?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Herpes zoster (HZ) is caused by VZV reactivation that is facilitated by a declined immunity against varicella-zoster virus (VZV), but also occurs in immunocompetent individuals. Cytomegalovirus (CMV) infection is associated with immunosenescence meaning that VZV-specific T-cells could be less responsive. This study aimed to determine whether CMV infection could be a risk factor for the development of HZ. CMV IgG serostatus was determined in stored serum samples from previously prospectively recruited ambulatory adult HZ patients in the UK (N = 223) in order to compare the results with those from UK population samples (N = 1545) by means of a logistic regression (controlling for age and gender). Furthermore, we compared the UK population CMV seroprevalence with those from population samples from other countries (from Belgium (N1 = 1741, N2 = 576), USA (N = 5572) and Australia (N = 2080)). Furthermore, CMV IgG titers could be compared between UK HZ patients and Belgium N2 population samples because the same experimental set-up for analysis was used. We found UK ambulatory HZ patients to have a higher CMV seroprevalence than UK population samples (OR 1.56 [1.11 2.19]). CMV IgG seropositivity was a significant risk factor for HZ in the UK (OR 3.06 [1.32 7.04]. Furthermore, high CMV IgG titers (exceeding the upper threshold) were less abundant in CMV-seropositive Belgian N2 population samples than in CMV-seropositive UK HZ patients (OR 0.51 [0.31 0.82]. We found CMV-seroprevalence to increase faster with age in the UK than in other countries (P < 0.05). We conclude that CMV IgG seropositivity is associated with HZ. This finding could add to the growing list of risk factors for HZ.
Introduction
Herpes zoster (HZ) is caused by the clinical reactivation of latent varicella-zoster virus (VZV). This reactivation is linked to a declined immunity against VZV as noted in immunocompromised individuals, but also in older individuals.Citation1,2 Interestingly, re-exposure to chickenpox patients has been hypothesized to boost T-cell immunity against VZV and subsequently postpone the risk on HZ.Citation3,4 Several clinical risk factors for HZ have been documented including auto-immune diseases, immunosuppressive medication, depression and asthma.Citation5,6 However, HZ is also known to occur in otherwise healthy ambulatory and young patients.Citation5,6 In addition, certain HLA types have been shown to be enriched in individuals with prolonged HZ (post-herpetic neuralgia).Citation7
Cytomegalovirus (CMV), another human herpesvirus (HHV) with latency-reactivation cycles, is well known for its modulating effects on immunity. For e.g., VZV-specific T-cells, but also T-cells against other HHV, from CMV-seropositive individuals are more mature, further differentiated and thus possibly less responsive compared to the VZV-specific T-cells from CMV-seronegative individuals.Citation8 From a clinical perspective it has been shown, although some debate is still ongoing, that CMV-seropositive older individuals have an increased frailty and mortality.Citation9 Recently, analyses using VZV-specific antibody titersCitation10 and herpes simplex virus type 1 (HSV-1) antibody titersCitation11 have indicated that CMV-seropositivity was significantly associated with reactivation of VZV and HSV-1.
The present study set out to assess the level of CMV-seropositivity in UK HZ patients compared to random population samples from the UK to determine whether there is an association with VZV reactivation. If so, this could be a major addition to the list of risk factors of HZ in otherwise healthy individuals.
