Abstract
To evaluate antibody persistence of Aleph inactivated split influenza vaccine, 3308 healthy Chinese people more than 3 years old were enrolled in a hemagglutination inhibition (HI) assay before vaccination, 641 were screened by HI assay negative, 437 of which received one dose of Aleph inactivated split influenza vaccine and 204 of which received one dose of control vaccine (recombinant hepatitis B). After vaccination, the receivers were collected blood at 1st month, 3rd month, 6th month and 12th month for Aleh influenza vaccine antibody persistence assess. The antibody test were determined by hemagglutination inhibition (HI) assay. There were significant difference in antibody geometric mean titer between experimental group and control at 1st month and 3rd month after vaccination. Influenza antibody could persist at least up to 3rd month. Because of the local spring influenza epidemic, we could not analyze the results of 6th and 12th month. Aleph influenza vaccines showed good immune persistence in healthy volunteers at least in the 3 months after vaccination. Influenza viruses are important human respiratory pathogens. Immunization is widely acknowledged to currently be the most effective method of minimizing the impact of pandemic influenza. Through we have checked many references about Influenza vaccine, the duration of protective antibody for influenza vaccines are still not available. Based on this situation and our previous work,Citation11 Influenza vaccine antibody duration analyze are necessary. This manuscript presents data on the persistence of Hemagglutination Inhibition (HI) immune response against the A/California/7/2009(H1N1), A/Peth/16/2009(H3N2) strain and B/Brisbane/60/2008. 641 were screened from 3302 volunteers by HI test of influenza A and confirmed enrollment based on the antibodies titer less than 1:10. After administered with one dose of Aleph influenza vaccine, blood samples were collected. 437 subjects (3–10y: 131; 11–17y: 110; 18–54y: 69; ≥55y: 127) were vaccinated influenza vaccine as test group. 204 subjects (3–10 y: 70; 11–17 y: 47; 18–54 y: 28; ≥55 y: 59) were vaccinated recombinant hepatitis B vaccine as control group. Immunogenicity end points were based on the European licensure criteria for pandemic influenza vaccines. The persistence of HI immune response against the vaccine strain was assessed through GMT. The immunogenicity of the Aleph influenza vaccine induced all reached the standards at 1st month and GMTs peak could persist at least up to 3rd month. (This study has been registered at clinicaltrials.gov under registration no. NCT01758185.) Because of the local spring influenza epidemic we could not analyze the results of 6th and 12th month. Aleph influenza vaccines showed good immune persistence in healthy volunteers at least in the 3 months after vaccination.
Introduction
Influenza (flu) was an acute respiratory infections caused by influenza virus (flu virus), which transmitted mainly through droplets and aerosols originating from the respiratory secretions of infected people, but occasionally also through contact with virus contaminated fomites. The clinical manifestations concluded fever, headache, muscle pain, fatigue, rhinitis, sore throat and cough. In temperate climates, seasonal epidemics are experienced mainly during the winter. While in tropical regions, influenza may occur throughout the year, causing outbreaks more irregularly. Influenza virus infection is a common cause of hospitalization and death, and worldwide the mortality from seasonal influenza virus infection is estimated to be 250,000 to 500,000 persons per year.Citation1 Vaccination is regarded as the most effective intervention to prevent and attenuate seasonal influenza and also reduced the seasonal influenza related complications.Citation2 Several domestic manufacturers had produced seasonal influenza vaccines in China.
Our previous work was to evaluate the safety and immunogenicity of our domestic seasonal influenza vaccine, the results showed that Aleph inactivated split influenza vaccine had good safety and immunogenicity,Citation3 from which we selected 641 participants for influenza antibody negative before vaccination and collected serum at 1st month, 3rd month, 6th month and 12th month for antibody persistence assay.
Through our checking for the antibody persistence of influenza, there was information about the immune persistence for pandemic influenza vaccine (H1N1),Citation4 there was limited references published for the persistence of the seasonal influenza vaccine manufactured in China. Moreover, the manufactured seed virus (recommended by World Health Organization yearly) for seasonal influenza vaccine was consistent from 2010 to 2012. Therefore, we intended to research for the persistence and the protection time for seasonal influenza vaccine.
