Curns AT, Parashar UD. Effectiveness and impact of rotavirus vaccines in the United States—2006–2012. Expert Rev Vaccines 2014; 13(3):365-76; PMID:24392657; http://dx.doi.org/10.1586/14760584.2014.877846
- Pitzer V, Viboud C, Lopman BA, Patel MM, Parashar UD, Grenfell B. Influence of birth rates and transmission rates on the global seasonality of rotavirus incidence. J R Soc Interface 2011; 8:1584-93; PMID:21508015; http://dx.doi.org/10.1098/rsif.2011.0062
- World Health Organization Global Advisory Committee on Vaccine Safety. J. Wkly Epidemiol Rec 2007; 82:252-9; PMID:17642098
- Bines J, Bentsi-Enchill A, Steele D. Post-marketing surveillance if rotavirus vaccine safety. In: Biologicals Va, editor. Geneva: World Health Organization; 2008.
- U.S. Food and Drug Administration. Update: Information on Rotarix - Labeling Revision Pertaining to Intussusception. In: Biologics VBa, editor. Washington, D.C.: U.S. Department of Health and Human Services; 201
- Brandt CD, Kim HW, Rodriguez WJ, Arrobio JO, Jeffries BC, Stallings EP, Lewis C, Miles AJ, Chanock RM, Kapikian AZ, et al. Pediatric viral gastroenteritis during eight years of study. J Clin Microbiol 1983; 18(1):71-8; PMID:6309901
- Zelman M, Sanford C, Neatby A, Halperin B, MacDougall D, Rowswell C, et al. Implementation of a universal rotavirus vaccination program: comparison of two delivery systems. TBD. TBD.
- Statistics Canada. Census Profile 2011. Ottawa, ON: Government of Canada; 2014 [updated 20111 January 2014]. Available from: http://www12.statcan.gc.ca/census-recensement/index-eng.cfm.
- GlaxoSmithKline Inc. Product Monograph Rotarix Mississauga, ON: GlaxoSmithKline Inc; 2014 [cited 2014 1 January]. Available from: http://www.gsk.ca/english/docs-pdf/product-monographs/Rotarix.pdf.
- Le Saux N, Bettinger JA, Halperin SA, Vaudry W, Scheifele DW, Canadian Immunization Monitoring Program A. Substantial morbidity for hospitalized children with community-acquired rotavirus infections: 2005-2007 IMPACT surveillance in Canadian hospitals. Pediatr Infect Dis J 2010; 29(9):879-82; PMID:20467353; http://dx.doi.org/10.1097/INF.0b013e3181e20c94
- Bines JE, Kohl KS, Forster J, Zanardi LR, Davis RL, Hansen J, Murphy TM, Music S, Niu M, Varricchio F, et al. Acute intussusception in infants and children as an adverse event following immunization: Case definition and guidelines of data collection, analysis, and presentation. Vaccine 2004; 22(5-6):569-74; PMID:14741146; http://dx.doi.org/10.1016/j.vaccine.2003.09.016