Abstract
Maternal immunization is an important strategy to prevent severe morbidity and mortality in mothers and their offspring. This study aimed to identify whether new parents were following immunization recommendations prior to pregnancy, during pregnancy, and postnatally. A cross-sectional survey was conducted by a questionnaire administered antenatally to pregnant women attending a maternity hospital with a follow-up telephone interview at 8–10 weeks post-delivery. Factors associated with uptake of pertussis vaccination within the previous 5 y or postnatally and influenza vaccination during pregnancy were explored using log binomial regression models. A total of 297 pregnant women completed the questionnaire. For influenza vaccine, 20.3% were immunized during pregnancy and 3.0% postnatally. For pertussis vaccine, 13.1% were vaccinated within 5 y prior to pregnancy and 31 women received the vaccine postnatally, 16 (51.6%) received the vaccine >4 weeks after delivery. Receiving a recommendation from a healthcare provider (HCP) was an independent predictor for receipt of both pertussis (RR 2.07, p < 0.001) and influenza vaccine (RR 2.26, p = 0.001). Non-English speaking mothers were significantly less likely to have received pertussis vaccination prior to pregnancy or postnatally (RR 0.24, p = 0.011). Multiparous pregnant women were less likely to have received an influenza vaccine during their current pregnancy (p = 0.015). Uptake of pregnancy related immunization is low and likely due to poor knowledge of availability, language barriers and lack of recommendations from HCPs. Strategies to improve maternal vaccine uptake should include education about recommended vaccines for both HCPs and parents and written information in a variety of languages.
Introduction
To reduce the morbidity and mortality from infectious diseases in pregnant women and their newborns, many countries recommend influenza immunization during pregnancy and pertussis immunization prior to pregnancy as part of pregnancy planning.Citation1,2 In Australia, the National Health and Medical Research Council guidelines recommend that a pre-conception health check should include assessment of measles, mumps, rubella (MMR), varicella, diphtheria, tetanus and pertussis immunization status.Citation1
The majority of hospitalisations and deaths from pertussis occur in infants less than 6 months of age as they have not received a complete course of pertussis immunization Citation3-5 and infection mainly occurs via transmission from parents with waning vaccine-induced immunity.Citation6-8 Based on this evidence, it is recommended that potential parents and other adults within the same household receive a pertussis containing vaccine if not received in the preceding 5 y as part of a “cocooning” strategy. In response to previous pertussis epidemics, authorities in the United States of America (USA), the United Kingdom (UK), New Zealand and Australia now recommend pertussis immunization in the third trimester of pregnancy to protect newborns.Citation9-11
Pregnant women are also at increased risk of severe illness, hospitalization and death from influenza, particularly evident during the H1N109 Influenza Pandemic.Citation12-14 Influenza immunization has been recommended as the most effective way of preventing hospitalizations and severe influenza-related complications in pregnant women and their infants to 6 months of age.Citation15-18 In Australia, seasonal influenza vaccine is available from March to October each year during the peak influenza season and is generally provided by family physicians.
This study aimed to determine whether parents were following the current immunization recommendations prior to, during and post-pregnancy. Our primary objective was to determine the proportion of pregnant women who had received a pertussis vaccine as part of the cocooning strategy, and/or influenza vaccine during pregnancy. We also sought to determine facilitators and barriers to uptake of maternal immunizations.
Results
Survey population and response rate
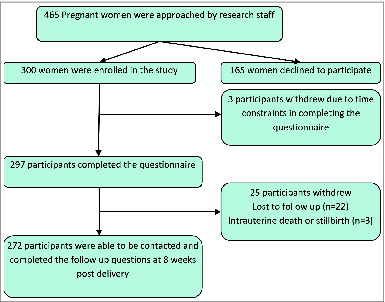
Of 465 pregnant women approached in the antenatal public and private obstetric clinics from December 2010-August 2011, 300 (64.5%) enrolled in the study and 297 completed the questionnaire (). Women were asked if they were interested in participating in a research study on immunisation. Postnatal follow-up telephone calls were completed for 272 (91.6%) enrolled participants.