Aleph influenza vaccine is a highly purified inactivated, egg-based trivalent influenza vaccine made in china. It was initially licensed in China in 2005. The aim of this post-marketing clinical study was to assess the antibody persistence produced by Aleph biomedical Co., Ltd.
This clinical trial (registration no. NCT01758185 [www.clinicaltrials.gov]) was conducted to assess long-term antibody persistence after immunized with one dose of seasonal influenza vaccine.
Results
Study population analysis and baseline analysis
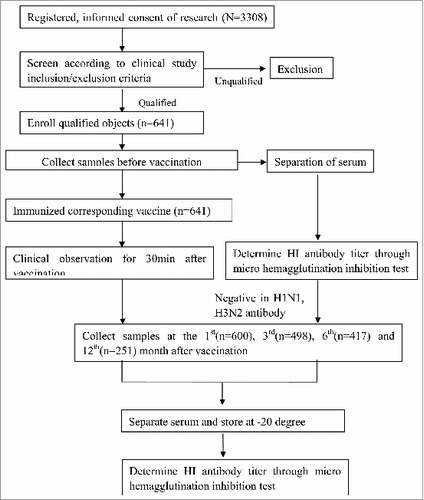
A total of 3308 healthy people more than 3 years old met the inclusion/exclusion criteria for the study and were randomized to the experimental (Influenza vaccine) and control (hepatitis B) groups (2205 in experimental group and 1103 in control group). Of the 3308 subjects that participated in the priming phase of the study,Citation11 only 641 subjects were identified HI antibody negative before vaccination (antibody to H1N1<1:5, H3N2 < 1:5 and B < 1:80). Therefore, through HI immune response test to A/H1N1, A/H3N2 virus and B, 641 were chosen as the subjects of the vaccine persistence study, of which 437 in experimental group and 204 in control group. During this study, 41 subjects were lost to follow-up at the 1st month serum collection after vaccination, 102 subjects were lost to follow-up at the 3rd month after the 1st month serum collection, 81 subjects were lost to follow-up at the 6th month after the 3rd serum collection, 166 subjects were lost to fellow-up at the 12th month after the 6th serum collection. Finally, a total of 251 subjects (600 at the 1st month, 498 at the 3rd month, 417 at the 6th month and 251 at the 12th month) completed the antibody persistency study. For the absence of the participants who were children taking part of a special activity, so there was much higher dropout rate in children group. Because of the difficulty for the longtime fellow-up evaluation, many subjects could be found or did not agree to attend fellow-up. There was no statistically significant difference in subjects' age among all age groups before vaccination, 1st serum collection, 3rd serum collection, 6th serum collection and 12th serum collection (P > 0.05; ).
Table 1. Numbers and mean age of subjects in the study groups
The baseline GMTs between the experimental and control groups varied from 1.59–1.79, 2.07–3.80 and 35.31–42.24 against H1N1 H3N2 and B respectively. There was no statistically significant differences between experimental and control groups in all subjects before vaccination for antibody against the H1N1strain of influenza geometric mean titer (GMT) (P > 0.05; ). Besides, the GMT of H3N2 and B strains between experimental and control groups in all subjects was significant statically different (P < 0.05; ). The main reason for this difference was the subjects were selected form 3308 subjects through HI assay with the negative standard for the possibility of this difference.
Table 2. GMTs by HI assay
Immunogenicity HI response
About seasonal vaccine strains, HI data for the seasonal strains were shown in . Geometric mean titers (GMTs) were calculated with their 95% confidence intervals (CI). There had been no criteria for the immune persistence of seasonal influenza vaccine.Citation5-8 28 days after the vaccination (1month, Day 35), the antibody level against H1N1 H3N2 and B strains of the test group increased to 25.6, 17.02 and 93.7 respectively. At 3 months after priming GMTs against H1N1 H3N2 and B strains were 36.07, 15.79 and 147.63 respectively. Meanwhile the GMTs of the control group barely changed. There was significant statistical difference between the 2 groups with respect to GMTs against all the A-type vaccine strains (H1N1, H3N2) and B-type strains (P < 0001, ) in 1st and 3rd antibody level. According to the data of 6th, 12th months, although there was statistical significant between the experimental group and the control group for antibody against A-type strains and B-type strain (P < 0.05, ) except for H3N2 in 12th month, the antibody titer in experimental and control group raised apparently, especially the GMTs of the experimental group and control group both changed distinctly. At 12th month, the GMTs in both groups for all strains declined significant, especially nearly to the pre-vaccination for H3N2 strain. We assumed that during the 3rd month and the 6th month, there was influenza prevalent led to natural infection in local place.
