Abstract
Studies that have evaluated the influenza vaccine effectiveness (VE) to prevent laboratory-confirmed influenza B cases are uncommon, and few have analyzed the effect in preventing hospitalized cases. We have evaluated the influenza VE in preventing outpatient and hospitalized cases with laboratory-confirmed influenza in the 2012–2013 season, which was dominated by a vaccine-matched influenza B virus. In the population covered by the Navarra Health Service, all hospitalized patients with influenza-like illness (ILI) and all ILI patients attended by a sentinel network of general practitioners were swabbed for influenza testing, and all were included in a test-negative case-control analysis. VE was calculated as (1-odds ratio)×100. Among 744 patients tested, 382 (51%) were positive for influenza virus: 70% for influenza B, 24% for A(H1N1)pdm09, and 5% for A(H3N2). The overall estimate of VE in preventing laboratory-confirmed influenza was 63% (95% confidence interval (CI): 34 to 79), 55% (1 to 80) in outpatients and 74% (33 to 90) in hospitalized patients. The VE was 70% (41 to 85) against influenza B and 43% (−45 to 78) against influenza A. The VE against virus B was 87% (52 to 96) in hospitalized patients and 56% in outpatients (−5 to 81). Adjusted comparison of vaccination status between inpatient and outpatient cases with influenza B did not show statistically significant differences (odds ratio: 1.13; p = 0.878). These results suggest a high protective effect of the vaccine in the 2012–2013 season, with no differences found for the effect between outpatient and hospitalized cases.
Introduction
Influenza is an important public health problem that affects a high proportion of the population every year, and its main preventive measure is the vaccine.Citation1 Since influenza vaccine composition is adapted every season to the viruses expected to be in circulation, its effectiveness varies.Citation2
Annual trivalent influenza vaccines contain hemagglutinin (HA) derived from influenza A(H1N1), A(H3N2) and B viruses, intended to be protective against the viruses in circulation each season. Influenza B viruses comprise 2 antigenically distinct lineages, Victoria and Yamagata, and only one of them is included in the trivalent influenza vaccine. When the lineage included in the seasonal vaccine matches the lineage of the circulating virus, the preventive effect of the vaccine is expected to be high against influenza B, whereas the level of cross-protection between the 2 lineages is not well known, but is assumed to be low,Citation3,4 for that reason a tetravalent vaccine has been recently formulated including both lineages.
In the 2012–2013 season the composition recommended for the influenza vaccine in the northern hemisphere included A/California/07/2009(H1N1)pdm09-like, A/Victoria/361/2011(H3N2)-like and B/Wisconsin/1/2010(Yamagata)-like viruses.Citation5 Virological surveillance of influenza during the 2012–2013 season in Spain detected that the influenza B virus was the predominant circulating influenza virus.Citation6-8 Studies that have evaluated the influenza vaccine effectiveness (VE) to prevent laboratory-confirmed influenza B cases in a season dominated by this type of virus are uncommon, and few have analyzed the effect in preventing inpatient cases.Citation9-12
The aim of this study was to evaluate the influenza VE in preventing outpatient and hospitalized cases with laboratory-confirmed influenza in the 2012–2013 season in Navarra, Spain, and to provide specific estimates of the VE against influenza B virus using the test-negative case-control design.
Results
Description of cases and controls
The weekly distribution of swabbed patients followed a similar pattern to the influenza-like illness (ILI) incidence in the population, peaking in weeks 8 and 7 of 2013, respectively ().
Figure 1. Number of influenza cases and test-negative controls, and incidence of influenza-like illness by week, 2012–2013 season in Navarra, Spain.
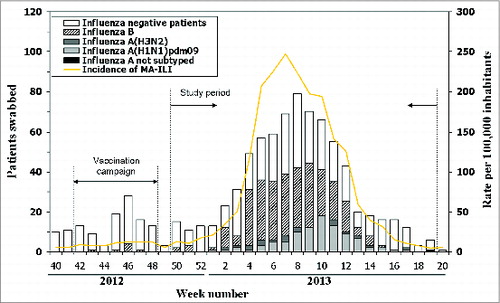
The study period was defined as week 50 of 2012 to week 19 of 2013. During this period 744 ILI patients were swabbed and 382 (51%) were confirmed for influenza virus: 268 (70%) with influenza B and 114 (30%) with influenza A. Among influenza A cases, 93 (82%) were subtyped as A(H1N1)pdm09, 20 (18%) as A(H3N2), and 1 strain was not subtyped. In the 97% of patients included in this study the date of symptom onset was recorded, and all of them had been swabbed during the first 5 d.