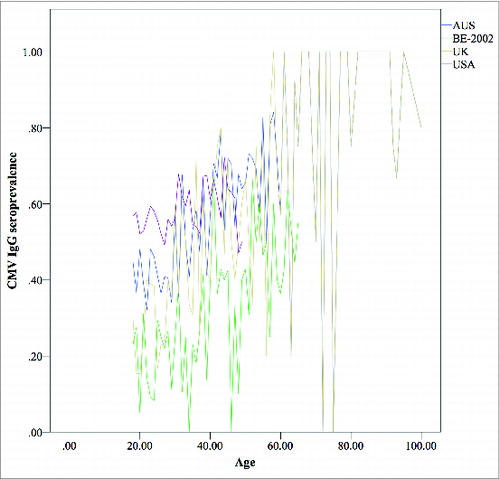
Furthermore, it is known that CMV-seropositivity is associated with ethnic and socio-economic factors,Citation12 as such we compared CMV-seroprevalence data between general population reference samples from the USA, Australia and Belgium. Differences in CMV-seroprevalence might explain the inter-country differences noted in HZ incidence.Citation13
Results
UK HZ patients vs. UK-reference sample
The best model choice (after step-wise logistic regression based on AIC):
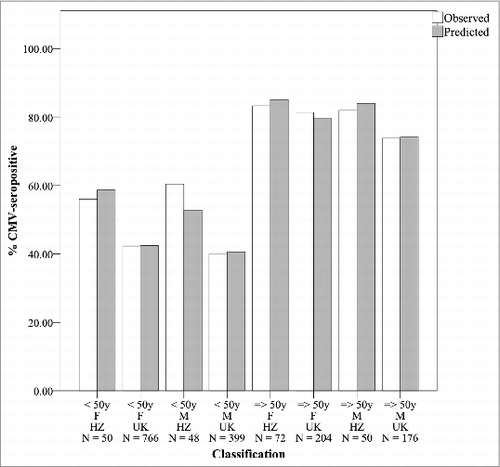
with i denoting individual i, logit (CMV = 1i) the probability individual i has a positive CMV IgG serostatus, βj=0,…,3 parameters, age as a continuous variable, Gender (Female = 0, Male = 1) and Dataset (HZ = 0, UK = 1) as dummy variables and εi error terms. Parameters were β0 = − 1.20 (SE 0.23, P < 0.001), β1 = 0.045 (SE 0.0033, P < 0.001), β2 = − 0.16 (SE 0.11, P = 0.13) and β3 = − 0.44 (SE 0.18, P = 0.011). We note that a sensitivity analysis focused only on HZ samples collected during acute HZ onset gave highly similar results. shows per classification (based on age < 50 or => 50, gender and data set) the observed versus predicted CMV-seroprevalence. We note that dichotomous age stratification (at 30 years, 40 years, 50 years, 60 years) did not present statistically significant differences in CMV IgG serostatus between the younger and older age groups most likely due to sample size restrictions. The parameter estimation illustrates that the likelihood for CMV-seropositivity increases with age and compared to the adult HZ patients our reference UK-population has a lower OR for CMV-seropositivity (0.64 [0.45–0.90]). suggests that the latter effect is mainly caused by individuals younger than 50 years.
Figure 1. Observed and predicted CMV-seroprevalence percentage for UK HZ patient and UK general population samples. Caption: Groups are classified using age (50 years as cut-off), gender, data set (UK HZ (HZ) or UK-reference (UK)). The number of participants per classification group is shown (N). For each classification group the left column shows the observed CMV-seroprevalence whereas the right column shows the CMV-seroprevalence as predicted by the logistic regression analysis.
CMV-seropositivity was associated with an increased risk of HZ (OR 3.06 [1.32–7.04], and the effect had a minor non-significant tendency to decrease per additional year of age (OR 0.99 [0.96–1.01]).
CMV-seropositivity as a function of country, age and gender
The comparisons between the UK-reference dataset and BE-2002, BE-2006, USA and AUS data sets illustrated that CMV-seroprevalence is not only a function of age and gender as was shown above, but also of country ( and Table S1). Our results show that CMV-seroprevalence increases slower with age in BE-2002, BE-2006, USA and AUS datasets compared to the UK-reference data set.
CMV IgG titers in UK HZ patients compared to Belgian population samples
Although CMV-seroprevalence data differed between countries, we nevertheless assumed that CMV-titers could be compared between the UK HZ and BE-2006 data sets. A logistic regression analysis (controlling for age and gender) showed that CMV IgG titers above the threshold of 500 U/ml occurred less frequently (OR 0.51 [0.31 0.82], P Value = 0.0050) in BE-2006 CMV-seropositive individuals than in UK HZ CMV-seropositive patients. In addition, we noted that 10/48 young (<50 years of age) UK HZ CMV-seropositive patients were CMV IgM positive, compared to 4/81 in the older (>= 50 years) age group (P = 0.0077).
Discussion
Both cytomegalovirus seropositivity and the CMV-specific IgG titer are associated with increased frailty and mortality.Citation14,15 Furthermore, CMV-seropositivity is associated with modified T-cell immunityCitation8 and increased serum antibody titers against VZVCitation10 and HSV-1.Citation11 These increased serum antibody titers in CMV-seropositive individuals could be caused by an increased reactivation rate in these individuals.Citation16 Our present study shows that CMV-seroprevalence is higher in samples from UK HZ patients (1998–2003) compared to samples of the general population in the UK (1991, but generally similar to 2002 as illustrated by Vyse et al.Citation17). Defining the UK HZ patients as cases and the UK population as controls, we found CMV-seropositivity to be associated with an increased risk of HZ with an OR of 3.06 [1.32 7.04].