Discussion
Influenza virus were enveloped ribonucleic acid viruses belonging to the family of orthomyxoviridae and were divided into 3 distinct types on the basis of antigenic differences of internal structural proteins.Citation15 Two influenza types, Type A and Type B, were responsible for yearly epidemic outbreaks of respiratory illness in humans and were further classified based on the structure of 2 major external glycoproteins, hemagglutinin (HA) and neuraminidase (NA). Type A strains infected a wide variety of avian and mammalian species. Type A strains were classified into many different serotypes, such as H1N1 and H3N2, which notably cause disease in humans.
Prevention of influenza is a major health issue worldwide, but providing protection by vaccination is challenging. The observed split influenza virus vaccine possessed good immunogenicity and safety confirmed in previous researches.Citation3
Our research results also showed that there was a sharp increase in GMTs of antibodies of A/H1N1, A/H3N2 and B at 6th month after vaccination in both control group and test group, suggesting a possible seasonal influenza in our chosen site during this study period. Since January the 3rd month after immunization, there had a period of high incidence of seasonal influenza, suggesting that there was natural infection in both control group and test group during 3rd month to 6th month after primary immunization, leading to a roaring increase of antibodies titer.
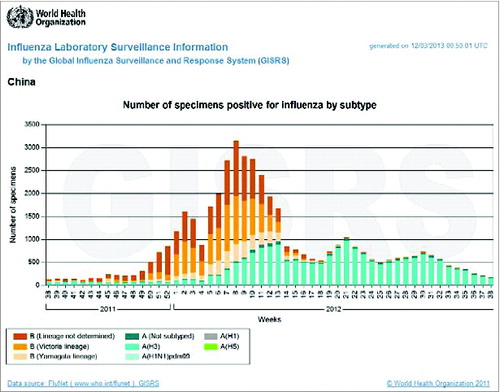
According to Influenza surveillance analysis in China from WHOCitation10 (), it was showed that the influenza began to be prevalent in the end of 2011 and in the February 2012 reached the peak level. During this prevalent time, the main epidemic strains were B (not determined, Victoria and Yamagata) and A (H3N2). The proved our assumption above. From , there was also A/H1N1 prevalent in this area during the 3rd and 6th after vaccination. According to references, though we could not find the reference of the local seasonal influenza epidemic situation in Zhuolu country, finally we found the one of seasonal influenza strain from 2012 to 2013 in Baoding where near our study site was A/H1N.Citation9 This indicated that influenza virus A/H1N1 was a local pandemic in our study site. Of the steep fall in antibody titers at 12th month post vaccination, the Figure 2 revealed that the 12th month after vaccination was not influenza prevalent transmission season, because the 12th month serum collection was held in 37, 38 weeks in 2012. The 37, 38 weeks in 2012 was not the influenza prevalent season.
Taking into account of existence of natural infection factors, peak level of H1N1 antibody of split influenza virus vaccine appeared at 1st to 3rd month after vaccination which was in accordance with results reported in some literatures.Citation11-13 Yet after vaccination, GMTs of H3N2 type antibody at 3rd month was lower than that at 1st month and peak level appeared at 1st month.
