Abstract
The use of the Tat protein of HIV in vaccines against AIDS showed promising results in primate and human studies. To characterize the impact of the administration route on the induction of humoral responses at systemic and mucosal levels, we compared intradermal, intramuscular and mucosal immunizations with Tat and a Tat-derived peptide. Mice were immunized with the Tat protein by different routes and the titer and isotype of anti-Tat antibodies were assessed in serum and mucosal lavages. Intramuscular and intradermal administrations showed comparable immunogenicity, while the mucosal administration was unable to induce IgM in serum and IgG at mucosal sites but showed superior immunogenicity in terms of IgA induction. Anti-Tat antibodies were also obtained upon vaccination with the immunodominant Tat 1–20 peptide which was, however, less immunogenic than the whole Tat protein.
Keywords:
The Tat protein of HIV plays a key role in the viral life cycle and progression to AIDSCitation1-4 and anti-Tat humoral and cellular responses correlate with disease control in HIV-infected individuals.Citation5-11 Thus, the inclusion of Tat in preventive and therapeutic vaccines has been pursued by several groups showing promising results in nonhuman primatesCitation12-16 and in phase I and II clinical trials.Citation16-19 However, the development of vaccines able to induce protective responses has to face some major challenges such as: i) the compliance requested for mass immunizations; ii) the induction of immune responses in mucosal tissues, which are the predominant sites of HIV acquisition;Citation20 and iii) the isotypes of antibodies elicited, which may influence the level of vaccine efficacy.Citation21 These factors may be modulated by the administration route. Intramuscular (IM) administration is widely used for vaccinations, although intradermal (ID) administration has been shown to be safe, well tolerated, less painfulCitation22-24 and to require lower dosesCitation22,25,26 due to the higher number of professional antigen presenting cells found in the skin than in the muscle.Citation27 Therefore, the ID route may be a relevant strategy for mass immunization, and clade B Tat protein has been administered by this route in 2 phase II clinical trialsCitation17 and unpublished data after having shown superior immunogenicity when administered ID rather than subcutaneously in phase I clinical trials.Citation18,19 At the same time, the oral administration is gaining interest for its high compliance and the capability of inducing mucosal responses.Citation28 However, to our knowledge, neither IM nor oral administration of the Tat protein have ever been compared to the ID route.
Thus, to assess the effects of the administration routes on the immunogenicity of the Tat protein, groups of mice were immunized with 30μg of TatCitation17 by ID, IM route or through the oral mucosa (OM) in the absence of adjuvants. ID and IM injections were performed in 2 sites on the back while, for OM immunization, mice were deprived of water 6 h before immunization and the immunogen was delivered in the mouth through a pipett tip. The dose of 30μg was chosen because preliminary experiments showed that ID immunization with 30μg of Tat protein induced higher anti-Tat IgG and IgM titers, as compared to immunization with 1 and 7.5μg of protein (), and its injection was safe and well tolerated by mice as demonstrated by periodical observation of spontaneous activity (Irwin Test) and histological studies of organs (not shown).Citation29
Figure 1. Characterization of anti-Tat humoral responses in sera. Serum samples of mice immunized 3 times ID with Tat protein at the dose of 1, 7.5 or 30μg were collected at day 42 from retro-orbital plexus and the presence of anti-Tat IgG and IgM was evaluated by Elisa test. Briefly, 96-well plates were coated with Tat (100ng/200μl/well) in 0.05 M carbonate buffer (pH 9.6) for 18 hours at 4°C. Plates were washed with PBS containing 0.05% Tween 20 (Sigma-Aldrich, Milan, Italy), and incubated for 90 minutes at 37°C with blocking buffer. The following blocking buffers were used: PBS containing 0.05% Tween 20 and 1% BSA (for IgG) and PBS containing 0.05% Tween 20 and 3% BSA (for IgM). After extensive washes, serial dilutions of each serum were dispensed in duplicate wells (100μl/well) and incubated for 90 minutes at 37°C. Plates were washed again before the addition of 100 μl/well of HRP-conjugated goat anti-mouse IgG or IgM (Sigma), and incubated at 37°C for 90 minutes. After incubation, plates were washed 5 times and subsequently a solution of 2,2'-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt (ABTS) substrate (Roche) was added. The absorbance values were measured at 405 nm with an automatic plate reader (SUNRISE TECAN Salzburg-Austria). The cut-off value was estimated as the mean OD of 3 negative control sera plus 0.05. Each OD value was subtracted of the blank and cut-off values to obtain a net OD value and IgG titers calculated by intercept function. (A) Titers of serum anti-Tat IgG. (B) Titers of serum anti-Tat IgM. *P < 0.05, **P < 0.01 according to 2-tailed Mann Whitney test. Results of 2 independent experiments are shown. Dots represent single mice and lines represent the means +/− SEM.