In comparison to test-negative controls, laboratory-confirmed cases with influenza were less frequently children under 5 y and adults aged 65 and over, persons with major chronic conditions and persons in the target population for influenza vaccination ().
Table 1. Baseline characteristics of laboratory-confirmed influenza cases and test-negative controls, 2012–2013 influenza season in Navarra, Spain
Among the 382 laboratory-confirmed cases there were 25 (7%) who had received the influenza vaccine during the 2012–2013 season compared to 84 (23%) of the 362 influenza-negative controls (P < 0.001).
Genetic characterization
Overall, 129 (34%) isolates obtained from the confirmed cases were further characterized by phylogenetic analysis of the HA1 sequence of the haemagglutinin gene in the National Influenza Center - Madrid laboratory. The 33 isolates of influenza A(H1N1)pdm09 virus were A/StPetersburg/27/2011(nH1N1)-like (79%) and A/StPetersburg/100/2011(nH1N1)-like (21%). All 17 A(H3N2) sequenced viruses were characterized as A/Victoria/361/2011(H3N2)-like. Among the 79 influenza B isolates with genetic characterization, the Yamagata lineage included in the trivalent vaccine predominated (92%), and the strains identified were B/Estonia/55669/2011(Yamagata)-like (49%), B/Wisconsin/1/2010(Yamagata)-like (43%) and B/Brisbane/60/2008(Victoria)-like (8%) ().
Table 2. Distribution by type/subtype and strain of influenza cases with genetic characterization during the 2012–2013 season in Navarra, Spain
VE in preventing laboratory-confirmed influenza
The adjusted estimate of the overall influenza VE was 63% (95% CI: 34 to 79). Among primary healthcare patients VE was 55% (95% CI: 1 to 80), while the VE estimate in preventing hospitalizations was 74% (95% CI: 33 to 90). Among persons aged 65 y and older the VE was 75% (95% CI: 22 to 92), and among people younger than 65 it was 59% (95% CI: 17 to 80). The specific estimates of the VE in preventing influenza A(H1N1)pdm09 and A(H3N2) suggested a moderate protective effect, but were not statistically significant ().
Table 3. Influenza vaccine effectiveness in preventing laboratory-confirmed influenza in the 2012–2013 influenza season in Navarra, Spain
VE in preventing laboratory-confirmed influenza B cases
To evaluate the influenza VE in preventing influenza B cases, the study period was defined as week 50 of 2012 to week 15 of 2013. During this period 597 ILI patients were swabbed and 268 (45%) were confirmed for influenza B virus. Among laboratory-confirmed B cases, 6% had received the influenza vaccine during the 2012–2013 season compared to 22% of the influenza-negative controls (P < 0.001) ().
The overall VE in preventing influenza B cases was 70% (95% CI: 41 to 85), similar to the VE among the target population for vaccination (70%; 95% CI: 37 to 86). The point estimate of VE was 89% (95% CI: 53 to 97) in persons aged 65 y and more and 87% (95% CI: 52 to 96) against hospitalizations with influenza B (). The comparison of vaccination status between inpatient and outpatient cases with laboratory-confirmed influenza B did not show any differences after adjustment for potential confounding factors (odds ratio (OR): 1.13; 95% CI: 0.24–5.43; p = 0.878).
Table 4. Influenza vaccine effectiveness in preventing influenza B cases in Navarra, Spain, week 50 of 2012 to week 15 of 2013
When taking into consideration vaccination in previous seasons in the model, the adjusted estimate of the 2012–2013 seasonal VE against influenza B virus was 69% (95% CI: 23 to 88), and the 2008–2009 influenza season vaccine, which was the last containing Yamagata lineage virus, yielded a residual preventive effect of 25%, although it did not reach statistical significance. Vaccination in previous seasons when Victoria lineage was included in the seasonal vaccine did not show a preventive effect ().