The mean age of participants was 30.4 y (range 17–44 years). The majority of respondents were Caucasian (86.9%; n = 258) with 10.1% (n = 30) of Asian ethnicity. The majority of participants were born in Australia (73.7%, n = 219), married (61.6%, n = 183) and almost half had no previous children (46.8%, n = 139) (). These sample characteristics are similar to South Australian or Australian population characteristics for pregnant women according to the 2013 Australian Bureau of Statistics (ABS) data.Citation19 The ABS data indicates the median age for South Australian pregnant women as 30.4 y and the proportion of South Australian pregnant women who were married as 63.9%. Country of Mothers birth was also similar between our sample and Australian ABS data with Australia as the predominant birth country of new mothers (73.7% vs 67.5%). The proportion of pregnant women with no previous children was also similar between our cohort (46.8%) and ABS data for Australian (44.7%).
Table 1. Demographic characteristic of respondents at antenatal interviews (n = 297)
Uptake of recommended vaccines
Pertussis
A total of 67 women (22.6%) had received pertussis vaccination within the previous 5 y (n = 39) or following birth (n = 31). Three of these participants received both pertussis vaccine prior to pregnancy and postnatally.
While 43.1% (n = 128) of respondents reported having received a pertussis (whooping cough) vaccine during their lifetime, only 1.3% (n = 4) received the immunization as part of their pregnancy planning. Almost a quarter (24.2%, n = 72) of participants were unsure whether they had previously received a “whooping cough” vaccine. Of the 128 women who reported receiving pertussis vaccination, 12.5% (n = 16) had done so within the preceding 12 months and a further 18.0% (n = 23) had been immunized between 12 months and 5 y prior. The remaining 64.1% (n = 82) received their last pertussis vaccine more than 5 y previously and 5.6% (n = 7) could not remember when it had been administered.
Factors associated with receiving pertussis vaccination prior to pregnancy or postnatally, were explored. Knowledge of pertussis vaccine availability prior to the study, a recommendation from a HCP, English as first language, age greater than 30 y and higher level of education were univariate predictors of having received pertussis vaccination. English as a first language and recommendation from a HCP remained significantly associated with uptake in a multiple log binomial regression analysis. Women who had English as a second language were almost 5 times less likely (RR 0.24, p = 0.011) to have received a pertussis vaccination within the previous 5 y or postnatally ().
Table 2. Predictors of receipt of pertussis vaccination within last 5 y or following birth
Influenza
Of 237 mothers whose influenza vaccination status was able to be determined, only 48 women (20.3%) received the influenza vaccine during their pregnancy, and an additional 7 mothers (3.0%) received the vaccine postnatally. In a multiple regression analysis, multiparous pregnant women were up to 68% less likely to receive influenza vaccine during pregnancy compared with nulliparous women (p = 0.017). Women who had received a recommendation to receive an influenza vaccine from a HCP were 2.26 times more likely to have received the vaccine during pregnancy than those who had not received a recommendation (p = 0.001). Women who were aware of the availability of influenza vaccination during pregnancy prior to study participation were 3.14 times more likely to be vaccinated (p = 0.026) ().
Table 3. Predictors of receipt of influenza vaccination during pregnancy
A total of 51.5% (n = 153) of mothers reported having received the influenza vaccine during their lifetime. Of these 153 individuals, 46.4% (n = 71) had received their last influenza vaccine in the preceding 12 months, with 41.2% (n = 63) having received it between 12 months and 5 y prior and 12.4% (n =19) having received an influenza vaccine more than 5 y prior.
Parents' knowledge of vaccine preventable diseases and corresponding immunizations
Pertussis (whooping cough)
Almost all respondents (95.0%, n = 282) indicated they had heard of whooping cough although only a minority of pregnant women (37.7%, n = 112) were aware that a pertussis vaccine was available prior to pregnancy or postnatally. Their source/s (multiple responses) of information included various HCPs (41.1%, n = 46; midwives and obstetricians (n = 19), family physicians (n = 27)), family/or friends (n = 24) and media (n = 18).