![Figure 1. Characterization of anti-Tat humoral responses in sera. Serum samples of mice immunized 3 times ID with Tat protein at the dose of 1, 7.5 or 30μg were collected at day 42 from retro-orbital plexus and the presence of anti-Tat IgG and IgM was evaluated by Elisa test. Briefly, 96-well plates were coated with Tat (100ng/200μl/well) in 0.05 M carbonate buffer (pH 9.6) for 18 hours at 4°C. Plates were washed with PBS containing 0.05% Tween 20 (Sigma-Aldrich, Milan, Italy), and incubated for 90 minutes at 37°C with blocking buffer. The following blocking buffers were used: PBS containing 0.05% Tween 20 and 1% BSA (for IgG) and PBS containing 0.05% Tween 20 and 3% BSA (for IgM). After extensive washes, serial dilutions of each serum were dispensed in duplicate wells (100μl/well) and incubated for 90 minutes at 37°C. Plates were washed again before the addition of 100 μl/well of HRP-conjugated goat anti-mouse IgG or IgM (Sigma), and incubated at 37°C for 90 minutes. After incubation, plates were washed 5 times and subsequently a solution of 2,2'-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt (ABTS) substrate (Roche) was added. The absorbance values were measured at 405 nm with an automatic plate reader (SUNRISE TECAN Salzburg-Austria). The cut-off value was estimated as the mean OD of 3 negative control sera plus 0.05. Each OD value was subtracted of the blank and cut-off values to obtain a net OD value and IgG titers calculated by intercept function. (A) Titers of serum anti-Tat IgG. (B) Titers of serum anti-Tat IgM. *P < 0.05, **P < 0.01 according to 2-tailed Mann Whitney test. Results of 2 independent experiments are shown. Dots represent single mice and lines represent the means +/− SEM.](/cms/asset/aea08b41-7e69-485b-9139-fe91ed6e64cf/khvi_a_1016676_f0001_b.gif)
Thus, animals were immunized at days 1, 14 and 28 by ID, IM or OM route. Serum and mucosal samples were collected at day 42 and tested for the presence of anti-Tat antibodies.Citation30,31
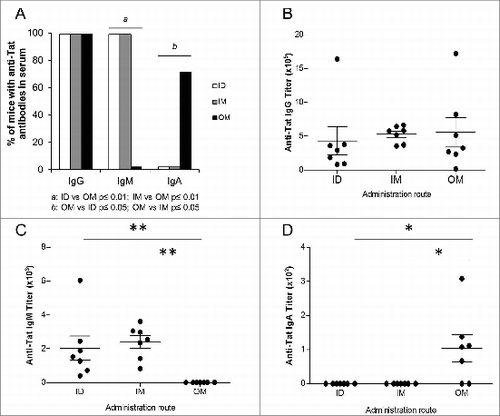
Anti-Tat IgG were present in sera of all animals () with titers comparable among groups (). In contrast, anti-Tat IgM in serum were present at similar levels in all mice injected IM or ID, but not in mice immunized OM (). Interestingly, the OM route was the only one capable of inducing serum anti-Tat IgA antibodies in 71% of vaccinated mice ().
