Abstract
Live attenuated influenza vaccines (LAIV) can prevent influenza illness and death in children. The absence of known correlates of protection induced by LAIV requires human studies of underlying mechanisms of vaccine-induced immunity, to further elucidate the immunological processes occurring. In this study, children scheduled for elective tonsillectomy were enrolled in a clinical trial to evaluate the immune response to LAIV, in order to compare T and B cell gene expression profiles. Twenty-three children (aged 3–17 years) were divided into 4 groups; unvaccinated controls, or vaccinated intranasally with LAIV at days 3–4, 6–7, and 12–15 before tonsillectomy. Total RNA extraction was performed on tonsillar tissue and high RNA quality was assured. The samples were then analyzed using a validated RT2 Profiler PCR Array containing 84 gene-specific primers involved in B and T cell activation, proliferation, differentiation, regulation and polarization. The gene expression after LAIV vaccination was subsequently compared to the controls. We observed that at d 3–4 post vaccination, 6 genes were down-regulated, namely APC, CD3G, FASLG, IL7, CD8A and TLR1. Meanwhile at 6–7 days post vaccination, 9 genes were significantly up-regulated, including RIPK2, TGFB1, MICB, SOCS1, IL2RA, MS4A1, PTPRC, IL2 and IL8. By days 12–15 the genes RIPK2, IL4, IL12B and TLR2 were overexpressed. RIPK2 was upregulated at all 3 time points. Our data suggests an overall proliferation, differentiation and regulation of B and T cells in the tonsils following LAIV, where the majority of genes were up-regulated at days 6–7 and normalized by days 12–15. These findings may provide a first step into defining future biomarkers or correlates of protection after LAIV immunization.
Introduction
The burden of global influenza epidemics is 3 to 5 million cases of severe illness and with estimated 250,000 to 500,000 deaths annually.Citation1-3 Since seasonal influenza can be prevented by vaccination, the World Health Organization (WHO) recommends annual vaccination of individuals at increased risk of the complications of influenza.Citation4-7 Particularly, young children under the age of 2 are a major source of viral transmission,Citation8 with the highest morbidity and mortality observed in older patients (>65 years old). Thus, the vaccination of young healthy children may reduce the levels of transmission in society.
Recently, the Live Attenuated Influenza Vaccine (LAIV) has been licensed in Europe for children.Citation9-11 LAIV is administered intranasally at the portal of entry of the virus, thereby inducing local immune responses in the draining lymph nodes and tonsils,Citation12,13 in turn leading to B and T cell activation.Citation14,15 Despite lower levels of serum haemagglutination inhibition (HI) antibody titres elicited by LAIV compared to the inactivated trivalent influenza vaccine (inactivated TIV), it has been shown to provide effective immunity in children, measured by reduction in virus survival and replication.Citation16,17 LAIV has therefore been incorporated into childhood vaccination programs in several countries, including USA, Canada, and several European countries.Citation18 Interestingly, one of the limitations to widespread inclusion of LAIV into national vaccination programs includes the lack of good correlate of protection.Citation19 Therefore, there is a need to further characterize and understand the underlying immunological mechanisms of action of LAIV in order to possibly find a reliable correlate of protection in the future.
Given our current understanding of LAIV and its ability to induce effective humoral and cell mediated immune responses in children, we aimed to investigate the dynamics of the locally induced B and T cell gene expression profiles in the tonsils following LAIV vaccination.Citation20 Our results indicate an overall proliferation, differentiation and regulation of B and T cells in the tonsils following LAIV, where dynamic changes in gene expression levels were identified. In particular RIPK2, IL-2 and IL-2RA were found to be highly upregulated. These findings are important starting points for unravelling the immunological processes that occur in the upper respiratory tract after LAIV immunization.
Material and Methods
Study population and experimental setup
Twenty-three children aged 3–17 years old and scheduled for tonsillectomy at the Department of Otorhinolaryngology, Haukeland University Hospital, Bergen, Norway were recruited for this study. A detailed explanation of the study prospective and protocols were explained to the recruited subjects and guardians upon enrolment. Written informed consent was obtained from patients and their guardians. The demographics of the subjects included in this study are presented in . The Regional Ethics Committee, and Norwegian Medicines Agency approved this study (EUDRACT # 2012-00284824 and www.clinicaltrials.gov NCT01866540).
Table 1. Demographics of patients included in the study
Vaccine strains
LAIV (Fluenz™ MedImmune LLC, USA), for 2012–13, is a seasonal trivalent influenza vaccine administered intranasally. The vaccine contained A/California/07/2009 (H1N1) pdm09-like strain, A/Victoria/361/2011 (H3N2)-like strain, and B/Massachusetts/2/2012-like strain.
Haemagglutination inhibition (HI) assay
Serum samples collected prior to vaccination and at tonsillectomy were tested by the HI assay. Serial dilutions of serum samples, 8 Hemagglutinating units of the homologous H1N1 and H3N2 vaccine strains and 0.7% turkey red blood cells were employed to measure the serum HI titers following standard procedure.Citation21,22
Tonsil tissue preparation and RNA isolation
A sectioned palatine tonsil (2 × 10 × 10 mm) was submerged in PAXgene® Blood RNA Tube reagent (PAXgene Blood RNA kit, PreAnalytiX GmbH, Hombrecht, Germany), in order to stabilize intracellular RNA by inhibiting Ribonuclease (RNase) activity and preserve ex-vivo gene expression. Lysing matrix D (MP Biomedicals, Santa Ana, California, USA) and small ceramic beads were added to the tissue and shaken for 30 seconds before storage at −20°C. Total RNA isolation was performed using the PAXgene Tissue RNA kit (PreAnalytiX GmbH, Hombrecht, Germany), according to the manufacturer's instructions.