Of interest, we also noted higher CMV IgG titers in CMV-seropositive HZ patients compared to CMV-seropositives in a Belgian general population sample. This finding could indicate that CMV reactivation, known to cause an increase in CMV-specific antibody titer via the secondary immune response,Citation16 (or perhaps even de novo infection) has occurred more recently or more frequently in HZ patients. This hypothesis suggests a co-susceptibility for human herpesvirus reactivation. We also assessed whether CMV-seroprevalence population data from other countries could be used as a control. However, we found CMV-seroprevalence to differ between countries even after controlling for age and gender. One could speculate whether socio-economic, cultural or other factors may contribute to these differences.Citation12 We cannot establish with certainty whether such factors also influenced the comparison between the UK HZ patients and UK general population. However, we used samples that maximized representativeness for both these populations within the limits of our control, and we found no indications that socio-economic or cultural factors differed between these datasets.
Further studies are needed to confirm these results. In particular, prospective case-control studies should reassess the magnitude of the risk caused by CMV-seropositivity on clinical VZV reactivation. Furthermore, a prospective study should also assess CMV presence in HZ patients via the documentation of the viral load at various sites (blood, saliva, urine, etc.).Citation18 Our study suggests that this risk could be higher in young individuals. Indeed, we also found a higher CMV IgM seroprevalence in young (<50 years) CMV-seropositive HZ patients (21%) compared to older HZ patients (5%), although we could not find an additional statistically significant effect of age on the difference in CMV IgG seroprevalence between HZ and control individuals (probably due to the limited sample size). Nevertheless, our finding regarding CMV IgM seroprevalence could support our speculation that not only CMV reactivation, but perhaps also de novo CMV infection could be implicated in VZV reactivation. These findings could help explain why a disease that is generally thought to be due to a decline in VZV-specific immunity also affects healthy young individuals. In the advent of a CMV vaccine that can prevent CMV infection, its benefits might therefore extend to a reduction in HZ-related morbidity. Indeed, our study identifies CMV-seropositive individuals, particularly under age 50 years, as a possible target group for HZ vaccination.Citation19,20 Furthermore, this study also provides support to add CMV-seropositivity to the growing list of confounders in clinical vaccine trials.Citation20 The currently reported 51% reduction of HZ incidence after HZ vaccination might actually be higher in CMV-seropositive individuals.
Although the analyses in the current paper focused on HZ, similar associations have also been shown for other human herpesviruses such as HSV-1. CMV-seropositive individuals not only have increased antibody titers against both VZV and HSV-1, but a recent epidemiological analysis showed strong associations between clinical HZ and HSV in the general population.Citation6 A possible explanation could be a shared increased susceptibility caused by CMV for example caused by T-cell exhaustion.Citation8
A limitation of our analysis is that the general population samples with which the HZ patients were compared contain an unknown proportion of individuals who have had a history of HZ (at most 10–30%Citation21,22). Clearly, this limitation makes the observation of a significant difference between the 100% HZ and the “general population” samples more convincing (and it may help explain why there was no significant difference with the US and Australian general population samples). Additionally, as recently shown, CMV-seronegative individuals can have CMV-responsive T-cells thereby suggesting a former encounter with CMV, and this might also have affected our analyses.Citation23,24 Finally, our study is the first to show an association between CMV infection and HZ, further causal relations should be studied in other studies.
We conclude that CMV-seropositivity is associated with HZ implying that CMV infection could be a possible clinical risk factor for HZ.
Methods
Sample collection
This analysis was restricted to adult (>= 18 year) samples.
223 adult HZ patients (age range = [18 89] with median 53 years; F/M = 55%) were obtained from 2 prospective longitudinal studies in England (samples from 1998–2003) as detailed elsewhere.Citation25,26 Serum samples were collected and frozen at various time points up to 12 months after acute HZ onset. CMV IgG measurements were performed on acute HZ onset serum samples (thus from the first time point = baseline). However, for 41/223 (=18%) patients CMV IgG measurements had to be performed on a sample post acute HZ onset (within 12 months), because there wasn't a baseline serum sample available. In order to minimize the risk of new CMV infections, CMV IgM was additionally determined on the 41 post acute HZ onset samples. Two of these samples had “gray zone” CMV IgM values and were excluded. Another sample from the total group of 223 was excluded because it had gray zone CMV IgG values. Thus we retained 220 adult HZ samples for further analysis. IgG directed against CMV pp150, pp28, p38, and p52 and IgM against CMV pp150, p52 were determined on the thawed samples in the Antwerp University Hospital laboratory (Roche Cobas, Basel, Switzerland).