Table 5. Effectiveness of the influenza seasonal vaccines received in the current and previous seasons against influenza B cases in Navarra, Spain, week 50 of 2012 to week 15 of 2013
Discussion
The results show a high effectiveness (63%) of the influenza vaccine in preventing laboratory-confirmed cases in the 2012–2013 season in Navarra, a season that was characterized by predominant circulation of Yamagata lineage influenza B virus, with good matching with the vaccine strain. The analysis restricted to the target population for vaccination, which includes persons aged 60 and over and those with major chronic conditions, showed similar effectiveness of the influenza vaccine. Our study included both outpatient and hospitalized patients recruited systematically in a population in accordance with influenza surveillance protocols. The vaccine showed an effectiveness of 55% in preventing outpatient cases and 74% in preventing hospitalizations due to infection with the influenza virus. The VE was high against virus B, whereas the estimates suggest a moderate effect in preventing influenza A(H1N1)pdm09 and A(H3N2). This effect in influenza A prevention is consistent with that reported in the same season by the I-MOVE network in various countries of Europe,Citation13 although it did not reach statistical significance in our case.
During the study period, influenza B virus predominated in Navarra (70%), similar to what was reported by the influenza surveillance system in Spain (76%).Citation14 A report from the European Center for Disease Prevention and Control notes that during the 2012–2013 season there was a dominance of B virus in Bulgaria, Italy, Ireland, Spain and the United Kingdom, and a co-dominance of B and A virus in central Europe.Citation15 Among type B viruses with known lineage in Navarra, 92% were B/Yamagata, the same lineage as included in the vaccine.Citation5 About 43% of the characterizations of influenza B corresponded to a B/Wisconsin/1/2010(Yamagata)-like strain, the strain that was included in the vaccine, while 49% were B/Estonia/55669/2011(Yamagata)-like strain, which was also considered to match well with the vaccine strain.Citation16
A study in Finland during 12 influenza seasons estimated that approximately 40% of all influenza B viruses causing infections in the population represented the other genetic lineage than the one included in the trivalent influenza vaccines.Citation17 To date, few studies have found a cross immunity between the 2 B virus lineages.Citation9,18 Since only 8% of the cases characterized genetically in our study were B/Victoria, we were unable to evaluate cross protection between influenza B lineages.
The residual effect of vaccination against influenza in previous seasons has been described, but few studies have evaluated this effect specifically against influenza B virus.Citation12,19 We evaluated the possible residual effect of the trivalent influenza vaccine in the last season in which the Yamagata lineage was included (season 2008–2009), and obtained a protective effect of 25%, which did not reach statistical significance. Conversely, influenza vaccination in the previous seasons in which the vaccine included the Victoria lineage (2009–2010 to 2011–2012 seasons) did not show any protective effect. In the absence of cross protection between the 2 B virus lineages, the quadrivalent vaccine against seasonal influenza, which includes both B virus lineages, could help reduce influenza-related morbidity and mortality in seasons with influenza B lineage-level mismatch.Citation17,20-22 A systematic review of influenza B suggests that this virus can pose a significant burden to the global population, although more research is needed in this field.Citation23
The present study estimates the influenza VE in a favorable scenario, marked by a vaccine virus that was matched with the circulating virus. Nonetheless, there remains margin for improvement, thus further research is needed to design better vaccines against influenza.
Unlike other studies,Citation9,11,13,18 we have analyzed, in the same season and the same place, the VE in preventing outpatient cases and hospitalizations with laboratory-confirmed influenza. Both endpoints are of interest when drawing conclusions about the effect of the vaccine. Although in all these settings the patients were recruited using the same ILI case definition, the percentage of influenza detection was higher in primary healthcare patients than in emergency room or hospitalized patients. Most primary healthcare patients were healthy people and, among them, the diagnosis of ILI is quite specific for influenza infection. However, in patients attended in hospital other respiratory illnesses such as pneumonia, which can also meet the ILI case definition, may be more frequent. Although the separate estimates seemed to indicate a slightly higher protective effect against hospitalized cases, owing to the different characteristics of the 2 populations, the difference between them disappears when inpatient and outpatient cases are directly compared and adjusted for the different population characteristics.
