?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Ricin toxin (RT) is an extremely potent toxin derived from the castor bean plant. As a possible bioterrorist weapon, it was categorized as a level B agent in international society. With the growing awareness and concerns of the “white powder incident” in recent years, it is indispensable to develop an effective countermeasure against RT intoxication. In this study we used site-directed mutagenesis and polymerase chain reaction (PCR) techniques to modify the gene of ricin A-chain (RTA). As a result, we have generated a mutated and truncated ricin A-chain (mtRTA) vaccine antigen by E.coli strain. The cytotoxicity assay was used to evaluate the safety of the as-prepared mtRTA antigen, and the results showed that there was no residual toxicity observed when compared to the recombinant RTA (rRTA) or native RT. Furthermore, BALB/c mice were subcutaneously (s.c.) vaccinated with mtRTA 3 times at an interval of 2 weeks, and then the survivals were evaluated after intraperitoneal (i.p.) or intratracheal challenge of RT. The vaccinated mice developed a strong protective immune response that was wholly protective against 40 × LD50 of RT i.p. injection or 20 × LD50 of RT intratracheal spraying. The mtRTA antigen has great potential to be a vaccine candidate for future application in humans.
Abbreviations:
- RT, ricin toxin
- RTA, ricin toxin A chain
- RTB, ricin toxin B chain
- rRTA, recombinant RTA
- mRTA, mutated RTA
- mtRTA, mutated and truncated RTA
- FBS, fetal bovine serum
- i.p., intraperitoneal
- i.p, intraperitoneally
- s.c., subcutaneously subcutaneous
- LD50, median lethal dose
- SD, standard deviation
- ELISA, enzyme-linked immunosorbent assay
- HRP, horseradish peroxidase
- IPTG, isopropyl-1-thio-β-galactopyranoside
Introduction
Ricin, a widely available plant toxin, belongs to a family of Type II Ribosome inactivate proteins (RIPs) that are able to kill eukaryotic cells. It consists of 2 peptide chains, an enzyme A chain (RTA) and a binding B chain (RTB), linked by a single disulfide bond.Citation1 The RTB is galactose binding lectin that binds to glycoproteins on cell surfaces, promoting entry of toxin into cells. Once inside the cell, the RTA specifically removes an adenine of the essential 28S rRNA, effectively inhibiting protein synthesis thereby resulting in the cell death.Citation2
The potency of ricin has been found with beneficial application in the construction of immunotoxins and other therapeutic agents.Citation3-5 However, the toxin was also exploited as a poison for biological warfare and bioterrorism due to its character of wide availability and extraordinary toxicity.Citation6 Inhalation of small amounts of RT can cause severe damage in the respiratory system or death. The reported estimated median lethal dose (LD50) in mice is approximately 3–10 μg/kg when inhaled or injected, and 30 mg/kg when ingested.Citation7 Because of its availability, easy accessibility, potential lethality, irreversible damage and lack of specific medical treatment, developing an effective vaccine becomes a reliable way against the potential biothreat.
Although there have been many studies focusing on the protection against RT, there is no vaccine available for human use currently. Early studies involved the formaldehyde-inactivated ricin toxoid.Citation8-10 However, there were some concerns that the toxoid could revert its toxicity under certain conditions and thus might not be a safe choice for human. It has been previously demonstrated that RTA subunit was generally more immunologically protective as an antigen than RTB subunit. Therefore, current vaccine studies aim at developing nontoxic versions of RTA.Citation11-12 There have been 2 most advanced ricin subunit vaccine candidates, named RiVax and RVEc. RiVax, which incorporates 2 point mutations (Y80A, V76M) to reduce its toxicity, can completely protect mice from a challenge with a 10 × LD50 of RT.Citation13-18 RVEc is a truncated derivative of RTA (RTA 1–33/44–198). It contains deletion of the C-terminal domain (199–267) and an exposed surface loop (34–43), and was found to have relatively high stability to thermal denaturation, no residual enzymatic activity, and the ability to generate full protection against 10 × LD50 of RT challenge in mouse model.Citation19-22
In this study, a novel vaccine candidate, named mutated and truncated RTA (mtRTA, D75A V76M Y80A, 1–198), has been designed by combining the merits of RiVax and RVEc. It introduces 3 important and nearby site mutations (D75A V76M Y80A) and deletes the C-terminal domain (199–267) of RTA. As compared to the recombinant RTA, the mtRTA has been found nontoxic both in vivo and in vitro. When administered subcutaneously (s.c.) to mice with Alum adjuvant, the mtRTA completely protected mice from a dose of 40 × LD50 of RT i.p. challenge and 20 × LD50 of RT intratracheal challenge, which showed that the mtRTA was an effective and nontoxic antigen.
