Abstract
Hepatitis B vaccines do not generate protective immune responses in older adults as effectively as they do in children and young adults. Improved formulations of existing vaccines may have the potential to improve this. This study investigated the persistence of serum antibodies against hepatitis B surface antigens (anti-HBs) 3.1–3.5 years following primary vaccination with 3 doses of HBvaxPRO® or Engerix B™ in healthy adults aged ≥50 years who were further challenged with 1 dose of recombinant hepatitis B antigen. This was an open-label extension study. Individuals (N = 204) with a mean (standard deviation) age at enrollment of 63.7 (7.0) years receiving HBvaxPRO® or Engerix B™ in a randomized, double-blind primary study were challenged with 1 dose of HBvaxPRO® (10 μg). Anti-HBs were measured pre- and 30 days post-challenge. 45.5% (34.8, 56.4 [95% CI]) of individuals who received HBvaxPRO® in the per protocol set (PPS) had anti-HBs titers ≥10 mIU/mL pre-challenge and 85.2% (76.1, 91.9) 1-month post-challenge. In those who received Engerix B™ in the primary vaccination series, the results were 58.8% (48.6, 68.5) and 88.3% (80.5, 93.8), respectively. The challenge dose of HBvaxPRO® was generally well tolerated. Subjects aged ≥50 years receiving a challenge dose of HBvaxPRO® demonstrated immune memory against hepatitis B 3 years after a 3-dose primary. The safety profile of this challenge dose of HBvaxPRO® was consistent with the well-established safety profile of the vaccine HBvaxPRO®.
Introduction
Hepatitis B is a major global health problem. It is estimated that about 2 billion people worldwide have been infected with hepatitis B virus; 360 million are chronically infected, of whom 600,000 individuals die each year from the acute or chronic consequences of hepatitis B.Citation1–4 Using data from the USA National Health and Nutrition Examination Survey for 1999–2006, Wasley and colleaguesCitation5 found there to be marked variations in the seroprevalence of the disease, defined as the presence of antibody to hepatitis B core antigen, among different age groups. Adults aged ≥50 years had a seroprevalence 1.7 and 12.8 times higher than did individuals aged 20–49 and 6–19 years, respectively.Citation5
Older adults (≥50 years) may be severely affected by hepatitis B infection, with consequences including fulminant hepatic necrosis, liver cirrhosis, chronic active hepatitis and hepatocellular carcinoma.Citation6 Prophylactic vaccination is effective in reducing morbidity and mortality due to hepatitis B infection, as well as reducing the chronic infections in populations.Citation7,8
However, an age-related decline in immune response in the elderly (immunosenescence) results in both greater susceptibility to infection and reduced response to vaccination.Citation9 A concentration of antibody against the hepatitis B surface antigens (anti-HBs) of ≥10 mIU/mL following vaccination is considered a reliable marker of protection against infection.Citation7 The rate of achieving this seroprotective response has been shown in several studies to decrease significantly with age, to 33–60% in people over 60 years of age compared with younger adults.Citation10–12
RECOMBIVAX HB™ (DNA recombinant hepatitis B vaccine, Merck & Co. Inc., New Jersey, USA), licensed in Europe as HBvaxPRO® (Sanofi Pasteur MSD SNC, Lyon, France), is a well-established hepatitis B vaccine that has been used extensively around the world since its initial licensure in 1986.
In modified process RECOMBIVAX HB™/HBvaxPRO®, the manufacturer altered the initial adjuvant by doubling the total phosphate concentration with the intention of achieving a more consistent immune response to the vaccine.Citation13 The antigenic components of RECOMBIVAX HB™/HBvaxPRO® were not changed from those present in the previous formulation of RECOMBIVAX HB™/HBvaxPRO®. The modification of the manufacturing process of RECOMBIVAX HB™/HBvaxPRO® was approved in Europe in 2008, and in the USA in 2011. Throughout this article, the modified process vaccine is referred to as HBvaxPRO®.