Total RNA quality control
The quantity, purity and integrity of the total RNA was measured using with the Nanodrop® ND1000 (Thermo Fisher) and the Agilent Bioanalyzer, using the Agilent RNA 6000 Nano Chip (Cat. no. 5067-1511, Agilent Technologies, USA) and the Agilent RNA 6000 Pico (Cat no. 5067-1513, Agilent Technologies, USA).
RT2 profiler PCR array analysis
Total RNA was transcribed into cDNA (cDNA) using the RT2 SYBR Green Mastermix and First strand cDNA kits (Cat. no. PAHS-0532, RT2 Profiler PCR Array Kit, Qiagen Sciences, Hilden, Germany). This was followed by cDNA amplification and quantification as recommended by the manufacturer.Citation23,24 This validated kit contains internal controls of validation (see Table S1 for full list of genes). The Cycle threshold (Ct) values were generated individually, for each subject and target gene, using the real-time PCR software Roche LC480 II (www.sabiosciences.com/pcrarrayprotocolfiles.php). These Ct values were then normalized using 3 housekeeping genes and proprietary software provided by the manufacturer.Citation25 Results were then treated group-wise, and presented in cluster-grams; with non-supervised hierarchical clustering of the entire dataset to display a heat map with dendograms and volcano plots.
Detection of influenza virus RNA in tonsillar tissue
Detection of influenza virus RNA was performed by running reverse transcription and quantitative PCR, using specific primers and probes on total RNA extracted from tonsillar tissue samples. We used validated reagents from BEI resources (NIAD, NIH: Influenza Virus Real-Time RT-PCR Assay, NR-15592) and following the recommended procedure. The kit enabled detection of A and B viruses, as well as subtyping the A viruses into H1, H3 and H5 strains.
Results
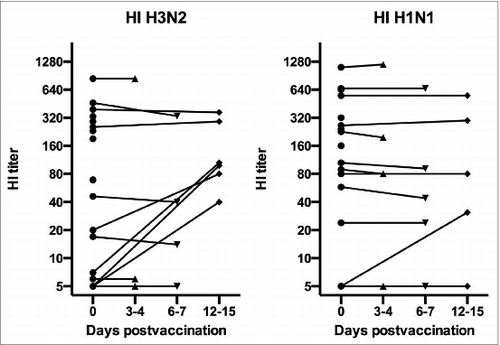
In this study we have vaccinated children scheduled for elective tonsillectomy with LAIV, and collected the tonsils for B and T cell gene array analysis (). Samples were collected in an unvaccinated control group (day 0) and at the operation day (variable days 3–15 post LAIV). The subjects were divided into the following 4 groups: unvaccinated controls (n = 6), tonsillectomised 3–4 days (group 1, n = 5), 6–7 days (group 2, n = 6), or 12–15 days (group 3, n = 6) post 1st vaccine dose. Apart from suffering from hypertrophic tonsils or recurrent tonsillitis, the patients were healthy and fulfilled the criteria for vaccination. Only a few patients had an increase in HI titer at the time of tonsillectomy ().
Figure 1. Serum hemagglutination inhibition (HI) antibody against the H1N1 and H3N2 vaccine viruses.
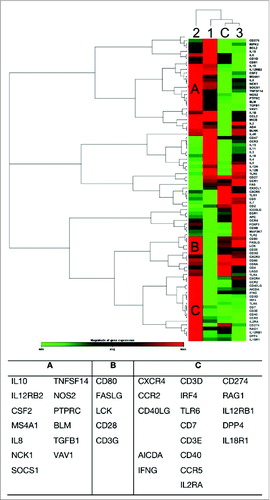
Identifying gene clusters that are involved in B and T cell activation
The dynamics of the locally induced B and T cell related gene expression profiles were investigated in the tonsils at 3 different times points following LAIV vaccination and compared to control unvaccinated children. Total RNA was extracted from the tonsillar tissue and a validated RT2 Profiler PCR Array analysis was used to analyze gene expression profiles. The Ct values were generated individually for each subject and target gene. The samples were then normalized using 3 housekeeping genes and the expression of 84 B and T cell specific genes (Tables S1 and S2) was studied by comparing the 3 post vaccination profiles with control unvaccinated children. A qualitative analysis of the data was conducted via cluster-grams, where genes within and across groups showing similar numerical data are clustered together to display a heat map with dendograms indicating co-regulated genes across groups or individual samples. Compared to the controls, we identified one gene cluster that is under-expressed at days 3–4. We observed an increased expression of genes on days 6–7, whereas the majority of these genes were under-expressed in the control group. However, at days 12–15 the number of under-expressed genes decreased but remained above baseline levels (). The dendograms in connect genes with similar numerical expression. The vertical dendogram form 2 main bifurcations segregating days 6–7 from the other time points. On the vertical dendogram, days 6–7 formed a cluster. Three clusters were observed at days 6–7 compared to controls: Cluster A (IL10-SOC1 and TNFSF14-VAV1), Cluster B (CD80-CD3G), and Cluster C (CXCR4-CD40LG, AICDA-IFNγ, CD3D-IL2RA and CD274-IL18R1). Together, our observations suggest a marked under-expression of genes on days 3–4. Whilst, 3 clusters of B and T cell activated genes were identified on days 6–7.
Figure 2. Identifying gene clusters that are involved in B and T cell activation. The colour gradient green-black-red represents relative level of gene expression, indicating under-even-over expression, respectively. The cluster gram shows a 2-way non-supervised hierarchical clustering of the entire data set, while displaying a heat map with a horizontal and vertical dendrogram indicating co-regulated genes across groups.