Though there was study of the immune persistence for Influenza H1N1 vaccine,Citation4 our study was the first immune persistence study about seasonal influenza split vaccine manufactured in China and could provide a strong basis for the formulation of influenza immunization strategy. Meanwhile, this study established control group to help us better understands the presence of confounding factors. For example, selected time points were, for September, October and December in 2011, March and September in 2012, respectively, which coincided with autumn and winter seasonal flu time in northern hemisphere. Regarding there has been a relative developed railway, highway or highway network around study site, a county in northern China, leading to an increasing mobility of the staff as well as a highly vulnerability to outside influence, therefore influenza outbreak at 6th month after vaccination in this study, affecting persistence observation of vaccine. Hence, there were still many problems need to be improved in the future, such as influenza cases surveillance in survey population in this persistence research to avoid the deficiency of epidemiological indicators at different time point after vaccination. In the same time, data of this study was repeated measurements, which couldn't meet individual requirements since there was internal relevant structure inside the data. Research in the future should work toward repeated measure analysis of variance to establish a generalized linear mixed model and thus understand the effect of the role played by studied vaccine.Citation14
Materials and Methods
Study design and subjects
The study was approved by the study unit ethics committee and conducted in accordance with Good Clinical Practice (GCP) and all applicable regulatory requirements including the Declaration of Helsinki. Before enrollment, written informed consent was obtained from all subjects or guardians.
This study design and subjects enrollment were part of our previously work,Citation3 which evaluate the safety, immunogenicity and batch consistency for Aleph inactivated influenza vaccine. This was a randomized, controlled, double-blinded study conducted in Zhuolu County in Hebei Province in China. 3302 healthy volunteers, who was aged 3 to 70 years without autoimmune diseases, acute illness and history of allergy and had no history of symptoms of upper respiratory infection within the last 6 month also had not previously received influenza vaccination since 2008 (), were divided randomly to experimental (influenza vaccine) and control (HBV) group in a 2:1 ratio via SAS 8.1 (45per block; 30 to experimental group and 15 to control group). Before vaccination, 3302 subjects were collected serum for Hemagglutination Inhibition (HI) assay, 641 of them were selected for being included in immune persistence study. The negative standard for Hemagglutination Inhibition (HI) assay of antibody before vaccination was H1,H3 antibody titer less than 1:5 and B antibody titer less than 1:80. If the H1, H3 and B antibody titer were appreciated for one of corresponding negative standards, the subject would be selected in our study. After collection of the antibody titer before vaccination, taking into account of the B strain antibody titer were almost more than 1:5 before vaccination and also the drop-following rate at 12th month, we selected 1:80 as the negative standard for B strain.
All immunizations completed before the validity date of the study vaccine, that is 30 Aril, 2012. The specific technical route was shown in .
This study was registered in Clinical Trails.gov with the identifier number is NCT01758185.
Vaccine
The inactivated split influenza vaccine used in the study was manufactured by Dalian Aleph Biomedical Co., Ltd. and contained A/California/7/2009(H1N1) 15 μg A/Victoria/210/2009(H3N2) 15 μg and B/Brisbane/60/2008 15 μg per dose(0.5 ml), after being propagated in embryonated eggs, inactivated, purified, split and re-purified containing 3 kinds of hemagglutinin flu strains 15 μg respectively. The vaccine batch number was 20110519, and it was valid until 30 April 2012. The recombinant hepatitis B vaccine used in the study was manufactured by Hissen, and contained HBsAg 10 μg per-dose (0.5 ml). The vaccine batch number was 2010031004, and it was valid until May 5, 2013.
Safety evaluation
Safety observation was performed by the investigators in all subjects at 30 min and at 6, 24, 48 and 72 h after vaccinations. Safety data after immunization had been published in another article.Citation3 Therefor, we don't provide safety data in this article.
Immunogenicity evaluation
Blood samples were collected at different time points before as well as 1, 3, 6, and 12 months after immunization. Serum were obtained by centrifuging at 3,000 g for 5 min; serum were then stored at –18°C. Serum influenza antibody against the vaccine strains of H1N1, H3N2 and B were measured by Hemagglutination inhibition assay (HI), according to standard methods All serum samples pre- and post-immunization were analyzed in Beijing LvZhu Biotechnology Co., Ltd. Laboratory. HI was assessed by a validated HI micro-titer assay using chicken erythrocytes against the vaccine strains.Citation4 Laboratory staff was blinded to the participants' vaccination group. Geometric mean titers (GMTs) were calculated with their 95% confidence intervals (CI).
Statistical analysis
The seroconvention rates and seroprotection rates of influenza antibody after immunization were determined.