For population groups with a potentially decreased immune response to hepatitis B vaccine, such as older adults, this modified process HBvaxPRO® vaccine may elicit an improved immune response. A primary study examined the immunogenicity and safety profiles of the modified process HBvaxPRO® in healthy adults (≥50 years). In this primary study, individuals received either the modified process HBvaxPRO® (10 μg hepatitis B surface antigen [HBsAg]), or the previous formulation of HBvaxPRO® (10 μg HBsAg) or Engerix B™ (20 μg HBsAg) following a 0-, 1- and 6-month schedule. The majority of individuals in each group achieved seroprotection (defined as anti-HBs titer ≥10 mIU/mL) regardless of the hepatitis B vaccine used, although lower seroprotection rates (SPRs) were observed in those aged ≥65 years than in younger individuals.Citation14
This extension study was designed to describe the persistence of anti-HBs 2 or more years following primary vaccination with the hepatitis B vaccine in the primary study, and the response to a challenge dose of HBvaxPRO® in healthy adults (≥50 years old). In this study, recombinant hepatitis B surface antigen (HBvaxPRO® 10 μg) was selected as the challenge dose for all groups, as it had previously been shown to boost seroprotection adequately in individuals primed with either Engerix B™ or HBvaxPRO®.Citation15 The study was also designed to provide safety data for HBvaxPRO®.
Results
Disposition of study participants
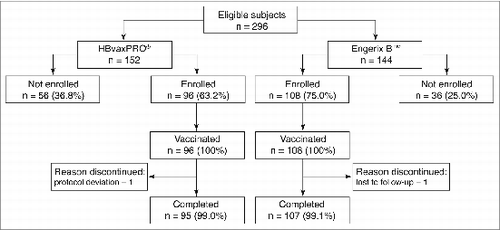
Of the 296 individuals eligible from the primary study, 204 (68.9%) were enrolled in the extension study (HBvaxPRO®, n = 96; Engerix B™, n = 108) (Fig. 1). The most frequent reasons for non-enrolment of the remaining 92 eligible subjects were consent not provided (n = 44), additional Hepatitis B vaccination after primary study (n = 18), concomitant participation in other clinical trials (n = 11) or lost to follow-up (n = 11). Only 2 individuals (1%, 1 in each group) did not complete the study. One individual was withdrawn at Visit 2 due to the blood withdrawal not being collected, and 1 was lost to follow-up.
The safety analysis set (SAS) comprised 203 (99.5%) individuals (HBvaxPRO®, n = 96; Engerix B™, n = 107). Thirteen individuals (6.4%) were excluded from the per protocol set (PPS; N = 191; HBvaxPRO®, n = 88; Engerix B™, n = 103), with a similar number excluded because of a protocol deviation (7 [3.4%]) and other conditions (6 [2.9%]), including HBsAg assay not having been done at Visit 1 (n = 3), HBsAg assay results equivocal at Visit 1 (n = 1) and anti-HBs missing at Visit 2 (n = 2).
Demographics of study population
The age of individuals ranged between 53 and 85 years, with a mean (standard deviation [SD]) age at enrollment of 63.7 (7.0) years (Table 1). Demographic characteristics were generally similar in the 2 vaccination groups. The mean (SD) time interval between completion of the primary vaccination series and receipt of the challenge dose was 3.3 (0.1) years in each group.
Table 1. Demographics of study population
Immunogenicity (PPS)
Primary analyses
A high SPR was achieved 1 month after a challenge dose of HBvaxPRO® in individuals aged ≥50 years who had received primary vaccination with hepatitis B vaccine. Regardless of the hepatitis B vaccine used in the primary vaccination series (), >85% of individuals had an anti-HBs titer ≥10 mIU/mL. The SPR pre- and 1 month post-challenge dose were numerically lower in individuals primed with HBvaxPRO® (45.5% [95% confidence interval {CI}: 34.8, 56.4] and 85.2% [76.1; 91.9], respectively) compared with those primed with Engerix B™ (58.8% [48.6, 68.5] and 88.3% [80.5, 93.8], respectively) ().