Figure 2. Characterization of anti-Tat humoral responses in sera. Serum samples of mice immunized 3 times ID, IM or OM with Tat protein (30μg) were collected at day 42 and the presence of anti-Tat IgG, IgM and IgA was evaluated by Elisa test. Elisa test for IgA evaluation was performed using PBS containing 0.1% Tween 20 and 1% BSA as block buffer. (A) Proportion of mice that developed serum anti-Tat IgG, IgM and IgA. Frequencies of anti-Tat positive mice were compared among different groups using 2-tailed Fisher's exact test. Anti-Tat positivity was determined by titers >100, 50 and 25 for IgG, IgM and IgA respectively. (B) Titers of serum anti-Tat IgG. (C) Titers of serum anti-Tat IgM. (D) Titers of serum anti-Tat IgA. * P < 0.05, **P < 0.01 according to 2-tailed Mann Whitney test. Results of 2 independent experiments are shown. Dots represent single mice and lines represent the means +/− SEM.
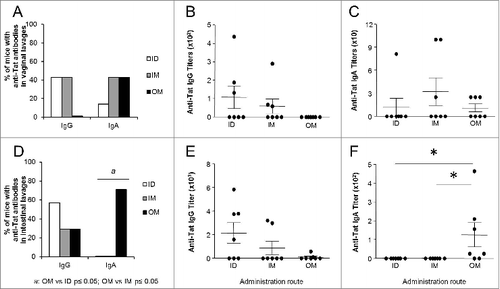
To determine whether Tat immunization elicited antibodies in mucosal secretions, we next assessed anti-Tat humoral responses in vaginal and intestinal lavages. As shown in , OM immunization did not induce anti-Tat IgG in vaginal secretions, that were barely detected in some mice injected ID or IM (), while the different routes of administration did not show significant differences in terms of anti-Tat IgA responses (). Analysis of intestinal lavages showed that the ID and IM routes induced higher titers of anti-Tat IgG than the OM administration (), whereas only the OM route of immunization induced anti-Tat IgA in intestinal secretions (, P < 0.05). Thus, while IM and ID administration of Tat were comparable for location and titers of antibodies elicited, the OM route showed a different pattern of responses inducing preferentially IgA both in serum and in mucosal lavages. Further studies aimed at assessing dose-dependence of immune responses and biodistribution of the Tat protein may be important to define the most effective vaccination protocol. This is particularly relevant for OM immunization since the antigen may have several fates (mainly adsorbed through the oral mucosa, but also part of it may be ingested).
Figure 3. Characterization of anti-Tat humoral responses in mucosal lavages. Mucosal samples of mice immunized 3 times ID, IM or OM with Tat protein (30μg) were obtained at day 42 by repeated flushing and aspiration with 0.5 ml of PBS containing a protease inhibitor cocktail (Roche, Mannheim, Germany). The presence of anti-Tat IgG and IgA was evaluated by Elisa test. Proportion of mice that developed anti-Tat IgG and IgA in (A) vaginal and (D) intestinal lavages. Frequencies of anti-Tat positive mice were compared among different groups using 2-tailed Fisher's exact test. Anti-Tat positivity was determined by titers >50 and 25 for IgG and IgA respectively. Titers of anti-Tat IgG in (B) vaginal and (E) intestinal lavages. Titers of anti-Tat IgA in (C) vaginal and (F) intestinal lavages. * P < 0.05 according to 2-tailed Mann Whitney test. Results of 2 independent experiments are shown. Dots represent single mice and lines represent the means +/− SEM.
