?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Mumps is caused by a paramyxovirus. It is an acute, but mild infectious disease. However, approximately 10% of patients with mumps can develop severe meningoencephalitis, disability, and death. Seasonal patterns in mumps vary across countries, but the reasons for this phenomenon remain unclear. The aim of this study was to assess the role of meteorological factors on mumps infection. We investigated the relationships between weather variability and the incidence of mumps in Taiwan using a Poisson regression analysis and case-crossover methodology. Between 2006 and 2011, 6,612 cases of mumps were reported to the Centers for Disease Control, Taiwan (Taiwan CDC). The incidence of mumps showed a significant seasonality in summertime (for oscillation, P < 0.001). The number of mumps started to increase at temperatures of 20°C (r2 = 0.73, P < 0.001), and the case count of mumps began to decline when the temperatures were higher than approximately 25°C (r2 = 0.24, p = 0.04), producing an inverted V-shaped relationship. Similarly, the number of mumps began to increase at a vapor pressure of 5–9 hPa (r2 = 0.87, P < 0.005) and decreased at a vapor pressure higher than 25–29 hPa (r2 = 0.21, p = 0.05). The number of mumps cases was positively associated with temperature and vapor pressure in the preceding period of the infection. In conclusion, this study showed that the occurrence of mumps is significantly associated with increasing temperature and vapor pressure in Taiwan. Therefore, these factors could be regarded as warning signals indicating the need to implement preventive measures.
Introduction
Mumps, caused by the mumps virus, is an acute but mild infectious disease. The mump virus, belonging to the Paramyxoviridae family, is an enveloped, single-stranded RNA virus.Citation1 Mumps is highly contagious and spreads rapidly in susceptible populations. Humans are the only known host of mumps. The transmission of mumps occurs by close contact. The period of infection is from 7 d before the onset of parotitis to 9 d afterwards.Citation2,3 The spectrum of illness is from subclinical infection to severe meningoencephalitis.Citation4 During the pre-vaccination era, the high risk group was among primary school children and some adolescents who had a previous infection.Citation4,5 Up to 42% of patients with mumps suffer from at least one of the following complications: orchitis, oophoritis, aseptic meningitis, encephalitis, pancreatitis, and deafness.Citation5-8
Similar to many infectious diseases, the number of reported mump cases exhibits a seasonal variation that has been well recognized in various countries. For example, a distinct seasonal variation was detected in the USA, with a peak occurring in April.Citation9 Other countries such as Ireland,Citation10 China,Citation11,12 and JordanCitation13 a peak in autumn, early-summer, winter and spring, respectively was also reported. Although these phenomena have not been well explained, the seasonality of mumps provides evidence that meteorological factors might play a key role in its occurrence.Citation14
The effects of weather factors on infectious diseases have received global attention in the context of climate change in recent years. A further understanding of the relationships between meteorological factors and occurrence of mumps could help improve both disease forecasting and preventive efforts. However, few studies have investigated the effects of meteorological factors on the occurrence of mumps. The purpose of this study was to assess the relationships between weather factors and the number of mumps cases in Taiwan.
Results
Epidemiological characteristics of patients with mumps
shows the number and annual incidence rate of mumps by gender, age, region, and season. Between January 2006 and December 2011, a total of 6,612 patients with mumps were diagnosed by physicians in Taiwan. The incidence rate (cases per 100,000 individuals per year) of mumps was 4.8 (range: 4.2 to 5.3). The mumps virus predominantly affected males compared to females (5.9 vs. 3.7). The incidence of mumps was decreasing as age was increasing, with a peak (28.4) in the age of 5 to 10 y old. The highest and lowest incidences of mumps were 7.8 and 3.5 in the eastern and the southern region, respectively, in Taiwan. Compared with in the winter (December, January, and February), the incidence of mumps increased in the summer (incidence rates ratio (IRR) = 1.61, 95% CI: 1.43 – 1.80), spring (IRR = 1.12, 95% CI: 0.99 – 1.26), and fall (IRR = 1.36, 95% CI: 1.21 – 1.53).