Table 2. Individuals with an anti-HBs titer ≥10 mIU/mL (primary endpoint) and geometric mean titers pre- and 1 month post-challenge with HBvaxPRO® (per protocol set)
Exploratory analyses
The geometric mean titers (GMT) pre- and 1 month post-challenge dose were numerically lower in individuals primed with HBvaxPRO® (12.3 mIU/mL [95% CI: 8.3; 18.4] and 957.4 mIU/mL [470.5; 1948.4], respectively) compared with those primed with Engerix B™ (24.1 mIU/mL [15.6; 37.3] and 1,497.6 mIU/mL [823.8; 2,722.5], respectively) ().
For individuals who were not seroprotected after the primary vaccination series (anti-HBs titer of <10 mIU/mL post-dose 3 of the primary vaccination series), response rates ≥10 mIU/mL were numerically higher for those who received HBvaxPRO® than for those who received Engerix B™ in the primary series (). For individuals who were seroprotected after the primary vaccination series and had a pre-challenge anti-HBs titer <10 mIU/mL, response rates were comparable with those in both groups ().
Table 3. Individuals with an anti-HBs titer ≥10mIU/mL pre- and 1 month post-challenge with HBvaxPRO®(exploratory analyses, individual subgroups) (per protocol set)
Safety
Data are summarized for the total population in the SAS, as all individuals received HBvaxPRO® in the extension study (). Adverse events (AEs) were reported by 87 of the 203 (42.9%) individuals included in the SAS.
Table 4. Summary of safety analysis (SAS)
Most of the injection-site AEs were solicited, and were reported by 32.5% of all vaccinated individuals, with pain being the most frequent. Injection-site AEs occurred mostly on the day of vaccination. All solicited injection-site AEs reported by ≥1% of individuals were of mild intensity, except for 4 cases of pain rated as moderate. Measurable solicited injection-site AEs had a maximum size of 2.5 cm. All injection-site AEs resolved, the majority within 3 days. No injection-site AE of severe intensity was reported. Of the 7 participants who reported unsolicited injection-site AEs, only pruritus was reported by ≥1% of individuals.
Systemic AEs were reported by 46 individuals (22.7%), with nasopharyngitis, back pain, nausea, headache, cough and oropharyngeal pain being the most frequently reported (≥2% of individuals). A total of 20 individuals (9.9%) had vaccine-related systemic AEs. Headache was the most frequently reported (5 individuals, 2.5%). There were no vaccine-related systemic AEs of severe intensity. Vaccine-related systemic AEs occurred mostly in the first 5 days following vaccination and resolved mostly within 3 days.
A maximum temperature ≥37.8°C was reported by 2 individuals (1.0%) in the 5 days following vaccination: an oral temperature of 37.9°C at Day 1 for 1 participant, and of 38.5°C at Day 5 for the other.
There was no vaccine-related serious AE (SAE) or death during the study, and no participant was withdrawn due to an AE. One systemic SAE of severe bipolar disorder due to hospitalization was reported during the study and resolved in 28 days. The individual was a 64-year-old woman with a long-standing medical history of depression.
Discussion
The main objectives of this extension study were to investigate immunogenicity and safety endpoints in individuals aged ≥50 years 2 or more years following completion of a 0-, 1- and 6-month series of HBvaxPRO® or Engerix B™ and 1 month following a challenge dose of HBvaxPRO®. The follow-up period more than 3 years in this current study was slightly longer than that in another study of antibody persistence.Citation16 The 1-month time interval following the challenge dose was consistent with studies of long-term immunogenicity for hepatitis B vaccines.Citation15,17–21 The PPS analyzed for immunogenicity was representative of the 296 eligible patients from the primary study with respect to the SPR following Dose 3.Citation14
The results of the study demonstrated high rates of individuals with anti-HBs ≥10 mIU/mL pre-challenge, which increased further at 1 month following a challenge dose of HBvaxPRO® in individuals ≥50 years of age who had received primary vaccination with hepatitis B vaccine >3 years previously. One month after challenge, the SPR was high in both groups, with more than 85% of individuals presenting an anti-HBs titer ≥10 mIU/mL, regardless of the hepatitis B vaccine used for the primary series. The immune responses (SPR and GMT) were numerically lower in subjects primed with HBvaxPRO® compared with Engerix B™, although the differences between the groups were smaller post-challenge than pre-challenge.