Influenza
The majority (70.0%, n = 208) of respondents were aware that influenza vaccine was available prior to, or during pregnancy. Almost half of these respondents (45.7%; n = 95) reported the source of information about influenza vaccine was their HCP. Other sources reported included media (n = 40), workplace (n = 23), family and friends (n = 22), posters and leaflets (n = 10) and university or school (n = 3).
Less than a quarter of respondents (21.9%, n = 65) had received a recommendation from a HCP to receive an influenza vaccine prior to conception or during pregnancy. The majority of these recommendations came from family physicians (90.8%, n = 59), with recommendations also reported from midwives (n = 4), a travel doctor (n = 1) and an obstetrician (n = 1).
Uptake of vaccines in the postpartum period
A total of 272 follow-up phone calls were completed. Of these, a total of 15.4% (n = 42) respondents indicated they had received one or more vaccines postnatally, including pertussis vaccine (n = 31), both influenza and pertussis (n = 5), influenza alone (n = 2) or both varicella and pertussis (n = 1). Pertussis immunization was confirmed either by date of administration or with the immunization provider for all 31 mothers. Furthermore, 12.7% (n = 35) of women reported their partner had received a pertussis vaccine. Three mothers received the vaccine within the first week after delivery with the remaining mothers being immunized between 8- 90 d post-delivery (median 38 d post-delivery). The most common reason cited for receiving a pertussis vaccine was for newborn protection (36.6%), with 33.3% of respondents receiving a recommendation from HCPs and 16.6% from family and friends. Three mothers stated their decision had been influenced by participation in the study and 3 were influenced by knowledge of the pertussis epidemic.
Of those who had not received a pertussis or influenza vaccine since the birth of their baby and were contactable (n = 230), commonly cited reasons were that vaccine/s were not offered or discussed, or they had no awareness of their need for immunization (30.9%, n = 71), a belief that immunization so soon after delivery was unnecessary (17.8%, n = 41), being time poor after delivery (17.4%, n = 40) or simply forgetting (13.5%, n = 31) ().
Table 4. Reasons why women did not receive any recommended vaccines in the postpartum period (n = 230)
Intention to accept the pertussis/influenza vaccines
A total of 73.4% (n = 218) of respondents indicated they would have received a pertussis vaccine prior to pregnancy had it been recommended to them. During antenatal care, only 16.2% (n = 48) had received a recommendation to receive pertussis vaccine postnatally. Of the 258 women who had not received a pertussis vaccine within the previous 5 years, 60.9% (n = 157) had intended to receive the pertussis vaccine after their baby was born, 28.7% (n = 74) were undecided and 9.3% (n = 24) had never intended to have the vaccine. One hundred and fifty four (59.7%) participants reported they would have received it had it been recommended, while 16.3% (n = 42) were undecided.
For the 226 women who had not received an influenza vaccine in the previous 12 months, 120 (53.1%) stated they would have received an influenza vaccine had it been recommended, and a further 41 (18.1%) were undecided.
Identifying concerns/barriers for maternal immunization
Over a third (35.1%, n = 104) of respondents indicated they had concerns about receiving a booster pertussis vaccine. The most common concerns were of potential side-effects of the vaccine to themselves (22.6%, n = 67) or their infants through breast feeding (12.2%, n = 36) or ineffectiveness of the vaccine (16.2%, n = 48). A minority indicated cost as a concerning factor (3.4%, n = 10) or disliked injections (5.7%, n = 17).
Over half of the women surveyed (54.2%, n = 161) indicated they had concerns about having any vaccine while pregnant. The most commonly reported concerns were potential side effects to themselves 46.7% (n = 134) or their unborn baby 40.1% (n = 115). Cost was a reported concern for 4.5% (n = 13) and 4.2% (n = 12) disliked injections.