In interpreting these results, some study limitations should be considered. The lineage could be determined in only 29% of influenza B cases, and 8% of these cases were of Victoria lineage, which was not included in the trivalent vaccine. This would have reduced the VE estimate. Furthermore, the sample size was insufficient to estimate the effect in preventing cases of A(H1N1)pdm09 and A(H3N2). Although we cannot totally rule out the possibility of false negatives, they are unlikely given that the diagnostic technique of reverse transcription polymerase chain reaction (RT-PCT) has high sensitivity for influenza detection, the physicians received training in swabbing techniques, and 2 swabs (pharyngeal and nasopharyngeal) were taken in all patients. Furthermore, of the patients included in this study with known time from symptom-onset to swabbing (97%), all were swabbed during the first 5 days, which would rule out the possibility that time to swabbing could have been a relevant confounding factor.Citation24-26
In conclusion, these results suggest a high protective effect of the influenza vaccine in the 2012–2013 season in Navarra, when a vaccine-matched influenza B virus predominated. After adjusting for different population characteristics, the VE was similar in outpatient and inpatient cases. Although the effectiveness is high, there is still room for improvement in influenza vaccine production technology. Our study highlights the importance of annual immunization against influenza in high-risk populations.
Methods
Study population and data collection
The present study was based on electronic clinical records in the region of Navarra, Spain. The Navarra Ethical Committee for Medical Research approved the study protocol. The Navarra Health Service gives care to 96% of the population of the region. Healthcare records include information from primary care, hospital admissions, laboratory test results and the vaccination register.
The seasonal influenza vaccination campaign took place from 15 October to 30 November 2012. The trivalent inactivated non-adjuvanted vaccine was recommended and offered free of charge to all subjects in the target groups, which include people aged 60 y or older and those with risk factors or major chronic conditions. Influenza vaccine status was obtained from the online regional vaccination register.Citation27 Subjects were considered to be protected 14 d after vaccine administration.
Influenza surveillance was based on automatic reporting of cases of ILI from all primary healthcare physicians, and searching of ILI cases by public health nurses in hospitals. A sentinel network composed of a representative sample of 79 primary healthcare physicians, covering 16% of the population, was asked to take nasopharyngeal and pharyngeal swabs, after obtaining verbal informed consent, from all their patients diagnosed with ILI whose symptoms had begun preferably less than 5 d previously. In hospitals, the protocol for managing influenza specified early detection and nasopharyngeal and pharyngeal swabbing of all hospitalized patients with ILI. Swabs were processed by RT-PCR assay. About one in 3 positive strains selected among culture-positive samples were sent to the National Influenza Center–Madrid laboratory for complete genetic characterization.
From the electronic healthcare records we obtained the following baseline variables: sex, age, migrant status, district of residence and major chronic conditions (heart disease, lung disease, renal disease, cancer, diabetes mellitus, cirrhosis, dementia, stroke, immunodeficiency, rheumatic disease and body mass index ≥40 kg/m2).
Test-negative case-control analysis
This analysis included all persons covered by the Regional Health Service, except for healthcare workers, persons living in nursing homes and children under 6 months of age. All outpatients and hospitalized patients who were swabbed during the study period were included in a test-negative case–control design.Citation28-31 The beginning and end of the study period was defined as 2 or more consecutive weeks with no detection of influenza virus of the type or subtype object of the analysis. To evaluate the VE we compared seasonal vaccination status in patients in whom any influenza virus was detected (cases) and in those negative for influenza (controls). Separate analyses were done by age group, type/subtype of influenza, healthcare setting, and in the target population for vaccination. We estimated the VE of influenza vaccination in the current season against influenza B cases. Additionally, the residual effect of previous influenza vaccination to prevent influenza B cases was evaluated. In a multivariate model we included influenza vaccination in the current season and influenza vaccination in the 4 previous seasons as 2 variables: one for the seasonal vaccines that included Yamagata lineage virus (2008–2009 season), and the other for the seasonal vaccines that included Victoria lineage virus (2009–2010, 2010–2011 and 2011–2012 seasons). Lastly, the difference in VE to prevent outpatient and hospitalized cases with laboratory-confirmed influenza B was evaluated in a head-to-head comparison of the vaccine status of hospitalized cases with primary healthcare cases.