The immune response observed for the 2 groups in the present study were consistent with what has been reported in other studies using different age groups that also demonstrated antibody persistence and immune response to a challenge dose of HBvaxPRO® and/or Engerix B™ several years after an initial vaccination.Citation15–20,22–26
The post-challenge SPRs and GMTs observed in this extension study were also comparable to or higher than those reported at the end of the primary vaccination series (Dose 3) for both groups (HBvaxPRO® group: 79.5% and 152.9 mIU/mL, respectively, at the end of the primary study, and 85.2% and 957.4 mIU/mL, respectively, post-challenge; Engerix B™group : 88.3% and 437.2 mIU/mL, respectively, at the end of the primary series, and 88.3% and 1,497.6 mIU/mL, respectively, post-challenge).Citation14 These results are comparable with those from a similar study conducted with HBvaxPRO® and Engerix B™ in children.Citation15
For individuals who had achieved seroprotection at the end of the primary series and who had an anti-HBs titer <10 mIU/mL before the challenge dose, the post-challenge immune responses were comparable between groups regardless of the hepatitis B vaccine used for the primary series. For individuals who were not seroprotected at the end of the primary series, numerically more individuals who received HBvaxPRO® in the primary series were seroprotected compared with those who had received Engerix B™, although the clinical importance of these small differences in overall immune response may therefore not be significant.
Overall, the results of the present study demonstrate that immune memory persists in adults aged ≥50 years who have previously been primed with 3 doses of a monovalent hepatitis B vaccine. However, the response to hepatitis B vaccination is known to decline with age,Citation10,12,14 probably due to age-related changes in the interactions between B and T lymphocytes.Citation27–29 Booster vaccinations are reported to be very effective in older individuals in case of inadequate response to primary vaccination.Citation12 Routine booster vaccination is not usually recommended for children or adultsCitation3,19,20,24–26 as it is difficult to identify individuals who would benefit from the procedure.
The safety profile of a challenge dose of HBvaxPRO® 10μg was consistent with that reported in the primary study,Citation14 and in a study of HBvaxPRO® in adults,Citation13 and also with the known safety profile of the vaccine HBvaxPRO®.Citation30 If a booster dose is deemed necessary, an extra dose of HBvaxPRO® is generally well tolerated.
Patients and Methods
Study vaccine
HBvaxPRO® (batch number WL00039773) was provided as a sterile suspension for injection in single-dose pre-filled syringes as a formulation of 10 μg of HBsAg in 1 mL without preservative. A single 1 mL dose of HBvaxPRO® contained 10.00 μg of recombinant HBsAg adsorbed on amorphous aluminum hydroxyphosphate sulfate (0.50 mg). Vaccine supplies were packaged in single-dose glass vials containing 1 mL of vaccine and stored at 2–8°C.
Study design and population
This was an open-label, multicenter extension of the randomized, double-blind, multicenter primary study.Citation14 This extension study was conducted from November 2010 to April 2011 in 20 centers from the primary study in Canada, Denmark, Sweden and the UK. The protocol was approved by national and local ethics review committees and the study was conducted in accordance with the Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association, Declaration of Helsinki, as amended in Seoul, October 2008, the International Conference on Harmonization (ICH), Good Clinical Practice and local laws, rules and regulations. All participants gave their written informed consent to take part in the study.
Among participants of the primary study, only those from the current HBvaxPRO® and Engerix B™ groups were selected, as both vaccines are currently licensed. Participants who had received 3 doses of either HBvaxPRO® or Engerix B™ at least 2 years prior to enrollment, who had completed the primary study as per protocolCitation14 and who complied with the current study protocol requirements were eligible for the extension study. All enrolled individuals received by intramuscular injection a single challenge dose of HBvaxPRO® (10 μg/mL) to mimic hepatitis B virus exposure at least 2 years after the primary vaccination. All individuals enrolled into this extension study retained the same allocation number that was assigned during their participation in the primary study; serum samples were not blinded during this study.
All individuals were required to record any AEs that occurred within 15 days following vaccination. Individuals were instructed to record oral evening temperature daily for the first 5 days following vaccination using a vaccination report card. The investigator was to be informed immediately in case of hospitalization or SAE.