Table 1. Demographic characteristics of patients with mumps in Taiwan, 2006–2011
Seasonality and effects of weather factors
We developed a Poisson regression model that incorporated terms for the calendar year, sine and cosine to assess time trends and seasonality of mumps using the monthly aggregate case number as the response variable. In addition to the time trends and seasonality, we added the mean temperature, mean humidity, vapor pressure, precipitation, sunshine hours, and kindergarten attendance into the Equationmodel I\bf model \,I
\bf model \,I using a backward-elimination algorism. After oscillatory or cubic spline smoothers were incorporated into the model, mean temperature and vapor pressure were the only factors found to be independently associated with mumps infection (Equationmodel II
\bf model \, II
\bf model \, II ). Therefore, Equationmodel II
\bf model \, II
\bf model \, II was used in this study.
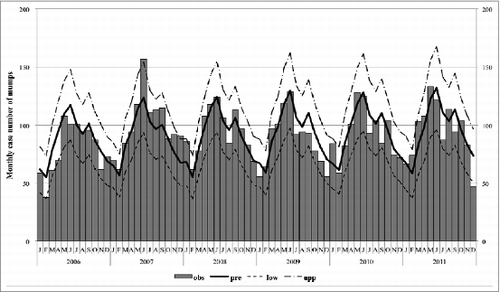
shows the actual and predicted monthly reported number of mumps. There was a seasonal pattern of mumps infection (for seasonal oscillation, P < 0.001) but no distinct annual trends in this model. In general, the overall trends of the predicted monthly reported case numbers fit the actual trends well (Pearson chi-square = 49.76; P > 0.05).
The association between meteorological factors and the incidence of mumps is presented in . Univariate analysis using Poisson models showed several meteorological factors associated with the incidence of mumps; however, after annual trends and cubic spline smoothers were incorporated into the models, only the mean temperature and vapor pressure were independently associated with the incidence of mumps (). Age and sex did not modify temperature or vapor pressure effects.
Table 2. Weekly weather pattern 8–14 d prior to symptom onset and the incidence of mumps virus infection in Taiwan, 2006–2011
Temperature, vapor pressure, and occurrence of mumps
The occurrence (case count) of mumps and temperature is shown in . The occurrence of mumps changes with different temperatures. The number of mumps cases began to rise at a temperature of 20°C (r2 = 0.73, P < 0.001). The occurrence of mumps began to decline if the temperature was higher than approximately 25°C (r2 = 0.24, P = 0.04), producing an inverted V-shaped relationship. The average case count (NT) increased by 3.9% (95% CI: 2.7–5.1%) for each 1°C increase in temperature.
Figure 2. (A) The average case count of mumps infections at various temperature domains. (B) The average case count of mumps infections at various vapor pressure domains.
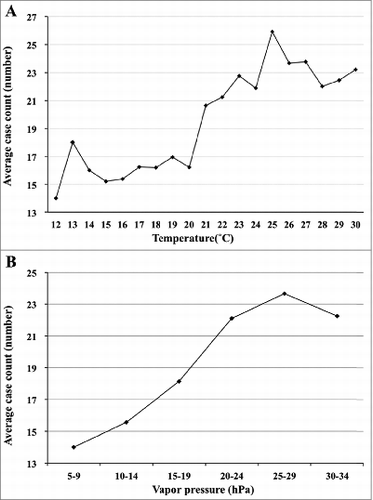
presents the association between the variation in the number of mumps and the various vapor pressure domains (NV). The number of mumps cases began to increase at a vapor pressure of 5–9 hPa (r2 = 0.87, P < 0.05). The occurrence of mumps began to decrease, when the vapor pressure was higher than 25–29 hPa (r2 = 0.21, p = 0.05). An increase in vapor pressure with 5-hPa was correlated with an increase of 2.8% in mumps cases (95% CI: 1.9–3.7%).
Acute meteorological effects using case-crossover analysis
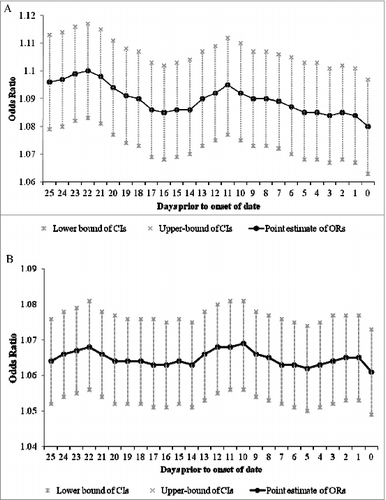
presents the relationship between the risk of mumps virus infection and per degree increase in temperature and per unit (5 hPa) increase in vapor pressure during the same day and in the subsequent 14 d The increased occurrence of the mumps was significantly associated with increased mean temperature and vapor pressure.