Crude and adjusted estimators of the effect were quantified by ORs with their 95% CIs, calculated using logistic regression models. The adjusted models included sex, age group, major chronic conditions, month of sample collection and healthcare setting (primary healthcare, hospitalization and emergency room). Percentages were compared by chi-square test. VE was estimated as a percentage: (1–OR)×100.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Note
Members of the Primary Health Care Sentinel Network of Navarra: I Abad, P Aldaz, E Álvarez, JJ Arana, I Arceiz, E Arina, I Arribas, MD Artajo, A Arza, B Azagra, FC Bartolome, C Bernués, C Bolea, A Brugos, B Cano, MV Castresana, JC Cenoz, F Cia, B Compains, JR Corpas, F Cortés, B Churío, PC Cuevas, EM Da Costa, J Díez Espino, M Doiz, FJ Escribano, MJ Esparza, V Etayo, C Fernández Alfaro, B Flamarique, J Gamboa, ML Garcés, L García Blanco, AB German, A Giner, M Gómara, N Goñi, MJ Guillorme, JO Guiu, JC Gurbindo, MJ Guruchaga, JA Heras, MC Hijos, MS Indurain, B Iñigo, SE Juan Belloc, JJ Jurio, MP León, JJ Longás, JJ Miner, M Moreno, MA Moros, U Navarro, FJ Orozco, M Orte, P Palacio, J Palau, C Pérez Lecumberri, P Pérez Pascual, B Pérez Sanz, A Prado Virto, M Prado Santamaria, A Puig Arrastia, E Ridruejo, M Ramos, BE Rípodas, M Rodríguez, MA Roncal, I Ruiz Puertas, C Sánchez Vázquez, P Sarrasqueta, MA Senosiain, J Sola, M Sota, ME Ursua, IA Urtasun, MJ Vigata, MT Virto, JM Vizcay.
Members of the Network for Influenza Surveillance in Hospitals of Navarra: P Artajo, X Beristain, E Bernaola, J Chamorro, M Esquiroz, P Fanlo, F Gil, M Gabari, J Hueto, C Martín, L Peña, C Pérez, M Ruiz (Complejo Hospitalario de Navarra), M Fernández-Alonso, J Núñez (Clínica Universidad de Navarra), JJ García Irure, M Torres, MT Ortega (Hospital Reina Sofía, Tudela), F Lameiro, L Barrado (Hospital García Orcoyen, Estella), N Alvarez (Servico Navarro de Salud), M Guevara, F Irisarri, M Arriazu, A Zabala, A Barricarte, J Castilla (Instituto de Salud Pública de Navarra).
Funding
This work was supported by the Carlos III Institute of Health (PS12/00087), the Spanish Ministry of Health (EC11–302), and by the I-MOVE program of the European Center for Disease Prevention and Control (ECDC).
References
- Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med 2007; 357:1373-81; PMID:17914038; http://dx.doi.org/10.1056/NEJMoa070844
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:36-44; PMID:22032844; http://dx.doi.org/10.1016/S1473-3099(11)70295-X
- Belshe RB, Coelingh K, Ambrose CS, Woo JC, Wu X. Efficacy of live attenuated influenza vaccine in children against influenza B viruses by lineage and antigenic similarity. Vaccine 2010; 28:2149-56; PMID:20003926; http://dx.doi.org/10.1016/j.vaccine.2009.11.068
- Skowronski DM, De Serres G, Dickinson J, Petric M, Mak A, Fonseca K, Kwindt TL, Chan T, Bastien N, Charest H, et al. Component-specific effectiveness of trivalent influenza vaccine as monitored through a sentinel surveillance network in Canada, 2006–2007. J Infect Dis 2009; 199:168-79; PMID:19086914; http://dx.doi.org/10.1086/595862
- World Health Organization. Recommended composition of the influenza virus vaccines for use in the 2012–2013 northern hemisphere influenza season. Wkly Epidemiol Rec 2012; 87:83-95; PMID:22462202
- Sistema de Vigilancia de la Gripe en España. Informe semanal 5/2013. [Report of the influenza surveillance system in Spain 5/2013]. Nº 337. 7 February 2013. Spanish. Available from: http://vgripe.isciii.es/gripe/documentos/20122013/boletines/grn0513.pdf
- Castilla J, Martínez-Baz I, Martinez-Artola V, Fernandez-Alonso M, Reina G, Guevara M, Garcia Cenoz M, Elia F, Alvarez N, Barricarte A, et al. Early estimates of influenza vaccine effectiveness in Navarre, Spain: 2012/13 mid-season analysis. Euro Surveill 2013; 18:2; PMID:23449182
- Torner N, Martínez A, Basile L, Marcos MA, Antón A, Mar Mosquera M, Isanta R, Cabezas C, Jané M, Domínguez A, Program Of Catalonia TP. Influenza vaccine effectiveness assessment through sentinel virological data in three post-pandemic seasons. Hum Vaccin Immunother 2015; 11:225-30; PMID:25483540.