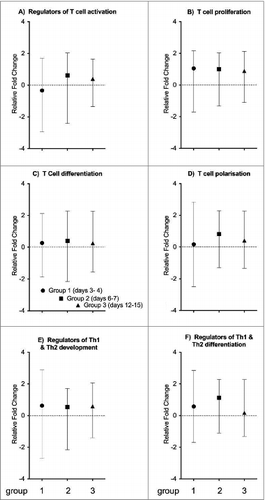
T cell specific gene expression
T cell specific genes were classified into 6 different functional categories, namely A) regulators of T cell activation, B) T cell proliferation, C) T cell differentiation, D) T cell polarization, E) regulators of Th1 and Th2 development, and F) Th1 and Th2 differentiation (). Genes involved in regulating T cell activation were downregulated at the earliest time point (3–4 days), yet were overexpressed at days 6–7 (), indicating an increase in T cell activation. The levels of genes related to T cell proliferation (), T cell differentiation (), and regulators of Th1/Th2 development () were relatively stably over-expressed in the 3 vaccination groups compared to the controls. The genes related to T cell polarization () and regulator genes of Th1/Th2 differentiation (), showed an increased in expression levels at days 3–4 compared to controls, they peaked at days 6–7 and returned to baseline levels at days 12–15.
Figure 3. T cell specific gene expression. T cell specific genes were classified into 6 different categories (A) regulators of T cell activation, (B) T cell proliferation, (C) T cell differentiation, (D) T cell polarization, (E) regulators of Th1 and Th2 development, and (F) Th1 and Th2 differentiation, as indicated above each plot. Each symbol represents the mean expression level (+/− range) of T cell specific genes compared to controls. The groups 1 to 3 are described in the text. The vertical scale indicates whether these genes are up- or downregulated, where positive values on the y-axis represent an up-regulation of genes, and negative values represent a down-regulation.
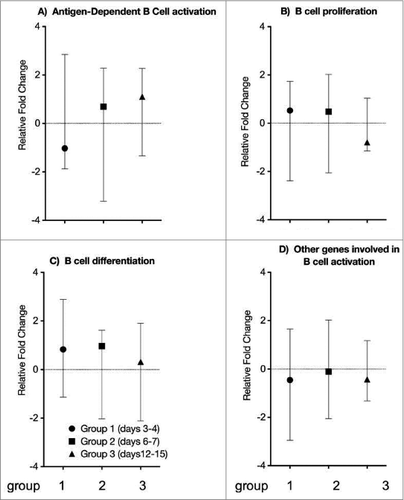
B cell specific gene expression
Genes involved in B cell activation were classified into 4 main groups, specifically A) antigen-dependent B cell activation, B) B cell proliferation, C) B cell differentiation and D) other genes involved in B cell activation. Fold change values for all the genes at each time point were plotted together and compared to the controls (). At 3–4 days post vaccination, we observed that genes involved in antigen-dependent B cell activation were under-expressed compared to the controls, but were overexpressed at days 6–7 (). Levels of B cell proliferation and differentiation genes were higher on days 3–4 and 6–7, yet became under-expressed at 12–15 days post vaccination (). Other genes involved in B cell activation were under expressed in all groups compared to controls, yet to a lesser extent at days 6–7 compared to days 3–4 and 12–15 post vaccination ().
Figure 4. B cell specific gene expression. Genes involved in B cell activation were classified into 4 main groups, specifically (A) antigen-dependent B cell activation, (B) B cell proliferation, (C) B cell differentiation and (D) other genes involved in B cell activation. Fold change values for all the genes combined at each time point were plotted and compared to the controls. Each symbol represents the mean level (+/− range) of B cell specific gene expression. The vertical scale indicates fold change of gene expression.
The expression of other immune cell related genes
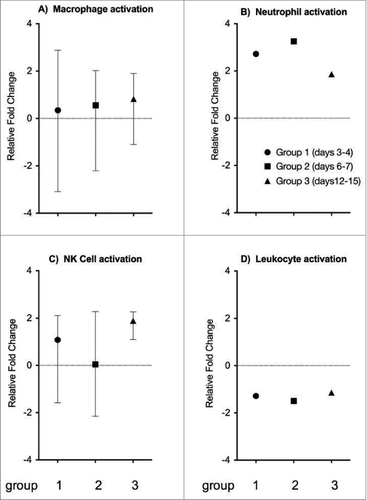
We investigated the expression of genes related to macrophage, neutrophil, natural killer cells and leukocyte activity (). The activation of macrophage-specific genes increased over time, and were highest at days 12–15 (), while neutrophil-specific genes peaked at days 6–7 (). Interestingly, natural killer cell activation genes were under-expressed at days 6–7, and then became highly over-expressed at days 12–15 (). Meanwhile, leukocyte-specific genes are under-expressed in all 3 groups compared to controls, most highly under-expressed at days 6–7 ().
Figure 5. The expression of other immune cell related genes. Genes involved in the activation of other immune cells include (A) macrophage activation, (B) neutrophil activation, (C) natural killer cell activation, and (D) leukocyte activation. Each plot represents the relative expression of the respective combined cell specific gene in the test groups compared to controls. The symbols indicate the mean gene expression level for the 3 groups (x-axis), and the range is indicated with vertical lines. The y-axis represents the fold change in gene expression levels.