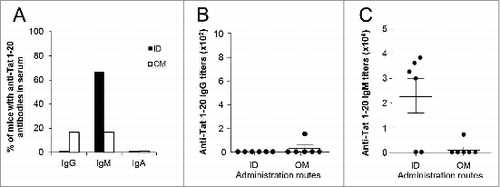
It has been recently shown that antibodies directed against the N-terminus region of Tat, which is the most immunogenic in terms of humoral responses,Citation32,33 protect monkeys from infection acquisition,Citation15 suggesting that the Tat 1–20 peptide constitutes an interesting candidate for a preventive vaccine. To assess how different routes of administration may affect humoral immunogenicity of peptide vaccines, mice were vaccinated 3 times (at days 1, 14 and 28), by ID or OM route, with Tat 1–20 peptide (EPVDPRLEPWKHPGSQPKT, synthesized by solid phase method and purified by HPLC to >98% purity by UF Peptides, University of Ferrara, Italy), encompassing an immunodominant Tat epitope,Citation32,33 at the dose of 7μg. This dose contains the same number of molecules of 30μg of Tat protein. Serum samples were collected at day 42 to assess systemic humoral responses against the Tat 1–20 peptide. As shown in , IgG antibodies were almost absent in mice vaccinated with the peptide, irrespective to the administration route, while they were present in all mice vaccinated with the whole protein (), suggesting that the peptide is, at least in the absence of a proper adjuvant or delivery system, much less immunogenic than the whole Tat protein. Anti-Tat 1–20 IgA were absent in all groups while, consistent with what previously observed, anti-Tat 1–20 IgM were present at higher frequencies in mice immunized ID rather than OM, although differences did not reach statistical significance (). All mice possessing antibodies against the peptide were also able to recognize the whole protein (not shown).
Figure 4. Characterization of anti-Tat 1–20 peptide humoral responses in sera. Serum samples of mice immunized 3 times ID or OM with the Tat 1–20 peptide (7μg) were collected at day 42 and the presence of anti-Tat 1–20 peptide IgG, IgM and IgA was evaluated by Elisa test (plates were coated with Tat 1–20 peptide at the dose of 100ng/200μl/well). (A) Proportion of mice that developed serum anti-Tat 1–20 peptide IgG, IgM and IgA. Frequencies of anti-Tat 1–20 peptide positive mice were compared among different groups using 2-tailed Fisher's exact test. Anti-Tat 1–20 peptide positivity was determined by titers >100, 50 and 25 for IgG, IgM and IgA respectively. (B) Titers of serum anti-Tat 1–20 peptide IgG. (C) Titers of serum anti-Tat 1–20 peptide IgM. Results of 2 independent experiments are shown. Dots represent single mice and lines represent the means +/− SEM.
Interestingly, our results show that the Tat protein is immunogenic when administered orally. OM administration of the Tat protein failed in eliciting IgM but induced serum IgG responses and both serum and mucosal IgA. Although we did not evaluate immune responses induced by OM immunization with other proteins, which may differ from Tat for water solubility, stability and cell penetration capacity, these results show that this strategy may be further explored as a way to induce IgA mucosal responses. This may be of particular relevance for the development of vaccines against HIV/AIDS or other infectious diseases, which are transmitted mostly by mucosal routes.Citation34
The immunogenicity of oral administration, which may be further boosted by proper adjuvants or different vaccination schedules,Citation28,35 could be due to the binding properties of Tat. Indeed, some studies have shown that the oral administration of antigens capable of binding to mucosal surface induces strong immune responses.Citation36 In fact, Tat has been reported to bind to heparan sulfate proteoglycans present in the extracellular matrix and on the cell membranesCitation37,38 as well as to integrins present on the cellular surface of professional antigen presenting cells.Citation39
However, some issues still need to be solved in the field of mucosal vaccines. Intriguingly, although mucosal responses were not investigated, data from the RV144 phase 3 trial suggests that serum IgA may actually abrogate vaccine efficacy conferred by serum anti-Env IgG.Citation40 Moreover, it is still unclear whether optimal mucosal immune responses are elicited by vaccines delivered mucosally or by systemic immunizations inducing strong immune responses crossing-over into mucosal sites. Although, ID, IM and OM routes induced comparable amounts of IgG in serum, the OM route almost failed in inducing IgG in intestinal and vaginal lavages, which were present at higher frequency and titers in ID and IM immunized animals. These differences may depend on the site of antigen presentation and thus to a different location of actively secreting plasmacells.