Comment
Our results show low uptake of all recommended immunizations related to pregnancy, particularly pertussis and influenza immunization. Despite most respondents being aware of the recommendation for influenza vaccine during pregnancy, of those who had not received it as part of pregnancy planning, the majority of women agreed they would have received the vaccine had it been recommended to them. Many respondents had concerns about potential side effects for themselves and/or their unborn child, and therefore avoided immunization during pregnancy.
In this study, a recommendation to receive vaccines provided greater likelihood of immunisation. This emphasizes the importance of knowledge provision from HCPs to improve immunization uptake for pregnant women and their partners when planning a pregnancy, with appropriate educational materials provided to HCPs to ensure they are aware of the current recommendations and reasons influencing decision making by parents/ mothers.
Women with English as a second language and lower educational levels were less likely to have received influenza or pertussis vaccinations. This suggests that current available information may be insufficient or inaccessible to these groups. Educational materials that are sensitive to ethnic diversity, easily readable and accessible to all new parents should be a priority for policy makers.
At the time of this study, cocooning was the only recommended strategy in Australia to provide protection to unimmunized or partly immunized infants. A recent study has shown evidence that pertussis immunization prior to conception or within 4 weeks after birth was protective against pertussis infection in infants.Citation20 Unfortunately, the majority of mothers in our study received the vaccine at least 4 weeks after delivery when there is less evidence of such benefit. Awareness of cocooning strategy is low in South Australia and this may relate to absence of funding for this progam. In 2011, all States in Australia, except South Australia and Tasmania, provided funding to subsidise the cost of the pertussis vaccine for new parents.Citation21 When a recommended vaccine is not funded it may be perceived as less important than funded vaccines, or become inaccessible due to financial difficulties, thus reducing uptake.Citation22 In addition, while the majority of women who had not recently been immunized with pertussis vaccine intended to receive the pertussis vaccine postnatally, very few followed through with this intention. Previous studies have shown that intent does not necessarily correlate with uptake.Citation23 Mothers indicated less concern about receiving pertussis immunization during pregnancy than other vaccines. The primary reason given for not having received pertussis vaccine postnatally was that it had not been offered to them or discussed with them, or that mothers were not aware of the health benefits of immunization in this setting. These are all potentially significant barriers to immunization receipt. A small number of women were alerted to the recommendation through participation in this study suggesting receipt of minimal information has the potential to improve uptake.
A recent study in the USA showed a high proportion (72%) of women received pertussis vaccine in the postpartum period when it was provided by the hospital before discharge. When women who had not been offered the vaccine were excluded from the analysis, however, uptake was 96.2%.Citation24 In this study, some women indicated that they did not receive the immunization after pregnancy because they were too busy after their baby was born. If pertussis immunization were available to postpartum women before hospital discharge this would be likely to increase uptake. This may also explain why multiparous women were less likely to receive influenza vaccine. Alternatively multiparous women may have considered repeat influenza immunization unnecessary. These data suggest that multiparous pregnant women should be targeted in influenza vaccine campaigns.
A limited proportion of mothers understood the importance or availability of pregnancy-related immunisation. A number of studies indicate that recommendations from HCPs play a major role in parents' decision making about vaccine acceptance.Citation25-29 A large proportion of the women in this study did not receive pre-pregnancy immunization planning, thus making maternal and postpartum pertussis immunization recommendation by HCPs even more important.
An effective maternal immunization program is reliant upon confident communication between HCP and prospective parents about the benefits and risks of pregnancy related immunization to optimise protection for pregnant women and their newborns. It is imperative that information to assist in vaccine awareness and vaccination decision making in Australian women with a non-English speaking background becomes widely available. This will begin to address the barriers to vaccination which may benefit all women and their newborns.
Materials and Methods
Study design
This cross-sectional observational study was undertaken between December 2010 and September 2011 at the Women's and Children's Hospital (WCH). The WCH is the largest of 3 major public maternity hospitals in South Australia, providing maternity care for metropolitan Adelaide and is the primary tertiary maternity hospital for complex care with approximately 5,000 births per year. Both public and private patients with diverse ethnicity and socio-economic status attend this obstetric hospital and were approached for participation in this study at any gestational period.