- McLean HQ, Thompson MG, Sundaram ME, Kieke BA, Gaglani M, Murthy K, Piedra PA, Zimmerman RK, Nowalk PK, Raviotta JM, et al. Influenza Vaccine Effectiveness in the United States During. 2012-13: Variable Protection by Age and Virus Type. J Infect 405 Dis 2015; 211:1529-40; PMID:25406334
- Lo YC, Chuang JH, Kuo HW, Huang WT, Hsu YF, Liu MT, Chen CH, Huang HH, Chang CH, Chou JH, et al. Surveillance and vaccine effectiveness of an influenza epidemic predominated by vaccine-mismatched influenza B/Yamagata-lineage viruses in Taiwan, 2011–12 season. PLoS One 2013; 8:e58222; PMID:23472161; http://dx.doi.org/10.1371/journal.pone.0058222
- Puig-Barberà J, Natividad-Sancho A, Launay O, Burtseva E, Ciblak MA, Tormos A, Buiques-Vila A, Martínez-∨beda S, Sominina A; GIHSN Group. 2012–2013 seasonal influenza vaccine effectiveness against influenza hospitalizations: results from the global influenza hospital surveillance network. PLoS One 2014; 9:e100497; PMID:24945510; http://dx.doi.org/10.1371/journal.pone.0100497
- Rondy M, Launay O, Puig-Barberà J, Gefenaite G, Castilla J, de Gaetano, de Gaetano Donati K, Galtier F, Hak E, Guevara M, Constanzo S, et al. European hospital IVE network. 2012/13 influenza vaccine effectiveness against hospitalised influenza A(H1N1)pdm09, A(H3N2) and B: estimates from a European network of hospitals. Euro Surveill 2015; 20: pii=21011; PMID:25613779
- Kissling E, Valenciano M, Buchholz U, Larrauri A, Cohen JM, Nunes B, Rogalska J, Pitigoi D, Paradowska-Stankiewicz I, Reuss A, et al. Influenza vaccine effectiveness estimates in Europe in a season with three influenza type/subtypes circulating: the I-MOVE multicentre case-control study, influenza season 2012/13. Euro Surveill 2014; 19. pii: 20701; PMID:24556348
- Instituto de Salud Carlos III. Informe de Vigilancia de la Gripe en España. Temporada 2012–2013 (Desde la semana 40/2012 hasta la semana 20/2013). [Report of the influenza surveillance system in Spain. 2012–2013 season]. Sistema de Vigilancia de la Gripe en España. Spanish. Available from: http://vgripe.isciii.es/gripe/documentos/20122013/InformesAnuales/Informe_Vigilancia_GRIPE_2012–13_18sep2013.pdf
- Snacken R, Broberg E, Beauté J, Lozano JE, Zucs P, Amato-Gauci AJ. Influenza season 2012–2013 in Europe: moderate intensity, mixed (sub)types. Epidemiol Infect 2014; 142:1809-12; PMID:24814635; http://dx.doi.org/10.1017/S0950268814001228
- European Centre for Disease Prevention and Control. Surveillance Report: Influenza virus characterisation. Stockholm: ECDC; Summary Europe, July 2013. Available from: http://www.ecdc.europa.eu/en/publications/Publications/influenza-virus-characterisation-July-2013.pdf
- Heikkinen T, Ikonen N, Ziegler T. Impact of influenza B lineage-level mismatch between trivalent seasonal influenza vaccines and circulating viruses, 1999–2012. Clin Infect Dis 2014; 59:1519-24; PMID:25139969; http://dx.doi.org/10.1093/cid/ciu664
- Skowronski DM, Janjua NZ, De Serres G, Sabaiduc S, Eshaghi A, Dickinson JA, Fonseca K, Winter AL, Gubbay JB, Krajden M, et al. Low 2012–13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS One 2014; 9:e92153; PMID:24667168; http://dx.doi.org/10.1371/journal.pone.0092153