Discussion
The epidemiological evidence revealed that mumps is still an important public health problem worldwide.Citation15,16 Using a Poisson regression analysis and a case-crossover methodology, we analyzed mumps data reported to the Taiwan CDC from 2006 to 2011. We found that the incidence of mumps was highest during the summer months but that incidence was positively correlated with increased mean temperature and vapor pressure in both the long-term and acute effect analyses. In this study, we also found the importance of the meteorological exposure in determining the incidence and seasonality of mumps in industrial countries such as Taiwan.
Mumps is a moderately to highly contagious infection among susceptible individuals. Mumps is infected through the nasal or upper respiratory tract mucosa.Citation17-19 Infection can be localized to the mucosa of the respiratory tract.Citation17 The virus is transmitted by various routes including direct contact, droplet spread, or contaminated fomites.Citation1 It has been shown that the incidence of mumps is influenced by the level of temperature and humidity.Citation14,20 Increases in temperature and humidity improve the duration of virus survival and virulence in the environment,Citation8,14 which consequently increases the opportunities for hosts to become infected with mumps.Citation21,22
In this study, the occurrence of mump virus infection began to increase at 20°C and began to decline if the temperatures were higher than approximately 25°C, producing an inverted V-shaped relationship. We also found that the number of mumps started to increase at a vapor pressure of 5–9 hPa and began to decrease if the vapor pressure was 25–29 hPa. Mumps is assumed to be transmitted from close contact with infected individuals via hands or from fomites.Citation8,14 The activity of the mumps virus can last for several months at 4°C yet only one day at 37°C; however, it cannot survive at high temperatures over 55°C.Citation23 The mechanism of temperature and vapor pressure in the transmission of mumps virus is still unclear. Although several prevention measures have been implemented for mumps in Taiwan including a vaccination program (coverage rate over 90%) and health education, cases of mumps have continued to develop every year.Citation24 More studies are needed to investigate whether a summer peak of mumps in Taiwan is a result of a different transmission route.
Mumps infection exhibits distinct seasonality in various countries. The mechanism of seasonality in infectious diseases can be complex and can be attributed to changes in environmental conditions, the prevalence or virulence of the mumps virus, or the life style of the host.Citation21 Particularly, in regard to respiratory viral pathogens, environmental factors may play an important role during transmission.Citation25,26 Various patterns of seasonality in the occurrence of mumps have been reported in various countries. In the USA, a seasonal pattern with significant peaks in April has been noted;Citation9 in the Ukraine, more cases of mumps occur in the winter and spring than in the summer and autumn;Citation27 in Jordan, the higher morbidity of mumps occurs in the winter and spring.Citation13 Our results showed a distinct seasonal pattern of mumps infection with a peak occurrence in the summer months. This result was similar to ChinaCitation11,12 but in contrast with that inJordan.Citation13 The discrepancy might be due to the effects of varied seasonal exposure and mutual confounding factors between meteorological factors, which are presented in different areas.Citation14
We found that the occurrence of mumps was associated with the mean temperature and the vapor pressure in both the regression analysis and the case-crossover study; this result was consistent with a previous study in JapanCitation14 but was inconsistent with another study in the Czech Republic that demonstrated that mumps was not related to warmer and dryer weather.Citation20 These discordant findings may have resulted from the different methodologies used or whether confounding factors were removed.
In our study, annual incidence of mumps in males was higher than in females. In a mumps outbreak in the United States,Citation28 females had a higher attack rate (64%) than males. In a mumps outbreak in Guam,Citation29 the ratio of male cases to female cases was 1:1. We are unable to explain this epidemiological finding but postulate that contributing factors may include different behavioral patterns or differential reporting patterns.