Serology samples were obtained on Day 1 prior to the challenge dose and 30 days post-challenge dose. Blood samples were tested at a central laboratory (PPD Vaccines and Biologics, Wayne, PA, USA) using hepatitis B-enhanced chemiluminescence assayCitation31 (Hep B ECi; Anti-HBs Quantitative Reagent Pack, Ortho Clinical Diagnostics, Inc..) to evaluate the serological response to the vaccine and to detect baseline anti-HBs.
Objectives and endpoints
Immunogenicity
The primary objective was to describe the SPR as measured by the percentage of individuals with an anti-HBs titer ≥10 mIU/mL at least 2 years following completion of a primary series of HBvaxPRO® or Engerix B™ and 1 month following a challenge dose of HBvaxPRO®.
Exploratory objectives were to assess the GMT at least 2 years following completion of a primary series of HBvaxPRO® or Engerix B™ and 1 month following a challenge dose of HBvaxPRO®.
Safety
Secondary objectives were to assess the safety and tolerability of the challenge dose of HBvaxPRO®. Safety endpoints were the percentages of individuals with solicited injection-site AEs (pain, erythema and swelling) and fever (oral temperature ≥37.8°C) from Day 1 to Day 5 inclusive; unsolicited injection-site and systemic AEs and SAEs from Day 1 to Day 15 inclusive; and vaccine-related SAEs (considered by the investigator to be possibly, probably or definitely related to the study vaccine) and deaths occurring at any time during the study.
Statistical analyses
Sample size
A total of 296 participants completed the primary study in accordance with the protocol. Of these, 152 received HBvaxPRO® and 144 received Engerix B™. Assuming that ≥50% of these individuals would re-enroll and complete this extension study after at least 2 years had elapsed following the initial primary vaccination series, at least 75 evaluable individuals per group were anticipated. It was estimated that the true SPR in individuals receiving a single challenge dose of HBvaxPRO® would be 50%, consistent with the overall lower immune responsiveness seen with increasing age.Citation32,33 This sample size would detect a CI half-width of approximately 13 percentage points for the SPR for each group. A larger sample size or a higher or lower SPR would lead to a smaller CI half-width around the estimate.
Immunogenicity
The primary immunogenicity analyses and summaries were conducted on the PPS, which excluded subjects with protocol deviations that could interfere with immune responses, and subjects generating positive (or equivocal) antibodies to hepatitis B core antigen or HBsAg before the challenge dose (results indicating a prior or ongoing hepatitis B infection).
The number and percentage of individuals with anti-HBs titer ≥10 mIU/mL and ≥100 mIU/mL, and the GMT were assessed for each group, defined as the vaccine received in the primary study. CIs for percentages were calculated based on the exact method for binomial data. The CIs for the GMTs were based on the natural log-transformed titers and the t-distribution. Data were summarized at pre- and post-challenge timepoints using the methodology described above, and were provided in each group for the full population and for the following 3 sub-populations.
| 1. | Individuals who were not seroprotected in the primary study (anti-HBs titer ≤10 mIU/mL) after receiving the primary vaccination series. | ||||
| 2. | Individuals who were seroprotected in the primary study after receiving the primary vaccination series and at the pre-challenge follow-up timepoint. | ||||
| 3. | Individuals who were seroprotected in the primary study after receiving a primary vaccination series, but had dropped below the seroprotection level at the pre-challenge follow-up timepoint. | ||||
Safety
All individuals who received a challenge dose in this study and had safety follow-up data were included in the safety and tolerability analysis (SAS). All data were summarized regardless of the vaccine received in the primary study. All injection-site AEs were considered as related to the study vaccine, while systemic AEs were considered unrelated or vaccine-related according to the investigator's assessment. Descriptive statistics were provided for safety endpoints.
Conclusions
The data from this open-label extension study of HBvaxPRO® vaccine showed it was well tolerated and highly immunogenic. A challenge dose of this vaccine demonstrated an immune memory against hepatitis B for up to 3 years after a 0-, 1- and 6-month vaccination schedule in adults aged ≥50 years.