- McLean HQ, Thompson MG, Sundaram ME, Meece JK, McClure DL, Friedrich TC, Belongia EA. Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin Infect Dis 2014; 59:1375-85; PMID:25270645; http://dx.doi.org/10.1093/cid/ciu680
- Belshe RB. The need for quadrivalent vaccine against seasonal influenza. Vaccine 2010; 28 (Suppl 4):D45-53; PMID:20713260; http://dx.doi.org/10.1016/j.vaccine.2010.08.028
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993-8; PMID:22226861; http://dx.doi.org/10.1016/j.vaccine.2011.12.098
- Bresee J, Hayden FG. Epidemic influenza–responding to the expected but unpredictable. N Engl J Med 2013; 368:589-92; PMID:23343038; http://dx.doi.org/10.1056/NEJMp1300375
- Paul Glezen W, Schmier JK, Kuehn CM, Ryan KJ, Oxford J. The burden of influenza B: a structured literature review. Am J Public Health 2013; 103:e43-51; PMID:23327249; http://dx.doi.org/10.2105/AJPH.2012.301137
- Martínez-Baz I, Martínez-Artola V, Reina G, Guevara M, Cenoz MG, Morán J, Irisarri F, Arriazu M, Castilla J. Primary Health Care Sentinel Network of Navarre. Effectiveness of the trivalent influenza vaccine in Navarre, Spain, 2010–2011: a population-based test-negative case-control study. BMC Public Health 2013; 13:191; PMID:23496887; http://dx.doi.org/10.1186/1471-2458-13-191
- Castilla J, Martínez-Baz I, Martínez-Artola V, Reina G, Pozo F, García Cenoz M, Guevara M, Morán J, Irisarri F, Arriazu M, et al. Primary Health Care Sentinel Network; Network for Influenza Surveillance in Hospitals of Navarre. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill 2013; 18. pii: 20388; PMID:23399423
- Castilla J, Martínez-Artola V, Salcedo E, Martínez-Baz I, Cenoz MG, Guevara M, Alvarez N, Irisarri F, Morán J, Barricarte A. Network for Influenza Surveillance in Hospitals of Navarre. Vaccine effectiveness in preventing influenza hospitalizations in Navarre, Spain, 2010–2011: cohort and case-control study. Vaccine 2012; 30:195-200; PMID:22100636; http://dx.doi.org/10.1016/j.vaccine.2011.11.024
- Aguilar I, Reyes M, Martinez-Baz I, Guevara M, Albeniz E, Belza MJ, Castilla J. Use of the vaccination register to evaluate influenza vaccine coverage in seniors in the 2010/11 influenza season, Navarre, Spain. Euro Surveill 2012; 17: pii=20154; PMID:22551499
- Valenciano M, Kissling E, Ciancio BC, Moren A. Study designs for timely estimation of influenza vaccine effectiveness using European sentinel practitioners networks. Vaccine 2010; 28:7381-8; PMID:20851086; http://dx.doi.org/10.1016/j.vaccine.2010.09.010
- Jackson LA, Jackson ML, Nelson JC, Neuzil KM, Weiss NS. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol 2006; 35:337-44; PMID:16368725; http://dx.doi.org/10.1093/ije/dyi274
- De Serres G, Skowronski DM, Wu XW, Ambrose CS. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro Surveill 2013; 18. pii: 20585; PMID:24079398
- Foppa IM, Haber M, Ferdinands JM, Shay DK. The case test-negative design for studies of the effectiveness of influenza vaccine. Vaccine 2013; 31:3104-9; PMID:23624093; http://dx.doi.org/10.1016/j.vaccine.2013.04.026