Interviews of pregnant women using a survey questionnaire
A questionnaire directed interview was held with pregnant women, to identify whether prospective parents were following or intending to follow immunization recommendations for pregnant women or those planning a pregnancy. This questionnaire was developed to identify demographic and other factors associated with uptake of cocooning strategy and influenza vaccination based on previous literature and questionnaires developed and published by the research team.Citation30 A mixture of yes/no response and open-ended questions were used. A follow-up telephone call was made to participants 8 to 10 weeks after the birth of their baby to record any vaccines that had been received since the initial interview and determine the actual uptake of the recommended immunizations. A non-medical researcher asked the survey questions without any additional information being provided about the diseases under consideration, so as not to bias the participants' decisions about receiving further vaccinations. Information was collected at a follow-up call to ascertain reasons why mothers received any further immunizations and to identify whether participation in the study had influenced their decision.
Participant recruitment
Women attending the public and private antenatal clinics at the Women's and Children's Hospital (WCH) were provided with study information and invited to participate following informed consent until the desired sample size (n = 297) was achieved. Pregnant women were eligible to participate regardless of gestation or expected delivery date (peak influenza season or otherwise) with language barriers being the only exclusion criteria. The questionnaire was only available in English, although an Asian language interpreter was available. Participants' demographic characteristics, immunization history, awareness and knowledge of, and attitudes toward pregnancy-related immunization were recorded.
Statistical analyses
The sample size was estimated on the primary outcome: the expected proportion of mothers who had received a pertussis vaccine either within the previous 5 y or following delivery. An adult vaccination survey in 2009,Citation31 estimated that 7.8% of South Australians received a pertussis vaccine as an adult. Using a sampling error of 0.03 (i.e. 3% above or below the expected estimate) a sample size of 297 was calculated.
Multivariable analysis was used to identify factors independently associated with pertussis and influenza vaccine uptake. Predictors that had a global p value <0.15 in univariate models were included into a multivariable binomial regression model and outcomes reported as risk ratios with 95% confidence intervals. A two-tailed p-value of less than 0.05 was considered to be statistically significant. Statistical analyses were performed using Stata statistical software, Version 11, College station: Stata corporation 2010.Citation32
The study protocol was approved by the Women's and Children's Health Network Human Research Ethics Committee. Informed consent was obtained from all participants.
Disclosure of Potential Conflicts of Interest
HM reports grants from Sanofi-Pasteur and GlaxoSmithKline for unrelated investigator led projects. The remaining authors report no conflict of interest.
Authors' Contributions
CW and NT assisted with study design, data collection, statistical analysis and prepared first draft of the manuscript under direct supervision of HM. CB contributed to study design and review and editing of the manuscript. JT assisted with data analysis and manuscript writing and editing. MC assisted in statistical design, analysis and manuscript writing. HM contributed to and reviewed and edited the manuscript. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed.
Acknowledgments
We would like to acknowledge and thank all the parents who participated in the questionnaire and the staff of the Vaccinology and Immunology Research Trials Unit (VIRTU) at the Women's and Children's Hospital.
Funding
Associate Professor Helen Marshall is supported by an NHMRC Career Development Fellowship No. 1016272.
References
- The Australian Government Department of Health and Ageing. The Australian Immunisation Handbook. 9th ed. Canberra; 2008.