Disclosure of Potential Conflicts of Interest
AD is an employee of Dynamic Research, Inc.. CT, ST and CE are employees of Sanofi Pasteur MSD. Andrew Wen-Tseng-Lee is a shareholder. RS, CA and LO have no other conflicts of interest.
Authors' Contributions
RS, CA, LO and AD were involved in patient enrolment and patient data acquisition. CT participated in the implementation and conduct of the study. ST was the biostatistician for the study, responsible for data analysis and interpretation. CE prepared the outline and first draft. All authors critically reviewed and approved the final version of the manuscript, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
The authors take full responsibility for the content of this contribution and thank Communigen® Ltd, Oxford, UK (supported by Sanofi Pasteur MSD SNC), for editorial support in preparing the drafts. The authors would like to thank the individuals who participated in the trial, as well as the investigators and their study-site personnel for their contribution to the study. They also wish to thank: Anne Fiquet, Xavier Cornen, Sylvie Dard and Elodie Turpeaud (Sanofi Pasteur MSD SNC) and ICON Clinical Research for their contribution to the conduct of the study; Andrew Wen-Tseng-Lee (Merck Research Laboratory) for his input during the study design phase; Jean Leparc, Priscilla Martinon and Julie Willingham-Peacock (Sanofi Pasteur MSD SNC) and Andrew Wen-Tseng-Lee (Merck Research Laboratory) for their critical review of the study results; and Cheryl L. Moyer (PPD Vaccine and Biologics LLC, Wayne, PA, USA) for overseeing the immune tests.
Funding
This study was supported by Sanofi Pasteur MSD SNC (sponsor). Although the sponsor reviewed drafts of the manuscript, the opinions expressed are those of the authors and may not necessarily reflect those of the sponsor. All authors approved the final version of the manuscript.
HBvaxPRO® Protocol rHB01C Study Group. Canada: T. Araki, H. Conter, A. Dowell, M. Ferguson, L. Frenette, B. Lasko, S. McNeil, D. Shu, E. St-Amour. Denmark: L. Ostergaard, S. Stenvang. Sweden: C. Ahlm, K. Pauksens, B. Strandell, P. Wågström. UK: A. Graham, P. Harvey, D. Haworth, R. Sharma, N. Wyatt.
References
- Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol 2005; 34(6):1329–39; PMID:16249217; http://dx.doi.org/10.1093/ije/dyi206
- World Health Organization. Hepatitis B vaccines WHO position paper. WER 2004; 79:255–63; PMID:15344666
- World Health Organization. Hepatitis B vaccines: WHO position paper – Recommendations. Vaccine 2010; 28(3):589–90; PMID:19896455; http://dx.doi.org/10.1016/j.vaccine.2009.10.110
- World Health Organization. Hepatitis B. Fact sheet no. 204. July 2013. Geneva: Switzerland.
- Wasley A, Kruszon-Moran D, Kuhnert W, Simard EP, Finelli L, McQuillan G, Bell B. The prevalence of hepatitis B virus infection in the United States in the era of vaccination. J Infect Dis 2010; 202(2):192–201; PMID:20533878; http://dx.doi.org/10.1086/653622
- Pan CQ, Zhang JX. Natural history and clinical consequences of hepatitis B virus infection. Int J Med Sci 2005; 2(1):36–40; PMID:15968338; http://dx.doi.org/10.7150/ijms.2.36
- Van Damme P, Ward J, Shouval D, Wiersma S, Zanetti A. Hepatitis B Vaccines. Vaccines. In: Plotkin SA, Orenstein WA, Offit PA, editors. Philadelphia: Saunders Elsevier; 2012; Chapter 15: 205–34.
- European Centre for Disease Prevention and Control (ECDC). Factsheet for general public: Hepatitis B. Available at: http://www.ecdc.europa.eu/en/healthtopics/hepatitis_B/factsheet-general-public/Pages/factsheet-general-public.aspx (accessed 17 June 2014).