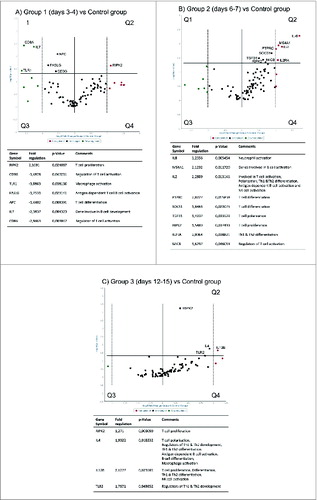
Significantly overexpressed genes and their contribution to tonsillar immune functions
The genes that were significantly expressed and their overall contribution to immune functions in the tonsils following LAIV were shown in the Q1 & Q2 regions of the volcano plots (). All 84 genes were almost equally distributed on both sides of the volcano plot at 3–4 day post vaccination (). Compared to controls, the expression of CD3G, TLR1, FASLG, APC, IL7 and CD8A located in the Q1 region were under-expressed by up to 3 fold. Meanwhile, RIPK2 was significantly overexpressed, thereby located in the Q2 region. Hence, as RIPK2 drives T cell proliferation the majority of these down-regulated genes reduce B and T cell activation at 3–4 days post vaccination. At 6–7 days after vaccination approximately 74% of the genes occupied the Q2 & Q4 regions of the plot with 26% in Q3 and leaving Q1 empty (). These overexpressed genes included IL8, MS4A1, IL2, PTPRC, SOCS1, TGFB1, RIPK2, IL2RA, MICB. Overall, we found that many B and T cell activation related genes including the aforementioned genes were upregulated 6–7 days post vaccination. A decrease occurred at days 12–15 (), signifying the contraction of the immune response and re-establishment of homeostasis. Here, 62 % of the entire gene set remained overexpressed compared to the controls. These significantly overexpressed genes included RIPK2, TLR2, IL4 and IL12B. Overall the contributions of these genes promoted B and T cell activation.
Figure 6. For figure legend, see page 1669Figure 6 (See previous page). Volcano plots of individual genes (dots) that are under and overexpressed compared to baseline (Controls). The horizontal axis indicates the relative level of expression with a vertical line dividing the under expressed genes to the left (green) and over expresses to the right (red). The y-axis indicates the statistical level of the change in gene expression with a horizontal line indicating the threshold (P-value < 0.05), and genes situated above the line are denoted significant using the student t-test. (A) gene expression in Group 1 (3–4 days post vaccination) compared to controls. The green (left, n = 45) and red (right, n = 39) dots represent the under and overexpressed genes respectively. (B) gene expression in Group 2 (6–7 days post-vaccination). The red (left, n = 62) and green (right, n = 22) dots represent genes that are over and under expressed respectively. (C) The genes that are over expressed (red, n = 52) and under expressed (green, n = 32) 12–15 days following vaccination compared to controls.
No Influenza virus RNA was detected in the tonsillar tissue
We tested for the presence of viral RNA in total RNA extracts from tonsillar tissue by using a validated real-time PCR assay using influenza specific primers and probes. No influenza specific RNA was detected in any of the samples using primers and probes against influenza A and B viruses as well as H1, H3 and H5 subtypes (data not shown).
Discussion
The tonsils are important in protection of the upper airways against respiratory pathogens such as influenza. This virus is major respiratory infection with up to 20% of children infected annually. LAIV is preferentially recommended for influenza prophylaxis in children in the UK, yet the mechanisms of immunological protection have not yet been defined. In this unique pediatric clinical trial, we have vaccinated children at various time intervals prior to elective tonsillectomy. We collected the tonsils at the time of operation and isolated high quality RNA to evaluate the dynamics of the B and T cell gene responses. Recently, we reported the systemic effect of the LAIV vaccine in a larger cohort including the children in the present study.Citation21 We found that LAIV elicited elevated B and T cell responses in these young children, persisting for 1 year after vaccination, with the highest responses observed against the B strain and the lowest to the Influenza A H1N1 strain. Our study has a high number of young children and is particularly unique in the collection of tonsils after vaccination allowing us to evaluate the local immune response in the upper airways.
In this study, we have performed a gene expression analysis with the goal of investigating the dynamics of B and T cell activation in tonsillar tissue following LAIV in children scheduled for elective tonsillectomy, in order to identify possible prognostic factors. Apart from fulfilling the criteria for tonsillectomy, our patients were otherwise healthy, showing no signs of immune compromise, as shown by stable immunoglobulin levels.Citation20 Hence, the fold change gene values derived from our analysis enable the investigation of the immunological processes in the upper respiratory tract after LAIV. Our main finding is that at 6–7 days post vaccination critical changes occur in gene expression patterns which are probably fundamental for development of vaccine induced immunity ().
The LAIV contains cold adapted vaccine viruses capable of limited replication in the upper respiratory tract. We were not able to detect influenza virus RNA in the tonsillar tissue samples, suggesting that the influenza virus antigen is taken up by dendritic cells in the nasal mucosa where the viruses replicate (). The viral antigen may be carried by the activated dendritic cells, to local lymphoid tissue such as the tonsils, where the viral antigen is presented to T cells.
Figure 7. A suggested working model for LAIV. Upon vaccination with LAIV by intranasal droplets (1), limited virus replication of the vaccine strains may occur in the nasal epithelial lining (2). Since we were not able to detect viral RNA in the tonsillar tissue, this indicates that viral antigens are transported to the tonsils by activated DCs (3) and not through local vaccine virus replication. We observed in this study that B and T cells are activated by changes in gene expression profiles, which are most pronounced at days 6–7 post vaccination (4, 5). An efflux of activated lymphocytes that may migrate to the site of infection/vaccination (6) as is supported by an earlier study.Citation45 Systemic and mucosal responses (7) were detected in these subjects after LAIV vaccination [21 and unpublished data].