In our study, a high percentage (56%) of mumps cases occurred in the northern region. In Taiwan, the population in the northern region has a high socioeconomic status, and approximately 40% or more of the Taiwanese population lives in this area.Citation30 The high proportion of mumps cases reflects this region's population density. In contrast, this study found that the highest incidence of mumps was found in the rural eastern region. The underlying cause of this high incidence rate remains unknown. Lower numbers of cases, as a result of the low population density in the eastern region (104 per km2), could be an explanation.
This study has several limitations. First, the public health surveillance data may be incomplete. It is believed that many notifiable infectious diseases (e.g., mumps) are underreported.Citation31 A reporting bias may occur anywhere in the reporting chain. However, this bias would occur only if weather effects were somehow correlated with the likelihood of disease reporting.Citation32 Next, it was difficult to obtain weather data in all counties in Taiwan. After excluding stations in isolated islands and areas in the mountains, only 15 weather stations were included in our analysis. These weather data may not represent the true status of weather exposure in the individual areas in Taiwan. The results supported the null hypothesis due to non differential misclassification. The effects of meteorological exposure on the occurrence of mumps in this study were most likely underestimated.Citation33 Third, most of the cases were reported through the clinical case definition instead of being confirmed by the laboratory criteria. However, typical cases often demonstrate distinct clinical features, which can usually be diagnosed on the basis of clinical findings.Citation34
In summary, the seasonal pattern of mumps infection in Taiwan was confirmed, and meteorological factors that might contribute to the observed seasonality, including the mean temperature and vapor pressure, were evaluated. Public health authorities could regard the results of the threshold estimation as a warning signal, and by applying these results with a prediction model for long-term trends, they could develop public health interventions before early-summer time to reduce the risk of infection and spread of the mumps virus. Mumps has attracted growing attention because several unexpected outbreaks occurred in many countries in the 2000s. We believe our findings demonstrate the importance of meteorological factors in determining the case occurrence and can help explain the notable seasonal pattern of mumps.
Methods
The population of Taiwan is approximately 2.3 million people with a total land area of 35,980 km2. Taiwan is in East Asia and is located between 21°45N and 25°56′N. The Tropic of Cancer (23.5° N) runs straight through Chiayi City, which is situated in south central Taiwan and divides the entire island into 2 climate zones. The northern part of Taiwan belongs to the sub-tropical monsoon climate zone, whereas the southern part belongs to the tropical monsoon climate zone. Consequently, the weather in Taiwan is relatively warm and high humidity occurs throughout the year.
This study was approved by the Institutional Review Board of the National Cheng Kung University Hospital.
Surveillance for mumps infection
Since 1999, mumps has been reported by the National Notifiable Disease Surveillance System (NNDSS) to the Centers for Disease Control, Taiwan (Taiwan CDC).Citation35 Until 2005, only the number of mump cases was reported to the Taiwan CDC. Since 2006, mumps has been a reportable disease by law in Taiwan. Physicians are required to report all cases of mumps to the Taiwan CDC within one week of case ascertainment.Citation35-37
We collected all mumps related data that were reported to the Taiwan CDC from January 2006 to December 2011. The data included the patient's age, gender, area of residence, and onset date for the swelling of parotid or other salivary glands. Additional clinical details were reported, including signs/symptoms and disease outcomes (either complications or death). Serum samples were also collected from the patients for serological confirmation of the diagnosis. Serological testing was performed by the Taiwan CDC mumps virus laboratory. Serum specimens were tested for mumps IgM and/or IgG using a commercially available capture IgM and/or IgG EIA kit (Denka Seiken Co., Ltd., Niigata, Japan) according to the manufacturer's instructions.Citation38
The definition of a probable case of mumps was a patient who was ill with an acute onset of unilateral or bilateral tenderness and self-limited swelling of the parotid or other salivary glands that lasted for at least 2 days, without other apparent causes.Citation28 A confirmed case was defined as a patient who had a positive laboratory test (presence of IgM antibodies and/or a fold4- increase in IgG antibodies) or who had the clinical features of mumps.Citation7,28
Meteorological data
The complete meteorological data including maximum and minimum daily mean temperature, relative humidity, vapor pressure, precipitation, and sunshine hours between January 2006 and December 2011 were obtained from the Taiwan Central Weather Bureau (http://www.cwb.gov.tw). Because Taiwan is a relatively small island, we used the mean meteorological data value for each calendar week obtained from all 15 weather stations across the island, excluding stations in isolated islands and areas in mountains