In conclusion, this study demonstrates that the IM administration of the Tat protein induced humoral responses comparable to those elicited by the ID route. Moreover, we explored the oral vaccination, a promising option which needs more formulation efforts to improve its efficiency further to the clarification of serum IgA role in protection from acquisition and progression to AIDS.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to acknowledge Dr. Noemi Vignoli for technical assistance.
Funding
This study was funded by the Italian Ministry of Health, “Special project on the development of a vaccine against HIV based on the Tat protein” and by the University of Ferrara.
References
- Berkhout B, Silverman RH, Jeang KT. Tat trans-activates the human immunodeficiency virus through a nascent RNA target. Cell 1989; 59:273-82; PMID:2478293; http://dx.doi.org/10.1016/0092-8674(89)90289-4
- Ott M, Emiliani S, Van Lint C, Herbein G, Lovett J, Chirmule N, McCloskey T, Pahwa S, Verdin E. Immune hyperactivation of HIV-1-infected T cells mediated by Tat and the CD28 pathway. Science 1997; 275:1481-5; PMID:9045614; http://dx.doi.org/10.1126/science.275.5305.1481
- Sforza F, Nicoli F, Gallerani E, Finessi V, Reali E, Cafaro A, Caputo A, Ensoli B, Gavioli R. HIV-1 Tat affects the programming and functionality of human CD8+ T cells by modulating the expression of T-box transcription factors. AIDS 2014; 28:1729-38; PMID:24841128; http://dx.doi.org/10.1097/QAD.0000000000000315
- Li L, Li HS, Pauza CD, Bukrinsky M, Zhao RY. Roles of HIV-1 auxiliary proteins in viral pathogenesis and host-pathogen interactions. Cell Res 2005; 15:923-34; PMID:16354571; http://dx.doi.org/10.1038/sj.cr.7290370
- van Baalen CA, Pontesilli O, Huisman RC, Geretti AM, Klein MR, de Wolf F, Miedema F, Gruters RA, Osterhaus AD. Human immunodeficiency virus type 1 Rev- and Tat-specific cytotoxic T lymphocyte frequencies inversely correlate with rapid progression to AIDS. J Gen Virol 1997; 78(Pt 8):1913-8; PMID:9266987
- Rodman TC, Pruslin FH, To SE, Winston R. Human immunodeficiency virus (HIV) Tat-reactive antibodies present in normal HIV-negative sera and depleted in HIV-positive sera. Identification of the epitope. J Exp Med 1992; 175:1247-53; PMID:1373758; http://dx.doi.org/10.1084/jem.175.5.1247
- Rezza G, Fiorelli V, Dorrucci M, Ciccozzi M, Tripiciano A, Scoglio A, Collacchi B, Ruiz-Alvarez M, Giannetto C, Caputo A, et al. The presence of anti-Tat antibodies is predictive of long-term nonprogression to AIDS or severe immunodeficiency: findings in a cohort of HIV-1 seroconverters. J Infect Dis 2005; 191:1321-4; PMID:15776379; http://dx.doi.org/10.1086/428909
- Re MC, Vignoli M, Furlini G, Gibellini D, Colangeli V, Vitone F, La Placa M. Antibodies against full-length Tat protein and some low-molecular-weight Tat-peptides correlate with low or undetectable viral load in HIV-1 seropositive patients. J Clin Virol 2001; 21:81-9; PMID:11255101; http://dx.doi.org/10.1016/S1386-6532(00)00189-X