- Mak TK, Mangtani P, Leese J, Watson JM, Pfeifer D. Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infect Dis 2008; 8(1): 44-52; PMID:18156088; http://dx.doi.org/10.1016/S1473-3099(07)70311-0
- Blumer C, Roche P, Spencer J, Lin M, Milton A, Bunn C, Gidding H, Kaldor J, Kirk M, Hall R, et al. Australia's notifiable diseases status, 2001: annual report of the national notifiable diseases surveillance system. Commun Dis Intell Q Rep 2003; 27(1): 1-78; PMID:12725505
- Chuk LM, Lambert SB, May ML, Beard FH, Sloots TP, Selvey CE, Nissen MD. Pertussis in infants: how to protect the vulnerable? Commun Dis Intell Q Rep 2008; 32(4):449-56; PMID:19374274
- Elliott E, McIntyre P, Ridley G, Morris A, Massie J, McEniery J, Knight G. National study of infants hospitalized with pertussis in the acellular vaccine era. PIDJ 2004;23(3):246-52; PMID:15014301
- Wendelboe AM, Njamkepo E, Bourillon A, Floret DD, Gaudelus J, Gerber M, Grimprel E, Greenberg D, Halperin S, Liese J, et al. Transmission of Bordetella pertussis to young infants. PIDJ 2007; 26(4):293-9; PMID:17414390
- Schellekens J, von König CH, Gardner P. Pertussis sources of infection and routes of transmission in the vaccination era. PIDJ 2005; 24(5 Suppl):S19-24; PMID:15876919
- Jardine A, Conaty SJ, Lowbridge C, Staff M, Vally H. Who gives pertussis to infants? Source of infection for laboratory confirmed cases less than 12 months of age during an epidemic, Sydney, 2009. Commun Dis Intell Q Rep 2010; 34(2):116-21; PMID:20677421
- Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged < 12 months - advisory committee on immunization practices (ACIP), 2011. MMWR Morb MortalWkly Rep 2011; 60(41):1424-6; PMID:22012116
- National Health Service. 2012 Whooping cough vaccination in pregnancy. 11th December 2012. Available at:http://www.nhs.uk/conditions/pregnancy-and-baby/Pages/Whooping-cough-vaccination-pregnant.aspx#Can [accessed 14 February 2013]
- New Zealand Ministry of Health. 20 Dec 2012. Fax to GPs - Free pertussis vaccination for pregnant women, 2013 flu programme, IMAC holiday hours. Available at: http://www.health.govt.nz/system/files/documents/pages/moh-gp-fax-20-12-2012.pdf [accessed 8 Jan 2013]
- Lindsay L, Jackson LA, Savitz DA, Weber DJ, Koch GG, Kong L, Guess HA. Community influenza activity and risk of acute influenza-like illness episodes among healthy unvaccinated pregnant and postpartum women. Am J Epidemiol 2006; 163(9):838-48; PMID:16554352; http://dx.doi.org/10.1093/aje/kwj095
- Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, Lindstrom S, Louie JK, Christ CM, Bohm SR, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009; 374(9688):451-58; PMID:19643469; http://dx.doi.org/10.1016/S0140-6736(09)61304-0
- Louie JK, Acosta M, Jamieson DJ, Honein MA; California Pandemic (H1N1) Working Group. Severe 2009 H1N1 influenza in pregnant and post-partum women in California. N Engl J Med 2010; 362(1):27-35; PMID:20032319; http://dx.doi.org/10.1056/NEJMoa0910444
- Thompson M, Williams J, Naleway A, Li DK, Chu S, Bozeman S, Hill HA, Cragan J, Shay DK; Pregnancy and Influenza Project Workgroup. The Pregnancy and Influenza Project: design of an observational case-cohort study to evaluate influenza burden and vaccine effectiveness among pregnant women and their infants. Am J Obstet Gynecol 2011; 204(6 Suppl 1):S69-76; PMID:21411050; http://dx.doi.org/10.1016/j.ajog.2011.01.006
- White SW, Petersen RW, Quinlivan JA. Pandemic (H1N1) 2009 influenza vaccine uptake in pregnant women entering the 2010 influenza season in Western Australia. Med J Aust 2010; 193(7):405-7; PMID:20919972
- Gall SA. Maternal immunization. Obstet Gynecol Clin North Am 2003; 30(4):623-36; PMID:14719841; http://dx.doi.org/10.1016/S0889-8545(03)00085-8
- Poehling KA, Szilagyi PG, Staat MA, Snively BM, Payne DC, Bridges CB, Chu SY, Light LS, Prill MM, Finelli L, et al. Impact of maternal immunization on influenza hospitalizations in infants. Am J Obstet Gynecol 2011; 204(6 Suppl 1):S141-8; PMID:21492825; http://dx.doi.org/10.1016/j.ajog.2011.02.042
- Australian Bureau of Statistics, 3301.0 - Births, Australia, 2013. Available from: http://www.abs.gov.au/AUSSTATS/[email protected]/DetailsPage/3301.02013?OpenDocument. [accessed 13 January 2015].