Statistical analysis
We calculated the annual incidence of mumps by dividing the number of reported mumps cases by the mid-year population of individuals of the same age, as reported between 2006 and 2011 in the Taiwan census data. It was expressed as the number of mumps cases per 100,000 individuals. Seasonal trends in the occurrence of mumps were assessed using Poisson regression models that incorporated sine and cosine oscillators, with yearly terms:Citation39,40\bf model \,I
\bf model \,I
\bf model \, II
\bf model \, II
Month (t) = 1 if t = 1, so month (t) = t in which E[Yi(t)] denotes the expected case counts at month t in year i. α is a constant value, each β term denotes a regression coefficient for a year or month, t indicates the months between January 2006 and December 2011, and i indicates the 5 y during the years 2006 and 2010. The function yeari(t) denotes whether it is year i (1 = yes, 0 = no). For example, year 2006 is represented by year1(t) = 1, year2(t) = 0, …., year6(t) = 0, and the reference year 2011 is represented by yeari(t) = 0 for all i. The function month(t) indicates a month number (i.e., 1 to 12 for January to December). Temperaturei(t) is the temperature at month t in year i; likewise, vapor pressurei(t) is the vapor pressure at month t in year i. We used the construction of univariable and multivariable Poisson regression models to evaluate the correlation between monthly number of mumps cases and weather exposure. We also used cubic splines for smoothing to account for annual variations during the 6-year study period. We used Akaike's information criterion (AIC) to optimize the knots within the spline model to avoid the pitfalls associated with both overfitting and underfitting.Citation32 A backward-elimination algorithm was conducted in multivariable models, with covariates retained for P ≤ 0.2.
To investigate the relationship between mumps infections and various temperatures and vapor pressure levels, we estimated the incidence of mumps at various temperatures and vapor pressure levels.Citation41 According to previous studies,Citation42,43 we assumed that the survival and transmission of the mumps virus would change as temperature and vapor pressure change and therefore might affect the infectivity of the mumps virus in a defined population. The average incidence of mumps (NT) in various temperature domains (T to T+ΔT) was estimated using the following formula:Citation40,41
Here, i denotes an index from 0 to n, ti is the average temperature for the ith 7-day period, Ci is the total cases of mumps for the i + 2nd 7-day period, and f(ti) is a function with the following results:
The numerator on the right side of the equation is the sum of all Ci comprising the 7-day average temperatures (ti) within the temperature domain of T to T+ΔT during the study period. The denominator is the total number of mumps with T < ti ≤ T+ΔT during the same study period.
Similarly, the average incidence of mumps (NV) in various vapor pressure domains (V to V+ΔV) was assessed using the following formula:
Here, i is a sequence from 0 to n, hi is the average vapor pressure for the ith 7-day period, Ci is the total cases of mumps from the i + 2nd 7-day period, and f(vi) is a function with the following results:
We used a case-crossover analysis to further investigate the acute effect of meteorological exposure on the occurrence of mumps.Citation44 This design is characterized by self-matching, in that cases serve as their own controls.Citation32,35 It specifically provides a mean for evaluating the acute effect of brief exposures.Citation44 Thereby, each subject's exposure prior to a case-defining event is compared with his or her own exposure during a control period when he/she had not yet been diagnosed as a case. A case day was the day on which the first symptom of mumps presented,Citation45,46 and the case period was 0–14 d prior to that day. The control day was selected 2 to 4 weeks before the case date (14–28 d prior to the case day). The possible effect period was estimated according to the incubation period of mumps, which is approximately 14 d (range: 10–20 days).Citation47 The average daily values of the meteorological factors were used as exposures, as were aggregated or mean values of the meteorological factors.
The analysis of case-crossover data are an application of standard methods for stratified data analysis. A conditional logistic regression was conducted to obtain exposure odds ratios (ORs) as estimates of incidence rate ratios and 95% confidence intervals (CIs) associated with meteorological variables.Citation48
To study the possibility of interaction (effect modification) by the demographic characteristics of patients, we developed multiplicative interaction terms and incorporated them into a logistic regression model.Citation49 We used software SAS Version 9.2 (SAS Institute Inc., Cary, NC, USA) to perform all statistical analyses. A P-value of <0.05 was considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by a grant (NSC 101 – 2314 – B – 006 – 055) from the National Science Council, Taiwan.