![Figure 7. A suggested working model for LAIV. Upon vaccination with LAIV by intranasal droplets (1), limited virus replication of the vaccine strains may occur in the nasal epithelial lining (2). Since we were not able to detect viral RNA in the tonsillar tissue, this indicates that viral antigens are transported to the tonsils by activated DCs (3) and not through local vaccine virus replication. We observed in this study that B and T cells are activated by changes in gene expression profiles, which are most pronounced at days 6–7 post vaccination (4, 5). An efflux of activated lymphocytes that may migrate to the site of infection/vaccination (6) as is supported by an earlier study.Citation45 Systemic and mucosal responses (7) were detected in these subjects after LAIV vaccination [21 and unpublished data].](/cms/asset/653f49e8-41b5-4f9e-a232-3380823ac9ad/khvi_a_1032486_f0007_oc.jpg)
Pivotal studies have recently focused on the importance of pre-existing CD4 and CD8 T cells in protection from influenza illness when neutralizing antibodies are absent.Citation26 Wilkinson et al. reported that pre-existing influenza specific CD4 T cells correlate with disease protection in a human challenge model, with lower levels of viral shedding and reduced severity of illness.Citation27 Furthermore, protection from the 2009 pandemic H1N1 virus was observed in individuals who had cross-reactive CD8 T cells to the conserved internal proteins of the virus but no detectable neutralizing antibodies.Citation28,29 In our current study, we observed a peak in the expression of genes involved in T cell differentiation, activation, polarization, and the regulation of Th1 and Th2 differentiation on days 6–7 post LAIV immunization, suggesting an induction of T lymphocyte commitment (). The commitment to the different T helper subsets is presently characterized by the expression of signature cytokines,Citation30 where we found that on days 6–7, increases in the expression of IL2 and IFN-γ were observed (). LAIV induced significant increases in Th1 cells secreting IFN-γ and IL-2 after the second dose of vaccine in these children.Citation21 Furthermore, these Th1 cells also secreted TNF-α, which was not included in the gene array in this study and should be studied in future trials. Influenza infection induces secretion of large amounts of IFN-γ, TNF-α and IL2, thereby promoting the Th1 pathway.Citation31,32 IL-2 is a central cytokine with a number of key roles in development of influenza immunity, where the IL-2 production from memory cells correlates to antibody levels.Citation33,34 Furthermore, the expression of IL-2 and IL-2RA on days 6–7 indicates recent T cell activation, as the kinetics of IL-2 is highest within the first 24 hours of infection.Citation12,35 Meanwhile, there was a significant increase in IL-4 expression at days 12–15 (). Since IL-4 is a key Th2 promoting cytokine,Citation32,36 our results suggest that LAIV is capable of inducing a mixed Th1/Th2 cytokine response. A study by Woods et al.,Citation37 with subjects inoculated with live influenza A (H1N1 or H3N2 ) virus found prominent change in interferon gene expression in the blood at 3–4 days post. Whereas in our study of live attenuated influenza viruses the greatest difference was found at 6–7 days post vaccination.
Overall, we found that the T cell dependent B cell activation and B cell differentiation initiated on days 3–4 increased up to days 6–7, where reactions like antibody isotype switching, affinity maturation and induction of J chain gene occur in the germinal center, and may be responsible for the slight decline in B cell proliferation (). Moreover, our data shows an increase in TGF-β on days 6–7. TGF-β is involved in isotype switching to IgA,Citation38,39 a process that occurs extensively in the germinal centers of the tonsilsCitation13 (). It is also involved in regulating anti-inflammatory processes. Hence, the increase in the secretion of TGF-β on days 6–7 suggests isotype switching from IgM to IgA. The subsequent secretion of IL4 on days 12–15 suggests that there may be subsequent B cell differentiation ().
In previous studies we have vaccinated adults with parenteral TIV. The systemic effect was detectable after 4–5 days post TIV vaccination with a peak in influenza specific antibody secreting cells (ASC) after one week, and a peak in serum antibodies after 2 weeks.Citation40 After TIV, mucosal antibodies appeared after 4–5 days post vaccination with a peak in influenza specific tonsillar ASC and salivary antibodies after one week.Citation41,42 We have also found a higher presence of influenza specific ASC in nasal tissue than in blood or tonsils, but this was not influenced by vaccination.Citation43,44 Parenteral vaccination also induced changes in cellular pattern of the tonsils.Citation45 Meanwhile, the LAIV vaccine elicits both systemic responsesCitation21 as well as local mucosal immune responses (data to be published).
When accounting for the involvement of other immune cells (), we see an increase in macrophage activation throughout all 3 time points. This is consistent with current findings suggesting that monocytes migrate to the site of infection, become activated and perform phagocytosis and present antigen. Neutrophils, on the other hand, migrate to the sites of infection, as shown by the increase in IL-8 expression on days 6–7 and phagocytose antigens (). The increase in NK cells at days 6–7 suggests these cells perform cytotoxic functions and assist the functions of effector B cells.
There may be several principal reasons for the observed changes in gene expression profiles in the tonsils; vaccination may induce activation, proliferation, influx/efflux of cells or a combination of these. Further studies have to be performed to reveal the exact mechanism behind the changes in the gene expression profile.