- Richardson MW, Mirchandani J, Duong J, Grimaldo S, Kocieda V, Hendel H, Khalili K, Zagury JF, Rappaport J. Antibodies to Tat and Vpr in the GRIV cohort: differential association with maintenance of long-term non-progression status in HIV-1 infection. Biomed Pharmacother 2003; 57:4-14; PMID:12642031; http://dx.doi.org/10.1016/S0753-3322(02)00327-X
- Zagury JF, Sill A, Blattner W, Lachgar A, Le Buanec H, Richardson M, Rappaport J, Hendel H, Bizzini B, Gringeri A, et al. Antibodies to the HIV-1 Tat protein correlated with nonprogression to AIDS: a rationale for the use of Tat toxoid as an HIV-1 vaccine. J Hum Virol 1998; 1:282-92; PMID:10195253
- Bellino S, Tripiciano A, Picconi O, Francavilla V, Longo O, Sgadari C, Paniccia G, Arancio A, Angarano G, Ladisa N, et al. The presence of anti-Tat antibodies in HIV-infected individuals is associated with containment of CD4+ T-cell decay and viral load, and with delay of disease progression: results of a 3-year cohort study. Retrovirology 2014; 11:49; PMID:24961156; http://dx.doi.org/10.1186/1742-4690-11-49
- Cafaro A, Caputo A, Fracasso C, Maggiorella MT, Goletti D, Baroncelli S, Pace M, Sernicola L, Koanga-Mogtomo ML, Betti M, et al. Control of SHIV-89.6P-infection of cynomolgus monkeys by HIV-1 Tat protein vaccine. Nat Med 1999; 5:643-50; PMID:10371502; http://dx.doi.org/10.1038/9488
- Maggiorella MT, Baroncelli S, Michelini Z, Fanales-Belasio E, Moretti S, Sernicola L, Cara A, Negri DR, Butto S, Fiorelli V, et al. Long-term protection against SHIV89.6P replication in HIV-1 Tat vaccinated cynomolgus monkeys. Vaccine 2004; 22:3258-69; PMID:15308348; http://dx.doi.org/10.1016/j.vaccine.2004.03.009
- Watkins JD, Lancelot S, Campbell GR, Esquieu D, de Mareuil J, Opi S, Annappa S, Salles JP, Loret EP. Reservoir cells no longer detectable after a heterologous SHIV challenge with the synthetic HIV-1 Tat Oyi vaccine. Retrovirology 2006; 3:8; PMID:16441880; http://dx.doi.org/10.1186/1742-4690-3-8
- Bachler BC, Humbert M, Palikuqi B, Siddappa NB, Lakhashe SK, Rasmussen RA, Ruprecht RM. Novel biopanning strategy to identify epitopes associated with vaccine protection. J Virol 2013; 87:4403-16; PMID:23388727; http://dx.doi.org/10.1128/JVI.02888-12
- Caputo A, Gavioli R, Bellino S, Longo O, Tripiciano A, Francavilla V, Sgadari C, Paniccia G, Titti F, Cafaro A, et al. HIV-1 Tat-based vaccines: an overview and perspectives in the field of HIV/AIDS vaccine development. Int Rev Immunol 2009; 28:285-334; PMID:19811313; http://dx.doi.org/10.1080/08830180903013026
- Ensoli B, Bellino S, Tripiciano A, Longo O, Francavilla V, Marcotullio S, Cafaro A, Picconi O, Paniccia G, Scoglio A, et al. Therapeutic immunization with HIV-1 Tat reduces immune activation and loss of regulatory T-cells and improves immune function in subjects on HAART. PLoS One 2010; 5:e13540; PMID:21085635; http://dx.doi.org/10.1371/journal.pone.0013540
- Ensoli B, Fiorelli V, Ensoli F, Lazzarin A, Visintini R, Narciso P, Di Carlo A, Monini P, Magnani M, Garaci E. The therapeutic phase I trial of the recombinant native HIV-1 Tat protein. AIDS 2008; 22:2207-9; PMID:18832884; http://dx.doi.org/10.1097/QAD.0b013e32831392d4