References
- Leinikki P. Mumps. In: Zuckerman AJ, Banatvala JE, Pattison JR. eds. Principles and Practice of Clinical Virology. 5th ed. Chichester: Wiley, 2004:459-66.
- Richardson M, Elliman D, Maguire H, Simpson J, Nicoll A. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J 2001; 20:380-91; PMID:11332662; http://dx.doi.org/10.1097/00006454-200104000-00004
- Seward J, Strebel P, Robertson S. Mumps. In: Heymann DL, ed. Control of Communicable Diseases Manual, 19th ed, American Public Health Association, Washington, DC, 2008:431-4.
- Galazka AM, Robertson SE, Kraigher A. Mumps and mumps vaccine: a global review. Bull World Health Organ 1999; 77:3-14; PMID:10063655
- Anonymous. A retrospective survey of the complications of mumps. J R Coll Gen Pract 1974; 24:552-6; PMID:4465449
- Watson-Creed G, Saunders A, Scott J, Lowe L, Pettipas J, Hatchette TF. Two successive outbreaks of mumps in Nova Scotia among vaccinated adolescents and young adults. CMAJ 2006; 175:483-8; PMID:16940266; http://dx.doi.org/10.1503/cmaj.060660
- Coffinières E, Turbelin C, Riblier D, Aouba A, Levy-Bruhl D, Arena C, Chiappe SG, Ferry JP, Hanslik T, Blanchon T. Mumps: burden of disease in France. Vaccine 2012; 30:7013-8; PMID:23059354; http://dx.doi.org/10.1016/j.vaccine.2012.09.070
- Hviid A, Rubin S, Mühlemann K. Mumps. Lancet 2008; 371:932-44; PMID:18342688; http://dx.doi.org/10.1016/S0140-6736(08)60419-5
- Shah AP, Smolensky MH, Burau KD, Cech IM, Lai D. Seasonality of primarily childhood and young adult infectious diseases in the United States. Chronobiol Int 2006; 23:1065-82; PMID:17050218; http://dx.doi.org/10.1080/07420520600920718
- Whyte D, O'Dea F, McDonnell C, O'Connell NH, Callinan S, Brosnan E, Powell J, Monahan R, FitzGerald R, Mannix M, et al. Mumps epidemiology in the mid-west of Ireland 2004–2008: increasing disease burden in the university/college setting. Euro surveill 2009; 14:1-5; PMID:19389339
- Cui A, Zhu Z, Chen M, Zheng H, Liu L, Wang Y, Ma Y, Wang C, Fang X, Li P, et al. Epidemiologic and genetic characteristics of mumps viruses isolated in China from 1995 to 2010. Infect Genet Evol 2014; 21:384-90; PMID:24355245; http://dx.doi.org/10.1016/j.meegid.2013.12.005
- Yang Q, Yang Z, Ding H, Zhang X, Dong Z, Hu W, Liu X, Wang M, Hu G, Fu C. The relationship between meteorological factors and mumps incidence in Guangzhou, China, 2005–2012. Hum Vaccin Immunother 2014; 10:2421-32; PMID:25424950; http://dx.doi.org/10.4161/hv.29286
- Batayneh N, Bdour S. Mumps: immune status of adults and epidemiology as a necessary background for choice of vaccination strategy in Jordan. APMIS 2002; 110:528-34; PMID:12390410; http://dx.doi.org/10.1034/j.1600-0463.2002.11007803.x
- Onozuka D, Hashizume M. Effect of weather variability on the incidence of mumps in children: a time-series analysis. Epidemiol Infect 2011; 139:1692-700; PMID:21211102; http://dx.doi.org/10.1017/S0950268810002967
- Parker Fiebelkorn A, Rosen JB, Brown C, Zimmerman CM, Renshowitz H, D'Andrea C, Gallagher KM, Harpaz R, Zucker JR. Environmental factors potentially associated with mumps transmission in yeshivas during a mumps outbreak among highly vaccinated students: Brooklyn, New York, 2009–2010. Hum Vaccin Immunother 2013; 9:189-94; PMID:23442590; http://dx.doi.org/10.4161/hv.22415
- Deeks SL, Lim GH, Simpson MA, Gagné L, Gubbay J, Kristjanson E, Fung C, Crowcroft NS. An assessment of mumps vaccine effectiveness by dose during an outbreak in Canada. CMAJ 2011; 183:1014-20; PMID:21576295
- Foy HM, Cooney MK, Hall CE, Bor E, Maletzky AJ. Isolation of mumps virus from children with acute lower respiratory tract disease. Am J Epidemiol 1971; 94:467-72; PMID:5120542
- Johnson CD, Goodpasture EW. An investigation of the etiology of mumps. J Exp Med 1934; 59:1-19; PMID:19870227; http://dx.doi.org/10.1084/jem.59.1.1
- Kilham L. Mumps meningoencephalitis with and without parotitis. Am J Dis Child 1949; 78:9324-33.