A very prominent enzyme gene was overexpressed at all time points in our study, namely RIPK2 (). The RIPK2 gene encodes a protein kinase that is an important activator of the NF-κB pathway and is implicated in the induction of apoptosis.Citation46,47 The function of RIPK2 in apoptosis has previously been tested in vitro, demonstrating that RIPK2 plays an important role in the transduction of Fas-FasL induced apoptosis (extrinsic pathway).Citation48 Moreover, RIPK2 has been shown to protect against severe influenza A virus infection by regulating the expression of IFN-γ and IL-18.Citation48 IL-18 over-expression is thought to cause increased morbidity and mortality in RIPK2 deficient mice by hyperactivating lymphocyte induced inflammation. In bacterial infections, RIPK2 plays an important role in activating the Nucleotide-binding Oligomerisation Domain 2 (NOD2) to induce autophagy; a cell stress-response induced in times of starvation and adaptation to limited resources in the milieu.Citation49 Hence, in our study the involvement of RIPK2 in B and T cell activation may be associated with the activation of transduction factor NFκB, which is involved in the expression of many genes including IL-2 ().
Conclusions
In conclusion, we have managed to gain a deeper understanding of the gene expression processes following LAIV immunisation. Our data suggests an overall proliferation, differentiation and regulation of B & T cells in the tonsils. Particularly, days 6–7 highlights important dynamic gene expression patterns that are probably fundamental for the development of vaccine induced immunity. Some of the more interesting and significantly upregulated genes were RIPK2, IL-2 and IL2-RA. Further studies are needed to explore the more detailed roles of RIPK2 in influenza infection, its involvement in severe inflammation reduction, viral clearance and cytokine expression patterns in tonsillar tissues.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Supplemental_Tables.zip
Download Zip (80.3 KB)Acknowledgments
We express our gratitude to the children who underwent tonsillectomy and their supportive parents, who altruistically joined our clinical trial. Their collaboration has been essential for the accomplishment of the study presented. We thank Dr. Hans J. Aarstad at the Ear, Nose and Throat division, Dr. Camilla Tøndel and the nurses at the Children Trial Unit, Haukeland University Hospital, for their help and collaboration, the laboratory staff at the Influenza Center, Gina Bergh and Kjerstin Jakobsen at the Broegelmann Research Laboratory, for excellent technical assistance. Finally, we would like to thank Professor Karl-Henning Kalland at the Department of Clinical Science for the valuable scientific discussions.
Funding
The Influenza Center is funded by the Ministry of Health and Care Services, Norway, the Norwegian Research Council Globvac program (220670/H10), the European Union (Univax 601738) and (EU IMI, FLUCOP 115672), Helse Vest and the K.G. Jebsen Center for Influenza Vaccines.
References
- Puig-Barbera J, Natividad-Sancho A, Launay O, Burtseva E, Ciblak MA, Tormos A, Buigues-Vila A, Martinez-Ubeda S, Sominina A, Group G. 2012–2013 Seasonal influenza vaccine effectiveness against influenza hospitalizations: results from the global influenza hospital surveillance network. PLoS One 2014; 9:e100497; PMID:24945510; http://dx.doi.org/10.1371/journal.pone.0100497
- Sokolow LZ, Naleway AL, Li DK, Shifflett P, Reynolds S, Henninger ML, Ferber JR, Odouli R, Irving SA, Thompson MG, et al. Severity of influenza and non-influenza acute respiratory illness among pregnant women, 2010–12. Am J Obstet Gynecol 2014; 212(2):e1-11; PMID:25111585
- Atkinson MP, Wein LM. Quantifying the routes of transmission for pandemic influenza. Bull Math Biol 2008; 70:820–67; PMID:18278533; http://dx.doi.org/10.1007/s11538-007-9281-2
- Strategic Advisory Group of Experts on Immunization - report of the extraordinary meeting on the influenza A (H1N1) 2009 pandemic, 7 July 2009. Wkly Epidemiol Rec 2009; 84:301–4; PMID:19630186
- Palache A, Oriol-Mathieu V, Abelin A, Music T, Influenza Vaccine Supply task f. Seasonal influenza vaccine dose distribution in 157 countries (2004–2011). Vaccine 2014; 32:6369–76; PMID:25442403; http://dx.doi.org/10.1016/j.vaccine.2014.07.012
- Krammer F, Jul-Larsen A, Margine I, Hirsh A, Sjursen H, Zambon M, Cox RJ. An H7N1 Influenza Virus Vaccine Induces Broadly Reactive Antibody Responses against H7N9 in Humans. Clin Vaccine Immunol 2014; 21:1153–63; PMID:24943383; http://dx.doi.org/10.1128/CVI.00272-14
- Kang HM, Lee EK, Song BM, Jeong J, Kim HR, Choi EJ, Shin YK, Lee HS, Lee YJ. Genetic and pathogenic characteristics of H1 avian and swine influenza A viruses. J Gen Virol 2014; 95(Pt 10):2118–26; PMID:24973238; http://dx.doi.org/10.1099/vir.0.065524-0
- Sugaya N, Takeuchi Y. Mass vaccination of schoolchildren against influenza and its impact on the influenza-associated mortality rate among children in Japan. Clin Infect Dis 2005; 41:939–47; PMID:16142657; http://dx.doi.org/10.1086/432938