- Ensoli B, Fiorelli V, Ensoli F, Lazzarin A, Visintini R, Narciso P, Di Carlo A, Tripiciano A, Longo O, Bellino S, et al. The preventive phase I trial with the HIV-1 Tat-based vaccine. Vaccine 2009; 28:371-8; PMID:19879233; http://dx.doi.org/10.1016/j.vaccine.2009.10.038
- Lehner T, Anton PA. Mucosal immunity and vaccination against HIV. AIDS 2002; 16(Suppl 4):S125-32; PMID:12699009
- Weaver EA, Nehete PN, Nehete BP, Yang G, Buchl SJ, Hanley PW, Palmer D, Montefiori DC, Ferrari G, Ng P, et al. Comparison of systemic and mucosal immunization with helper-dependent adenoviruses for vaccination against mucosal challenge with SHIV. PLoS One 2013; 8:e67574; PMID:23844034; http://dx.doi.org/10.1371/journal.pone.0067574
- Eble PL, Weerdmeester K, van Hemert-Kluitenberg F, Dekker A. Intradermal vaccination of pigs against FMD with 1/10 dose results in comparable vaccine efficacy as intramuscular vaccination with a full dose. Vaccine 2009; 27:1272-8; PMID:19114077; http://dx.doi.org/10.1016/j.vaccine.2008.12.011
- Kunzi V, Klap JM, Seiberling MK, Herzog C, Hartmann K, Kursteiner O, Kompier R, Grimaldi R, Goudsmit J. Immunogenicity and safety of low dose virosomal adjuvanted influenza vaccine administered intradermally compared to intramuscular full dose administration. Vaccine 2009; 27:3561-7; PMID:19464535; http://dx.doi.org/10.1016/j.vaccine.2009.03.062
- Kulkarni PS, Sapru A, D'Costa PM, Pandit A, Madhusudana SN, Yajaman AB, Mangrule S, Gunale B, Bavdekar AR. Safety and immunogenicity of a new purified vero cell rabies vaccine (PVRV) administered by intramuscular and intradermal routes in healthy volunteers. Vaccine 2013; 31:2719-22; PMID:23583817; http://dx.doi.org/10.1016/j.vaccine.2013.03.050
- Frenck RW, Jr., Belshe R, Brady RC, Winokur PL, Campbell JD, Treanor J, Hay CM, Dekker CL, Walter EB, Jr., Cate TR, et al. Comparison of the immunogenicity and safety of a split-virion, inactivated, trivalent influenza vaccine (Fluzone(R)) administered by intradermal and intramuscular route in healthy adults. Vaccine 2011; 29:5666-74; PMID:21699951; http://dx.doi.org/10.1016/j.vaccine.2011.06.010
- Young F, Marra F. A systematic review of intradermal influenza vaccines. Vaccine 2011; 29:8788-801; PMID:21968444; http://dx.doi.org/10.1016/j.vaccine.2011.09.077
- Glenn GM, Kenney RT. Mass vaccination: solutions in the skin. Curr Top Microbiol Immunol 2006; 304:247-68; PMID:16989274
- Barackman JD, Ott G, Pine S, O'Hagan DT. Oral administration of influenza vaccine in combination with the adjuvants LT-K63 and LT-R72 induces potent immune responses comparable to or stronger than traditional intramuscular immunization. Clin Diagn Lab Immunol 2001; 8:652-7; PMID:11329476
- Caputo A, Brocca-Cofano E, Castaldello A, De Michele R, Altavilla G, Marchisio M, Gavioli R, Rolen U, Chiarantini L, Cerasi A, et al. Novel biocompatible anionic polymeric microspheres for the delivery of the HIV-1 Tat protein for vaccine application. Vaccine 2004; 22:2910-24; PMID:15246628; http://dx.doi.org/10.1016/j.vaccine.2003.12.025