- Hubálek Z. North Atlantic weather oscillation and human infectious diseases in the Czech Republic, 1951–2003. Eur J Epidemiol 2005; 20:263-70; http://dx.doi.org/10.1007/s10654-004-6518-3
- Dowell SF. Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg Infect Dis 2001; 7:369-74; PMID:11384511; http://dx.doi.org/10.3201/eid0703.017301
- Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P. Seasonality and the dynamics of infectious diseases. Ecol Lett 2006; 9:467-84; PMID:16623732; http://dx.doi.org/10.1111/j.1461-0248.2005.00879.x
- Cantell K. Mumps virus. Adv Virus Res 1961; 8:123-64; PMID:13876259; http://dx.doi.org/10.1016/S0065-3527(08)60684-3
- Chen CC, Lu CC, Su BH, Chen KT. Epidemiologic features of mumps in Taiwan from 2006 to 2011: a new challenge for public health policy. World J Pediatr 2014 ( in press); PMID:25416005
- Tang JW. The effect of environmental parameters on the survival of airborne infectious agents. J R Soc Interface 2009; 6:S737-46; PMID:19773291; http://dx.doi.org/10.1098/rsif.2009.0227.focus
- Fisman D. Seasonality of viral infections: mechanisms and unknowns. Clin Microbiol Infect 2012; 18(10):946-54; PMID:22817528; http://dx.doi.org/10.1111/j.1469-0691.2012.03968.x
- Hrynash Y, Nadraga A, Dasho M. Effectiveness of a vaccination program against mumps in Ukraine. Eur J Clin Microbiol Infect Dis 2008; 27:1171-6; PMID:18618156; http://dx.doi.org/10.1007/s10096-008-0575-6
- Dayan GH, Quinlisk MP, Parker AA, Barskey AE, Harris ML, Schwartz JM, Hunt K, Finley CG, Leschinsky DP, O'Keefe AL, et al. Recent resurgence of mumps in the United States. N Engl J Med 2008; 358:1580-9; PMID:18403766; http://dx.doi.org/10.1056/NEJMoa0706589
- Nelson GE, Aguon A, Valencia E, Oliva R, Guerrero ML, Reyes R, Lizama A, Diras D, Mathew A, Camacho EJ, et al. Epidemiology of a mumps outbreak in a highly vaccinated island population and use a third dose of measles-mumps-rubella vaccine for outbreak control – Guam 2009 to 2010. Pediatr Infect Dis J 2013; 32:374-80; PMID:23099425; http://dx.doi.org/10.1097/INF.0b013e318279f593
- Department of Household Registration Affair. Ministry of Interior, R.O.C. National Statistics, R.O.C. (Taiwan). www.ris.gov.tw/ch4/static/st10-0.htm (accessed August 2014)
- Doyle TJ, Glynn MK, Groseclose SL. Completeness of notifiable infectious disease reporting in the United States: an analytical literature review. Am J Epidemiol 2002; 155:866-74; PMID:11978592; http://dx.doi.org/10.1093/aje/155.9.866
- Kinlin LM, Spain CV, Ng V, Johnson CC, White AN, Fisman DN. Environmental exposures and invasive meningococcal disease: an evaluation of effect on varying time scales. Am J Epidemiol 2009; 169:588-95; PMID:19164421; http://dx.doi.org/10.1093/aje/kwn383
- Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet 2002; 359:248-52; PMID:11812579; http://dx.doi.org/10.1016/S0140-6736(02)07451-2
- Greenwood D. Medical microbiology: a guide to microbial infections: pathogenesis, immunity, laboratory investigation and control. 18th ed. Churchill Livingstone 2012.