- Grubeck-Loebenstein B, Della Bella S, Iorio AM, Michel JP, Pawelec G, Solana R. Immunosenescence and vaccine failure in the elderly. Aging Clin Exp Res 2009; 21(3):201–9; PMID:19571643; http://dx.doi.org/10.1007/BF03324904
- deRave S, Heijtink RA, Bakker-Bendik M, Boot J, Schalm SW. Immunogenicity of standard and low dose vaccination using yeast-derived recombinant hepatitis B surface antigen in elderly volunteers. Vaccine 1994; 12(6):532–4; PMID:8036828; http://dx.doi.org/10.1016/0264-410X(94)90313-1
- Looney RJ, Hasan MS, Coffin D, Campbell D, Falsey AR, Kolassa J, Agosti JM, Abraham GN, Evans TG. Hepatitis B immunization of healthy elderly adults; relationship between naïve CD4+ T cells and primary immune response and evaluation of GM-CSF as an adjuvant. J Clin Immunol 2001; 21:30–6; PMID:11321236; http://dx.doi.org/10.1023/A:1006736931381
- Wolters B, Junge U, Dziuba S, Roggendorf M. Immunogenicity of combined hepatitis A and B vaccine in elderly persons. Vaccine 2003; 21(25–26):3623–8; PMID:12922091; http://dx.doi.org/10.1016/S0264-410X(03)00399-2
- Van Damme P, Minervini G, Liss C, McCarson B, Vesikari T, Boslego JW, Bhuyan PK. Safety, tolerability and immunogenicity of a recombinant hepatitis B vaccine manufactured by a modified process in healthy young adults. Hum Vaccin 2009; 5(2):92–7; PMID:18690015; http://dx.doi.org/10.4161/hv.5.2.6587
- Gilbert CL, Klopfer SO, Martin JC, Schödel FP, Bhuyan PK. Safety and immunogenicity of a modified process hepatitis B vaccine in healthy adults ≥50 years. Hum Vaccin 2011; 7(12):1336–42; PMID:22185811; http://dx.doi.org/10.4161/hv.7.12.18333
- Diez-Domingo J, Flores SA, Martin JC, Klopfer SO, Schödel FP, Bhuyan PK. A randomized, multicenter, open-label clinical trial to assess the anamnestic immune response 4 to 8 years after a primary hepatitis B vaccination series. Pediatr Infect Dis J 2010; 29(10):972–4; PMID:20724955; http://dx.doi.org/10.1097/INF.0b013e3181f1b3b6
- Cassidy WM, Watson B, Ioli VA, Williams K, Bird S, West DJ. A randomized trial of alternative two- and three- dose hepatitis B vaccination regimens in adolescents: antibody responses, safety, and immunogenic memory. Pediatrics 2001; 107(4):626–31; PMID:11335734; http://dx.doi.org/10.1542/peds.107.4.626
- Giambi C, Bella A, Barale A, Montù D, Marchisio M, Oddone M, Zito S, Rapicetta M, Chionne P, Madonna E, Ciofi degli Atti ML. A cohort study to evaluate persistence of hepatitis B immunogenicity after administration of hexavalent vaccines. BMC Infect Dis 2008; 8:100; PMID:18662386; http://dx.doi.org/10.1186/1471-2334-8-100
- Gilca V, De Serres G, Boulianne N, Murphy D, Ouakki M, De Wals P, Trudeau G, Masse R, Dionne M. Long-term persistence of immunity after vaccination of pre-adolescents with low doses of a recombinant hepatitis B vaccine. Hum Vaccin Immunother 2013; 9(8):1685–90; PMID:23744506; http://dx.doi.org/10.4161/hv.25015
- Reinert P, Cinquetti S, Soubeyrand B, Biasio LR, Meghlaoui G, Thomas S, Watson M. Challenge with hepatitis B vaccine in children previously vaccinated with an hepatitis B containing combination vaccine. Adv Ther 2010; 27:28–38. [Erratum in Adv Ther 2010;27:192.]; PMID:20182924; http://dx.doi.org/10.1007/s12325-010-0005-x
- Zanetti A, Parlato A, Roman∫ L, Desole MG, Ferrera G, Giurdanella F, Zuliani M, Richard P, Thomas S, Fiquet A. Challenge with a hepatitis B vaccine in two cohorts of 4–7-year-old children primed with hexavalent vaccines: an open-label, randomised trial in Italy. Vaccine 2012; 30(39):5770–5; PMID:22789511; http://dx.doi.org/10.1016/j.vaccine.2012.06.078