Reference adult population CMV-seroprevalence data were obtained for 1545 individuals (age range = [18 100] with median 34 years; 55% women) in the UK (1991 - nearly identical to the 2002 UK data set - CMV-specific IgG ELISA (Enzygnost, Dade Behring),Citation17 1741 individuals (age range = [18 71] with median 32 years; F/M = 51%) in Belgium (2002 - CMV IgG (Enzygnost, Dade Behring) ),Citation10 576 individuals (age range = [18 65] with median 29 years; F/M = 50%) in Belgium (2006) (seeCitation27 for sampling method; CMV testing is the same as for UK HZ samples), 5635 individuals (age range = [18 49] with median 31 years; F/M = 54%) from the USA National Health and Nutrition Examination Survey (NHANES)Citation28 (1999–2002 - multistep IgG detection involving SeraQuest enzyme immunoassay (Quest International) and VIDAS test (bioMerieux Vitek) for more information see Bate et alCitation28) and 2080 individuals (age range = [18 59] with median 33 years; F/M = 50%) in Australia (2006 - CMV IgG ELISA (Medac)).Citation29 See for more information.
Table 1. CMV-seroprevalence shown per dataset
Data analysis
First, multiple logistic regression was performed with CMV IgG serostatus as dependent variable and age (as a continuous variable), gender, data set (HZ or UK-reference ‘UK’) as predictor variables. The inclusion of age and gender allows us to control for possible confounding factors. Furthermore, interaction terms between age, gender and dataset were tested (age*gender, age*data set, gender*dataset and age*gender*data set). Interaction terms define how the effect of the predictor variable X1 on the dependent variable changes conditional on the value of another predictor variable X2. For example, the term age*gender defines how the effect of age on CMV-seroprevalence differs between men and women. Logistic regression was performed as (1) a step-wise logistic regression including 2-way interactions between all variables and (2) a logistic regression without these interactions. Akaike's Information Criterion (AIC)-based stepwise logistic regression analyses were performed using the Generalized Linear Model software in Matlab 2013b. Confidence intervals shown are 95% CI.
Next, we reversed our analysis and defined the UK HZ group as cases and the UK-reference group as controls, thus assuming that only a minority (maximum of 10–30%)Citation21,22 of the UK-reference sample donors had a HZ history. In this case-control analysis we assessed the importance of CMV-seropositivity as a risk factor for HZ (also controlling for age and gender).
Subsequently, we performed a between-countries comparison regarding CMV seroprevalence by means of multiple logistic regression including the following datasets: UK-reference (baseline), BE-reference 2002 ‘BE-2002’, BE-reference 2006 ‘BE-2006’, USA-reference ‘USA’ and Australia-reference ‘AUS.
Finally, we also assessed whether high CMV IgG titers (defined as exceeding the upper limit of detection, i.e. >= 500 IU/ml) occurred more frequently in HZ CMV-seropositive patients (UK HZ) than in CMV-seropositive general population samples (BE-2006). The latter comparison is possible because HZ UK and BE-2006 CMV IgG titers were obtained in the same lab using the same technology, although differences in CMV epidemiology between the UK and Belgium might bias our analysis. Higher CMV IgG titers in HZ patients could indicate a recent exposure to CMV (either de novo or reactivationCitation16). We also assessed CMV IgM serostatus, occurring during de novo CMV infection or CMV reactivation,Citation30 seroprevalence in these CMV IgG seropositive individuals.
Disclosure of Potential Conflicts of Interest
J.B. has received funding from SPMSD for the VZV Identification Program, which undertakes genotyping of VZV from vaccine adverse events. J.B. also heads the VZV reference laboratory, which has genotyped VZV for GlaxoSmithKline. B.O., N.H., R.P., H.J., H.S., M.Q., H.G., H.T. and P.B. report no conflicts of interest.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Ethical Approval
East London and the city research ethics committee LREC R&WF2002/38 (UK HZ patient samples).