- Centers for Disease Control, Department of Health, Taiwan. Notifiable infectious disease statistical system. Available at http://nidss.cdc.gov.tw (accessed on 14 May 2013)
- Chang YK, Chen JY, Chang HL, Yu MC, Hsiao HF, Hou CC, Liu SY, Chen KT. Absence of endemic measles transmission in a highly vaccinated population from 1999 to 2008: implications of sustained measles elimination in Taiwan. Vaccine 2010; 28:5532-7; PMID:20665978
- Chen KT, Chang HL, Wang ST, Cheng YT, Yang JY. Epidemiologic features of hand-foot-mouth disease and herpangina caused by enterovirus 71 in Taiwan, 1998–2005. Pediatrics 2007; 120: e244-52; PMID:17671037; http://dx.doi.org/10.1542/peds.2006-3331
- Kidokoro M, Tuul R, Komase K, Nymadawa P. Characterization of mumps viruses circulating in Mongolia: identification of a novel cluster of genotype H. J Clin Microbiol 2011; 49:1917-25; PMID:21411578; http://dx.doi.org/10.1128/JCM.02387-10
- Fisman DN, Lim S, Wellenius GA, Johnson C, Britz P, Gaskins M, Maher J, Mittleman MA, Spain CV, Haas CN, et al. It's not the heat, it's the humidity: wet weather increases legionellosis risk in the greater Philadelphia metropolitan area. J Infect Dis 2005; 192:2066-73; PMID:16288369; http://dx.doi.org/10.1086/498248
- Chang HL, Chio CP, Su HJ, Liao CM, Lin CY, Shau WY, Chi YC, Cheng YT, Chou YL, Li CY, et al. The association between enterovirus 71 infections and meteorological parameters in Taiwan. PLoS One 2012; 7:e46845; PMID:23071650; http://dx.doi.org/10.1371/journal.pone.0046845
- Chan PK, Mok HY, Lee TC, Chu IM, Lam WY, Sung JJ. Seasonal influenza activity in Hong Kong and its association with meteorological variations. J Med Virol 2009; 81:1797-806; PMID:19697414; http://dx.doi.org/10.1002/jmv.21551
- Arita M, Shimizu H, Nagata N, Ami Y, Suzaki Y, Sata T, Iwasaki T, Miyamura T. Temperature-sensitive mutants of enterovirus 71 show attenuation in cynomolgus monkeys. J Gen Virol 2005; 86:1391-401; PMID:15831951; http://dx.doi.org/10.1099/vir.0.80784-0
- Bi P, Zhang Y, Parton KA. Weather variables and Japanese encephalitis in the metropolitan area of Jinan city, China. J Infect 2007; 55:551-6; PMID:17714787; http://dx.doi.org/10.1016/j.jinf.2007.07.004
- Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol 1991; 133:144-53; PMID:1985444
- Levy D, Lumley T, Sheppard L, Kaufman J, Checkoway H. Referent selection in case-crossover analyses of acute health effects of air pollution. Epidemiology 2001; 12:186-92; PMID:11246579; http://dx.doi.org/10.1097/00001648-200103000-00010
- Murray EL, Klein M, Brondi L, McGowan JE Jr, van Mels C, Brooks WA, Kleinbaum D, Goswami D, Ryan PB, Bridges CB. Rainfall, household crowding, and acute respiratory infections in the tropics. Epidemiol Infect 2012; 140:78-86; PMID:21371367; http://dx.doi.org/10.1017/S0950268811000252
- Prince A. Infectious disease. In Nelson Essentials of Pediatrics. Behrman RE, Kliegman RM, eds., Fourth edition, W.B. Saunders Company, Philadelphia, Pennsylvania, USA; 2002:436.
- Woodward M. Epidemiology: Study Design and Data Analysis, 2nd ed. Boca Raton, FL: Chapman & Hall/CRC; 2005:515-600.
- Cameron AC, Trivedi PK. Regression Analysis of Count Data. 1st ed. Cambridge: Cambridge University Press, 1998.