- Zinke M, Kappes R, Kindler K. Paulus-Koschik A, Goering U, Disselhoff J, Soemantri P, Grunert D, Laakmann KH, Gunasekaran R, et al. Immune memory to hepatitis B virus in 4–9-year-old children vaccinated in infancy with four doses of hexavalent DTPa-HBV-IPV/Hib vaccine. Hum Vaccin 2009; 5:592–8; PMID:19535920; http://dx.doi.org/10.4161/hv.9051
- Carollo M, Palazzo R, Bianco M, Pandolfi E, Chionne P, Fedele G, Tozzi AE, Carsetti R, Romano L, Ausiello CM. Hepatitis B specific T cell immunity induced by primary vaccination persists independently of the protective serum antibody level. Vaccine 2013; 31:506–13; PMID:23174200; http://dx.doi.org/10.1016/j.vaccine.2012.11.029
- Zanetti AR, Roman∫ L, Giambi C, Pavan A, Carnelli V, Baitelli G, Malchiodi G, Valerio E, Barale A, Marchisio MA, et al. Hepatitis B immune memory in children primed with hexavalent vaccines and given monovalent booster vaccines: an open-label, randomised, controlled, multicentre study. Lancet Infect Dis 2010; 10:755–761; PMID:20884297; http://dx.doi.org/10.1016/S1473-3099(10)70195-X
- Poovorawan Y, Chongsrisawat V, Theamboonlers A, Bock HL, Leyssen M, Jacquet J-M. Persistence of antibodies and immune memory to hepatitis B vaccine 20 years after infant vaccination in Thailand. Vaccine 2010; 28:730–6; PMID:19892043; http://dx.doi.org/10.1016/j.vaccine.2009.10.074
- Poovorawan Y, Chongsrisawat V, Theamboonlers A, Leroux-Roels G, Crasta PD, Hardt K. Persistence and immune memory to hepatitis B vaccine 20 years after primary vaccination of Thai infants, born to HBsAg and HBeAg mothers. Hum Vaccin Immunother 2012; 8(7):896–904; PMID:22777097; http://dx.doi.org/10.4161/hv.19989
- Van Damme P, Leroux-Roels G, Crasta P, Messier M, Jacquet J-M, Van Herck K. Antibody persistence and immune memory in adults, 15 years after a three-dose schedule of a combined Hepatitis A and B vaccine. J Med Virol 2012; 84:11–17; PMID:22052690; http://dx.doi.org/10.1002/jmv.22264
- Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis 2008; 46(7):1078–84; PMID:18444828; http://dx.doi.org/10.1086/529197
- Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol 2009; 9(3):185–94; PMID:19240757; http://dx.doi.org/10.1038/nri2508
- Haynes L, Swain SL. Why aging T cells fail: implications for vaccination. Immunity 2006; 24(6):663–6; PMID:16782020; http://dx.doi.org/10.1016/j.immuni.2006.06.003
- HBVaxPro: Summary of product characteristics Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000373/human_med_000813.jsp&mid=WC0b01ac058001d124 (accessed 24 October 2014).
- Lee AW, Vesikari T, Gilbert CL, Klopfer SO, Schödel FP, Bhuyan PK. Immunogenicity and safety of a Haemophilus influenzae B (Hib)-hepatitis B vaccine with a modified process hepatitis B component administered with concomitant pneumococcal conjugate vaccine to infants. Vaccine 2011; 29(45):7942–8; PMID:21875633; http://dx.doi.org/10.1016/j.vaccine.2011.08.071
- Ginaldi L, De Martinis M, D'Ostilio A. The immune system in the elderly: I Specific humoral immunity. Immunol Res 1999; 20(2):101–8; PMID:10580635; http://dx.doi.org/10.1007/BF02786466
- Paganelli R, Scala E, Quinti I, Ansotegui IJ. Humoral immunity in aging. Aging Clin Exp Res 1994; 6(3):143–50; PMID:7993921; http://dx.doi.org/10.1007/BF03324229