- Caputo A, Brocca-Cofano E, Castaldello A, Voltan R, Gavioli R, Srivastava IK, Barnett SW, Cafaro A, Ensoli B. Characterization of immune responses elicited in mice by intranasal co-immunization with HIV-1 Tat, gp140 DeltaV2Env and/or SIV Gag proteins and the nontoxicogenic heat-labile Escherichia coli enterotoxin. Vaccine 2008; 26:1214-27; PMID:18243435; http://dx.doi.org/10.1016/j.vaccine.2007.12.030
- Nicoli F, Finessi V, Sicurella M, Rizzotto L, Gallerani E, Destro F, Cafaro A, Marconi P, Caputo A, Ensoli B, et al. The HIV-1 Tat protein induces the activation of CD8(+) T cells and affects in vivo the magnitude and kinetics of antiviral responses. PLoS One 2013; 8:e77746; PMID:24223723; http://dx.doi.org/10.1371/journal.pone.0077746
- Butto S, Fiorelli V, Tripiciano A, Ruiz-Alvarez MJ, Scoglio A, Ensoli F, Ciccozzi M, Collacchi B, Sabbatucci M, Cafaro A, et al. Sequence conservation and antibody cross-recognition of clade B human immunodeficiency virus (HIV) type 1 Tat protein in HIV-1-infected Italians, Ugandans, and South Africans. J Infect Dis 2003; 188:1171-80; PMID:14551888; http://dx.doi.org/10.1086/378412
- Moreau E, Belliard G, Partidos CD, Pradezinsky F, Le Buanec H, Muller S, Desgranges C. Important B-cell epitopes for neutralization of human immunodeficiency virus type 1 Tat in serum samples of humans and different animal species immunized with Tat protein or peptides. J Gen Virol 2004; 85:2893-901; PMID:15448351; http://dx.doi.org/10.1099/vir.0.80365-0
- Wu L. Biology of HIV mucosal transmission. Curr Opin HIV AIDS 2008; 3:534-40; PMID:18802490; http://dx.doi.org/10.1097/COH.0b013e32830634c6
- Zhou M, Zhang G, Ren G, Gnanadurai CW, Li Z, Chai Q, Yang Y, Leyson CM, Wu W, Cui M, et al. Recombinant rabies viruses expressing GM-CSF or flagellin are effective vaccines for both intramuscular and oral immunizations. PLoS One 2013; 8:e63384; PMID:23700422; http://dx.doi.org/10.1371/journal.pone.0063384
- de Aizpurua HJ, Russell-Jones GJ. Oral vaccination. Identification of classes of proteins that provoke an immune response upon oral feeding. J Exp Med 1988; 167:440-51; PMID:3346623; http://dx.doi.org/10.1084/jem.167.2.440
- Chang HC, Samaniego F, Nair BC, Buonaguro L, Ensoli B. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS 1997; 11:1421-31; PMID:9342064; http://dx.doi.org/10.1097/00002030-199712000-00006
- Ensoli B, Gendelman R, Markham P, Fiorelli V, Colombini S, Raffeld M, Cafaro A, Chang HK, Brady JN, Gallo RC. Synergy between basic fibroblast growth factor and HIV-1 Tat protein in induction of Kaposi's sarcoma. Nature 1994; 371:674-80; PMID:7935812; http://dx.doi.org/10.1038/371674a0
- Fanales-Belasio E, Moretti S, Fiorelli V, Tripiciano A, Pavone Cossut MR, Scoglio A, Collacchi B, Nappi F, Macchia I, Bellino S, et al. HIV-1 Tat addresses dendritic cells to induce a predominant Th1-type adaptive immune response that appears prevalent in the asymptomatic stage of infection. J Immunol 2009; 182:2888-97; PMID:19234184; http://dx.doi.org/10.4049/jimmunol.0711406
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 2012; 366:1275-86; PMID:22475592; http://dx.doi.org/10.1056/NEJMoa1113425