Epidata software was used for data entry and SAS16.0 statistical software was applied for data processing. Comparisons of GMT between long-term antibody persistence were tested by mixed linear model among groups, with an inspection level of α = 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We appreciated all the investigators from Zhuolu Center for Disease Control and Prevention in Hebei province for their contribution to the study. We also appreciate Dr. Jian Kong for his help in HI assays.
Funding
The Capital Medical Scientific Development Research Funds No. 2011-7023-01 supported this study. This study was also funded by an unrestricted research grant from Dalian Aleph Biomedical Co., Ltd.
References
- Bhat N, Wright JG, Broder KR, Murray EL, Greenberg ME, Glover MJ, Likos AM, Posey DL, Klimov A, Lindstrom SE, et al. Influenza-associated deaths among children in the United States, 2003–2004. N Engl J Med 2005; 353:2559-67; PMID:16354892; http://dx.doi.org/10.1056/NEJMoa051721
- Van Essen GA, Palache AM, Forleo E, Fedson DS. Influenza vaccination in 2000: recommendations and vaccine use in 50 developed and rapidly developing countries. Vaccine 2003; 21:1780-5; PMID:12686094; http://dx.doi.org/10.1016/S0264-410X(03)00072-0
- Li S Li L, Ai X, Yang L, Bai Y, Wang Z, Han H, Lu Q, Luo F, Zhang Z, et al. A randomized, controlled, blinded study of the safety, immunogenicity and batch consistency of Aleph inactivated split influenza vaccine made in China in Chinese people. Hum Vaccin Immunother 2014; 10 (3):1-9; PMID:24301228
- Launay O, Duval X, Fitoussi S, Jilg W, Kerdpanich A, Montellano M, Schwarz TF, Watanveerade V, Wenzel JJ, Zalcman G, et al. Extended antigen sparing potential of AS03-adjuvanted pandemic H1N1 vaccines in children, and immunological equivalence of two formulations of AS03-adjuvanted H1N1 vaccines: results from two randomised trials. BMC Infect Dis 2013; 16(13):435; PMID:24041010; http://dx.doi.org/10.1186/1471-2334-13-435
- World Health Organization. Wkly Epidemiol Rec 2012; 87: 233-40; PMID:22715518
- Hehme NW, Künzel W, Petschke F, Türk G, Raderecht C, Van Hoecke C, Sänger R. Ten years of experience with the trivalent split-influenza vaccine, fluarix(TM). Clin Drug Investig 2002; 22:751-69; http://dx.doi.org/10.2165/00044011-200222110-00004
- Committee for Proprietary Medicinal Products (CPMP): Note for guidance on harmonisation of requirements for influenza vaccines. The European Agency for the Evaluation of Medicinal Products. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf
- Center for Biologics Evaluation and Research (CBER). Guidance for Industry: clinical data needed to support the licensure of seasonal inactivated influenza vaccines. Food and Drug Administration (FDA). http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091990.pdf
- Wang L, Li J-J, Zu W-G, Cheng Y, Tian B. The analyze for Influenza cases in Bao Ding City. Mod Prevent Med; 2014(14)
- World Health Organization. Influenza Laboratory Surveillance Information by the Global Influenza Surveillance and Response System(GISRS). 2013.
- Cheng K, Shen X, Yang S, Zhou Z, Xie J, Chen W, Weng Y, Yan Y. Research on growth and decline of antibody in H1N1 vaccine serum. Chinese Zoonosis 2012; 06:566-9
- Liu X, Lv J, Yang M, Chen X. Immune effect analysis in Daxinganling crowd, Inner Mongolia with H1N1 influenza vaccine in 21 to 93 days. Contemp Med 2010; 27:159-60.
- Zheng K, Yang R, Zhang X, Huang R, Chai B. Immunization persistence evaluation of H1N1 influenza vaccine after one year. Prevent Med Infor 2012; 10:825-8
- Huang K, Ni Z, Cheng W. Mixed linear model application in repeated measurement data of clinical trials. Mod Prevent Med 2005; 11:166-7.
- Lamb RA, Krug RM. Orthomyxoviridae: The Virus and Their replication. In: Fields Virology, Editors-in-Chief: Knipe DM, Howley PM. 4th Edition. Philadelphia, PA: Lippincott Williams and Wilkins, Publishers; 2001:1487-531.