Supplemental_Table.zip
Download Zip (8.7 KB)Acknowledgments
We thank Helena Tutil and Veronique De Vroey for their assistance in HZ patient sample management. We also thank the laboratories and staff that processed and tested the samples of the previously published general population sera in Belgium, The UK and Australia. We specifically acknowledge Pierre Van Damme, Dominic Dwyer and Gwendolyn Gilbert.
Funding
This work was supported by the Research Foundation Flanders (FWO) [personal grants to B.O. and H.T. and project grant G040912N]; the University of Antwerp [Pfizer-sponsored scientific chair to N.H.]; The Wellcome Trust [081703/B/06/Z] and the National Institute for Health Research (NIHR) UCL/UCLH Comprehensive Biomedical Research Center [to J.B.].
References
- Dolin R, Reichman RC, Mazur MH, Whitley RJ. NIH conference. Herpes zoster-varicella infections in immunosuppressed patients. Ann Intern Med 1978; 89:375-88; PMID:210697; http://dx.doi.org/10.7326/0003-4819-89-3-375
- Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, Caulfield MJ, Irwin MR, Smith JG, Clair J, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis 2008; 197:825-35; PMID:18419349; http://dx.doi.org/10.1086/528696
- Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med 1965; 58:9-20; PMID:14267505
- Ogunjimi B, Van Damme P, Beutels P. Herpes zoster risk reduction through exposure to chickenpox patients: a systematic multidisciplinary review. PLoS One 2013; 8:e66485; PMID:23805224; http://dx.doi.org/10.1371/journal.pone.0066485
- Forbes HJ, Bhaskaran K, Thomas SL, Smeeth L, Clayton T, Langan SM. Quantification of risk factors for herpes zoster: population based case-control study. BMJ 2014; 348:g2911; PMID:25134101; http://dx.doi.org/10.1136/bmj.g2911
- Ogunjimi B, Buntinx F, Bartholomeeusen S, Terpstra I, De Haes I, Willem L, Elli S, Bilcke J, Van Damme P, Coenen S, et al. Herpes zoster is associated with herpes simplex and other infections in under 60 year-olds. J Infect 2015; 70:171-7; PMID:25218425; http://dx.doi.org/10.1016/j.jinf.2014.08.016
- Meysman P, Ogunjimi B, Naulaerts S, Beutels P, Van Tendeloo V, Laukens K. Varicella-zoster virus-derived major histocompatibility complex class I-Restricted peptide affinity is a determining factor in the HLA risk profile for the development of postherpetic neuralgia. J Virol 2015; 89:962-9; PMID:25355886; http://dx.doi.org/10.1128/JVI.02500-14
- Fletcher JM, Vukmanovic-Stejic M, Dunne PJ, Birch KE, Cook JE, Jackson SE, Salmon M, Rustin MH, Akbar AN. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J Immunol 2005; 175:8218-25; PMID:16339561; http://dx.doi.org/10.4049/jimmunol.175.12.8218
- Pawelec G, McElhaney JE, Aiello AE, Derhovanessian E. The impact of CMV infection on survival in older humans. Curr Opin Immunol 2012; 24:507-11; PMID:22541724; http://dx.doi.org/10.1016/j.coi.2012.04.002
- Ogunjimi B, Theeten H, Hens N, Beutels P. Serology indicates cytomegalovirus infection is associated with varicella-zoster virus reactivation. J Med Virol 2014; 86:812-9; PMID:24037981; http://dx.doi.org/10.1002/jmv.23749
- Stowe RP, Peek MK, Cutchin MP, Goodwin JS. Reactivation of herpes simplex virus type 1 is associated with cytomegalovirus and age. J Med Virol 2012; 84:1797-802; PMID:22997083; http://dx.doi.org/10.1002/jmv.23397
- Dowd JB, Aiello AE, Alley DE. Socioeconomic disparities in the seroprevalence of cytomegalovirus infection in the US population: NHANES III. Epidemiol Infect 2009; 137:58-65; PMID:18413004; http://dx.doi.org/10.1017/S0950268808000551
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 2014; 4:e004833; PMID:24916088; http://dx.doi.org/10.1136/bmjopen-2014-004833