- Quinn HE, Snelling TL, Habig A, Chiu C, Spokes PJ, McIntyre PB. Parental Tdap Boosters and Infant Pertussis: A Case-Control Study. Pediatrics 2014; 134(4):713-20; In press 21; PMID:25225136
- Australian Government Department of Health and Ageing. Pertussis (Whooping Cough) immunisation funded under the Immunise Australia Program Available from: http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/immunise-pertussis [accessed 12 October 2011]
- Marshall H, Ryan P, Roberton D. Uptake of varicella vaccine-a cross sectional survey of parental attitudes to nationally recommended but unfunded varicella immunisation. Vaccine 2005; 23(46-47):5389-97; PMID:16039020; http://dx.doi.org/10.1016/j.vaccine.2005.05.033
- Rickert VI, Auslander BA, Cox DS, Rosenthal SL, Rickert JA, Rupp R, Zimet GD. School-based vaccination of young US males: impact of health beliefs on intent and first dose acceptance. Vaccine 2014 Apr 7; 32(17):1982-7; PMID:24492015; http://dx.doi.org/10.1016/j.vaccine.2014.01.049
- Healy CM, Rench MA, Castagnini LA, Baker CJ. Pertussis immunization in a high-risk postpartum population. Vaccine 2009; 27(41):5599-602; PMID:19647062; http://dx.doi.org/10.1016/j.vaccine.2009.07.030
- Cheng PJ, Huang SY, Shaw SW, Kao CC, Chueh HY, Chang SD, Hsu TY, Kung FT, Hsieh TT. Factors influencing women's decisions regarding pertussis vaccine: a decision-making study in the Postpartum Pertussis Immunization Program of a teaching hospital in Taiwan. Vaccine 2010; 28(34):5641-7; PMID:20600516; http://dx.doi.org/10.1016/j.vaccine.2010.05.078
- Gust DA, Kennedy A, Shui I, Smith PJ, Nowak G, Pickering LK. Parent attitudes toward immunizations and healthcare providers the role of information. Am J Prev Med 2005; 29(2):105-12; PMID:16005806; http://dx.doi.org/10.1016/j.amepre.2005.04.010
- Kharbanda EO, Vargas CY, Castaño PM, Lara M, Andres R, Stockwell MS. Exploring pregnant women's views on influenza vaccination and educational text messages. Prev Med 2011; 52(1):75-7; PMID:21047526; http://dx.doi.org/10.1016/j.ypmed.2010.10.009
- Lau JT, Cai Y, Tsui HY, Choi KC. Prevalence of influenza vaccination and associated factors among pregnant women in Hong Kong. Vaccine 2010; 28(33):5389-97; PMID:20542072; http://dx.doi.org/10.1016/j.vaccine.2010.05.071
- Forsyth KD, Campins-Marti M, Caro J, Cherry JD, Greenberg D, Guiso N, Heininger U, Schellekens J, Tan T, von König CH, et al. New pertussis vaccination strategies beyond infancy: recommendations by the global pertussis initiative. Clin Infect Dis 2004; 39(12):1802-9; PMID:15578403; http://dx.doi.org/10.1086/426020
- Marshall H, Ryan P, Roberton D, Baghurst P. A cross sectional survey to assess community attitudes to introduction of human papillomavirus vaccine. Aust N Z J Public Health 2007; 31(3):235-242; PMID:17679241; http://dx.doi.org/10.1111/j.1467-842X.2007.00054.x
- Australian Institute of Health and Welfare. 2009 Adult Vaccination Survey: summary results. Cat. no. PHE 135.released 03 Mar 2011. Available at: http://www.aihw.gov.au/publication-detail/?id=10737418409 [accessed 07 February 2014]
- Stata statistical software, Version 11, College station: Stata corporation; 2010