- Finch C, Li W, Perez DR. Design of alternative live attenuated influenza virus vaccines. Curr Top Microbiol Immunol 2015; 386:205–235; PMID:25005928
- Coelingh KL, Luke CJ, Jin H, Talaat KR. Development of live attenuated influenza vaccines against pandemic influenza strains. Expert Rev Vaccines 2014; 13:855–71; PMID:24867587; http://dx.doi.org/10.1586/14760584.2014.922417
- Harvey R, Johnson RE, MacLellan-Gibson K, Robertson JS, Engelhardt OG. A promoter mutation in the haemagglutinin segment of influenza A virus generates an effective candidate live attenuated vaccine. Influenza Other Respir Viruses 2014; 8(6):605–12; PMID:25087607; http://dx.doi.org/10.1111/irv.12274
- Brandtzaeg P. Potential of nasopharynx-associated lymphoid tissue for vaccine responses in the airways. Am J Respir Crit Care Med 2011; 183:1595–604; PMID:21471092; http://dx.doi.org/10.1164/rccm.201011-1783OC
- Perry M, Whyte A. Immunology of the tonsils. Immunol Today 1998; 19:414–21; PMID:9745205; http://dx.doi.org/10.1016/S0167-5699(98)01307-3
- Palomares O, Ruckert B, Jartti T, Kucuksezer UC, Puhakka T, Gomez E, Fahrner HB, Speiser A, Jung A, Kwok WW, et al. Induction and maintenance of allergen-specific FOXP3+ Treg cells in human tonsils as potential first-line organs of oral tolerance. J Allergy Clin Immunol 2012; 129:510–20, 520:e511–519; PMID:22051696; http://dx.doi.org/10.1016/j.jaci.2011.09.031
- Keijzer C, Haijema BJ, Meijerhof T, Voorn P, de Haan A, Leenhouts K, van Roosmalen ML, van Eden W, Broere F. Inactivated influenza vaccine adjuvanted with bacterium-like particles induce systemic and mucosal influenza A virus specific T-cell and B-cell responses after nasal administration in a TLR2 dependent fashion. Vaccine 2014; 32:2904–10; PMID:24598720; http://dx.doi.org/10.1016/j.vaccine.2014.02.019
- Carter NJ, Curran MP. Live attenuated influenza vaccine (FluMist(R); Fluenz): a review of its use in the prevention of seasonal influenza in children and adults. Drugs 2011; 71:1591–622; PMID:21861544; http://dx.doi.org/10.2165/11206860-000000000-00000
- Belshe RB, Toback SL, Yi T, Ambrose CS. Efficacy of live attenuated influenza vaccine in children 6 months to 17 years of age. Influenza Other Respir Viruses 2010; 4:141–5; PMID:20409210; http://dx.doi.org/10.1111/j.1750-2659.2009.00124.x
- Jefferson TO, Rivetti D, Di Pietrantonj C, Rivetti A, Demicheli V. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2007:CD001269; PMID:17443504
- Jefferson T, Rivetti A, Di Pietrantonj C, Demicheli V, Ferroni E. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev 2012; 8:CD004879; PMID:22895945
- Pidelaserra Marti G, Isdahl Mohn KG, Cox RJ, Brokstad KA. The influence of tonsillectomy on total serum antibody levels. Scand J Immunol 2014; 80:377–9; PMID:25039393; http://dx.doi.org/10.1111/sji.12213
- Mohn KG, Bredholt G, Brokstad KA, Pathirana RD, Aarstad HJ, Tøndel C, Cox RJ. Longevity of B-cell and T-cell responses after live attenuated influenza vaccination in children. J Infect Dis. 2014 Nov 25. 211(10):1541-9 PMID:25425696
- Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum heamagglutination inhibition antibody in protection against challenge infection with influenza A and B viruses. J Hyg 1972; 70:767–77; PMID:4509641; http://dx.doi.org/10.1017/S0022172400022610
- Bustin SA, Nolan T. Pitfalls of quantitative real-time reverse-transcription polymerase chain reaction. J Biomol Tech 2004; 15:155–66; PMID:15331581
- Fleige S, Pfaffl MW. RNA integrity and the effect on the real-time qRT-PCR performance. Mol Aspects Med 2006; 27:126–39; PMID:16469371; http://dx.doi.org/10.1016/j.mam.2005.12.003
- Imbeaud S, Graudens E, Boulanger V, Barlet X, Zaborski P, Eveno E, Mueller O, Schroeder A, Auffray C. Towards standardization of RNA quality assessment using user-independent classifiers of microcapillary electrophoresis traces. Nucleic Acids Res 2005; 33:e56; PMID:15800207; http://dx.doi.org/10.1093/nar/gni054
- Halbroth BR, Heil A, Distler E, Dass M, Wagner EM, Plachter B, Probst HC, Strand D, Hartwig UF, Karner A, et al. Superior in vitro stimulation of human CD8+ T-cells by whole virus versus split virus influenza vaccines. PLoS One 2014; 9:e103392; PMID:25072749; http://dx.doi.org/10.1371/journal.pone.0103392
- Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, Lambkin-Williams R, Gilbert A, Oxford J, Nicholas B, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med 2012; 18:274–80; PMID:22286307; http://dx.doi.org/10.1038/nm.2612
- Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, Bean T, Barclay W, Deeks JJ, Lalvani A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med 2013; 19:1305–12; PMID:24056771; http://dx.doi.org/10.1038/nm.3350
- McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T-cell immunity to influenza. N Engl J Med 1983; 309:13–7; PMID:6602294; http://dx.doi.org/10.1056/NEJM198307073090103