- Gkrania-Klotsas E, Langenberg C, Sharp SJ, Luben R, Khaw KT, Wareham NJ. Seropositivity and higher immunoglobulin g antibody levels against cytomegalovirus are associated with mortality in the population-based European prospective investigation of Cancer-Norfolk cohort. Clin Infect Dis 2013; 56:1421-7; PMID:23442763; http://dx.doi.org/10.1093/cid/cit083
- Wang GC, Kao WH, Murakami P, Xue QL, Chiou RB, Detrick B, McDyer JF, Semba RD, Casolaro V, Walston JD, et al. Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol 2010; 171:1144-52; PMID:20400465; http://dx.doi.org/10.1093/aje/kwq062
- Mehta SK, Stowe RP, Feiveson AH, Tyring SK, Pierson DL. Reactivation and shedding of cytomegalovirus in astronauts during spaceflight. J Infect Dis 2000; 182:1761-4; PMID:11069250; http://dx.doi.org/10.1086/317624
- Vyse AJ, Hesketh LM, Pebody RG. The burden of infection with cytomegalovirus in England and Wales: how many women are infected in pregnancy? Epidemiol Infect 2009; 137:526-33; PMID:18789177; http://dx.doi.org/10.1017/S0950268808001258
- Cannon MJ, Hyde TB, Schmid DS. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev Med Virol 2011; 21:240-55; PMID:21674676; http://dx.doi.org/10.1002/rmv.695
- Bilcke J, van Hoek AJ, Beutels P. Childhood varicella-zoster virus vaccination in Belgium: cost-effective only in the long run or without exogenous boosting? Hum Vaccin Immunother 2013; 9:812-22; PMID:23321955; http://dx.doi.org/10.4161/hv.23334
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271-84; PMID:15930418; http://dx.doi.org/10.1056/NEJMoa051016
- Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis 2004; 4:26-33; PMID:14720565; http://dx.doi.org/10.1016/S1473-3099(03)00857-0
- Yawn BP, Saddier P, Wollan PC, Sauver JLS, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007; 82:1341-9; PMID:17976353; http://dx.doi.org/10.4065/82.11.1341
- Loeth N, Assing K, Madsen HO, Vindelov L, Buus S, Stryhn A. Humoral and cellular CMV responses in healthy donors; identification of a frequent population of CMV-specific, CD4+ T cells in seronegative donors. PLoS One 2012; 7:e31420; PMID:22347475; http://dx.doi.org/10.1371/journal.pone.0031420
- Ogunjimi B, Smits E, Heynderickx S, Van den Bergh J, Bilcke J, Jansens H, Malfait R, Ramet J, Maecker HT, Cools N, et al. Influence of frequent infectious exposures on general and varicella-zoster virus-specific immune responses in pediatricians. Clin Vaccine Immunol 2014; 21:417-26; PMID:24429070; http://dx.doi.org/10.1128/CVI.00818-13
- Quinlivan M, Hawrami K, Barrett-Muir W, Aaby P, Arvin A, Chow VT, John TJ, Matondo P, Peiris M, Poulsen A, et al. The molecular epidemiology of varicella-zoster virus: evidence for geographic segregation. J Infect Dis 2002; 186:888-94; PMID:12232828; http://dx.doi.org/10.1086/344228
- Quinlivan ML, Ayres KL, Kelly PJ, Parker SP, Scott FT, Johnson RW, Maple C, Breuer J. Persistence of varicella-zoster virus viraemia in patients with herpes zoster. J Clin Virol 2011; 50:130-5; PMID:21093356; http://dx.doi.org/10.1016/j.jcv.2010.10.014
- Theeten H, Hutse V, Hens N, Yavuz Y, Hoppenbrouwers K, Beutels P, Vranckx R, Van Damme P. Are we hitting immunity targets? The 2006 age-specific seroprevalence of measles, mumps, rubella, diphtheria and tetanus in Belgium. Epidemiol Infect 2011; 139:494-504; PMID:20587123; http://dx.doi.org/10.1017/S0950268810001536
- Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988–2004. Clin Infect Dis 2010; 50:1439-47; PMID:20426575; http://dx.doi.org/10.1086/652438
- Seale H, MacIntyre CR, Gidding HF, Backhouse JL, Dwyer DE, Gilbert L. National serosurvey of cytomegalovirus in Australia. Clin Vaccine Immunol 2006; 13:1181-4; PMID:16957061; http://dx.doi.org/10.1128/CVI.00203-06
- Prince HE, Lape-Nixon M. Role of cytomegalovirus (CMV) IgG avidity testing in diagnosing primary CMV infection during pregnancy. Clin Vaccine Immunol 2014; 21:1377-84; PMID:25165026; http://dx.doi.org/10.1128/CVI.00487-14