- Gaur P, Singh AK, Shukla NK, Das SN. Inter-relation of Th1, Th2, Th17 and Treg cytokines in oral cancer patients and their clinical significance. Hum Immunol 2014; 75:330–7; PMID:24486578; http://dx.doi.org/10.1016/j.humimm.2014.01.011
- Weaver JM, Yang H, Roumanes D, Lee FE, Wu H, Treanor JJ, Mosmann TR. Increase in IFNgamma(-)IL-2(+) cells in recent human CD4 T cell responses to 2009 pandemic H1N1 influenza. PLoS One 2013; 8:e57275; PMID:23526940; http://dx.doi.org/10.1371/journal.pone.0057275
- Falchetti R, Lanzilli G, Casalinuovo IA, Gaziano R, Palamara AT, Di Francesco P, Ravagnan G, Garaci E. Splenic CD4+ and CD8+ T cells from influenza immune mice concurrently produce in vitro IL2, IL4, and IFN-gamma. Cell Immunol 1996; 170:222–9; PMID:8660821; http://dx.doi.org/10.1006/cimm.1996.0155
- Litjens NH, Huisman M, Hijdra D, Lambrecht BM, Stittelaar KJ, Betjes MG. IL-2 producing memory CD4+ T lymphocytes are closely associated with the generation of IgG-secreting plasma cells. J Immunol 2008; 181:3665–73; PMID:18714042; http://dx.doi.org/10.4049/jimmunol.181.5.3665
- Cox RJ, Madhun AS, Hauge S, Sjursen H, Major D, Kuhne M, Hoschler K, Saville M, Vogel FR, Barclay W, et al. A phase I clinical trial of a PER.C6 cell grown influenza H7 virus vaccine. Vaccine 2009; 27:1889–97; PMID:19368768; http://dx.doi.org/10.1016/j.vaccine.2009.01.116
- Lessard CJ, Li H, Adrianto I, Ice JA, Rasmussen A, Grundahl KM, Kelly JA, Dozmorov MG, Miceli-Richard C, Bowman S, et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjogren's syndrome. Nat Genet 2013; 45:1284–92; PMID:24097067; http://dx.doi.org/10.1038/ng.2792
- Karpala AJ, Bingham J, Schat KA, Chen LM, Donis RO, Lowenthal JW, Bean AG. Highly pathogenic (H5N1) avian influenza induces an inflammatory T helper type 1 cytokine response in the chicken. J Interferon Cytokine Res 2011; 31:393–400; PMID:21194349; http://dx.doi.org/10.1089/jir.2010.0069
- Woods CW, McClain MT, Chen M, Zaas AK, Nicholson BP, Varkey J, Veldman T, Kingsmore SF, Huang Y, et al. A host transcriptional signature for presymptomatic detection of infection in humans exposed to influenza H1N1 or H3N2. PLoS One 2013; 8(1):e52198; PMID:23326326; http://dx.doi.org/10.1371/journal.pone.0052198
- Zhu HT, Ru L, Guo YF. [Clinical significance of TGF-beta1 in children with primary IgA nephropathy[. Zhongguo Dang Dai Er Ke Za Zhi 2014; 16:749–53; PMID:25008886
- Shlomchik MJ, Weisel F. Germinal center selection and the development of memory B and plasma cells. Immunol Rev 2012; 247:52–63; PMID:22500831; http://dx.doi.org/10.1111/j.1600-065X.2012.01124.x
- Cox RJ, Brokstad KA, Zuckerman MA, Wood JM, Haaheim LR, Oxford JS. An early humoral immune response in peripheral blood following parenteral inactivated influenza vaccination. Vaccine 1994 Aug; 12(11):993–9; PMID:7975853; http://dx.doi.org/10.1016/0264-410X(94)90334-4
- Brokstad KA, Cox RJ, Olofsson J, Jonsson R, Haaheim LR. Parenteral influenza vaccination induces a rapid systemic and local immune response. J Infect Dis 1995 Jan; 171(1):198–203; PMID:7798664; http://dx.doi.org/10.1093/infdis/171.1.198
- Brokstad KA, Cox RJ, Oxford JS, Haaheim LR. IgA, IgA subclasses, and secretory component levels in oral fluid collected from subjects after parental influenza vaccination.J Infect Dis 1995 Apr; 171(4):1072–4; PMID:7706797; http://dx.doi.org/10.1093/infdis/171.4.1072-a
- Brokstad KA, Cox RJ, Eriksson JC, Olofsson J, Jonsson R, Davidsson A. High prevalence of influenza specific antibody secreting cells in nasal mucosa. Scand J Immunol 2001 Jul–Aug; 54(1–2):243–7; PMID:11439173; http://dx.doi.org/10.1046/j.1365-3083.2001.00947.x
- Brokstad KA, Eriksson JC, Cox RJ, Tynning T, Olofsson J, Jonsson R, Davidsson A. Parenteral vaccination against influenza does not induce a local antigen-specific immune response in the nasal mucosa. J Infect Dis 2002 Apr 1; 185(7):878–84; PMID:11920311; http://dx.doi.org/10.1086/339710
- Eriksson JC, Davidsson A, Garberg H, Brokstad KA. Lymphocyte distribution in the tonsils prior to and after influenza vaccination. Vaccine 2003 Dec 8; 22(1):57–63; PMID:14604571; http://dx.doi.org/10.1016/S0264-410X(03)00540-1
- McCarthy JV, Ni J, Dixit VM. RIP2 is a novel NF-kappaB-activating and cell death-inducing kinase. J Biol Chem 1998; 273:16968–75; PMID:9642260; http://dx.doi.org/10.1074/jbc.273.27.16968
- Furman D, Jojic V, Kidd B, Shen-Orr S, Price J, Jarrell J, Tse T, Huang H, Lund P, Maecker HT, et al. Apoptosis and other immune biomarkers predict influenza vaccine responsiveness. Mol Syst Biol 2013; 9:659; PMID:23591775; http://dx.doi.org/10.1038/msb.2013.15
- Lupfer C, Thomas PG, Anand PK, Vogel P, Milasta S, Martinez J, Huang G, Green M, Kundu M, Chi H, et al. Receptor interacting protein kinase 2-mediated mitophagy regulates inflammasome activation during virus infection. Nat Immunol 2013; 14:480–8; PMID:23525089; http://dx.doi.org/10.1038/ni.2563
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ: Autophagy fights disease through cellular self-digestion. Nature 2008; 451:1069–75; PMID:18305538; http://dx.doi.org/10.1038/nature06639