Results
Preparation and confirmation of the mtRTA protein
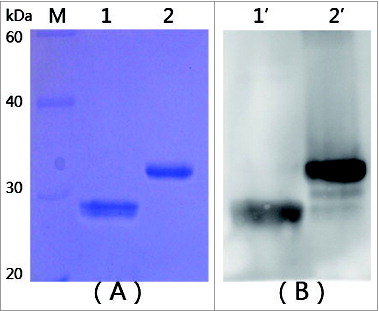
mtRTA (22kDa) was successfully expressed in E. coli as soluble form. The bacterial cells were sonicated and the lysates were subjected to nickel-NTA affinity chromatography. The purity of the protein was found to be greater than 98% pure as determined by SDS-PAGE (). The antigenicity of the purified mtRTA protein was then confirmed by Western blotting (). The results showed that the mtRTA was similarly antigenic as rRTA.
Cytotoxicity
The mtRTA was measured for cytotoxicity to compare with rRTA and RT (Fig. S2). The result in revealed that the cytotoxicity of mtRTA was significantly lower in BEAS-2B cells compare to rRTA and RT (P < 0.05). At all the protein concentrations, the viability of the cells in mtRTA group was approximate 100%, but the viability of the cells in RT groups was approximately ranged from 10% to 50%. The cytotoxicity of rRTA was significantly stronger than mtRTA and weaker than RT (P < 0.05). The result indicates that mtRTA is safe and non-toxic to BEAS-2B cells.
Figure 2. Cytotoxic effects of proteins in the BEAS-2B cells. The toxicities of target proteins, mtRTA (•
 ) and RT (▾
) and RT (▾ ), were tested using the CellTiter 96® AQueous One Solution Cell Proliferation Assay, by measuring the toxicities in the human bronchial epithelial cell-line BEAS-2B. The X-axis represents the concentration of different proteins (mtRTA, rRTA and RT), and the Y-axis represents the cell viability. Each point represents the arithmetic mean ± SD of triplicate determinations. “*” represents P < 0.05.
), were tested using the CellTiter 96® AQueous One Solution Cell Proliferation Assay, by measuring the toxicities in the human bronchial epithelial cell-line BEAS-2B. The X-axis represents the concentration of different proteins (mtRTA, rRTA and RT), and the Y-axis represents the cell viability. Each point represents the arithmetic mean ± SD of triplicate determinations. “*” represents P < 0.05.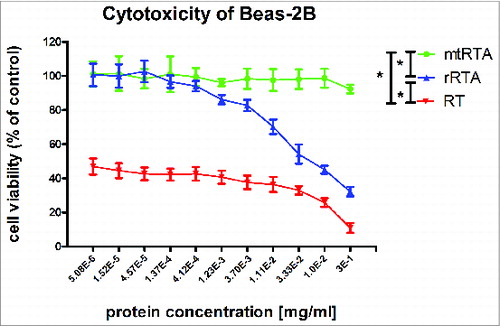
Toxicity assay in the mouse
Two different doses (0.5 and 0.1 mg) of 2 distinct proteins (mtRTA and rRTA) were i.p. injected into mice respectively. The status of these mice was recorded daily at 10 d post injection and the results were listed in . Mice injected with mtRTA appeared as normal as before. However, mice that were injected with rRTA showed signs of intoxication between 48 h and 72 h post injection, including reduced activity, reduced eating of provided food, piloerection. In addition, one mouse in the rRTA (0.5 mg/mouse) group died between 48 h and 72 h. The histopathology analysis showed that obvious pathological alterations were detected in the lungs of the mice in rRTA groups, which including epithelial necrosis, partial consolidation and generalized interstitial edema (), while no abnormality was found in the lungs of mice injected with mtRTA () when compared with the normal mouse lung histology shown in . Also, this resultindicated that the mtRTA antigen provoked no obviously toxicity in mice even at the dose of 0.5 mg/mouse, which is approximate 50 times the dose we proposed to used in the human clinical trial (10 μg).
Figure 3. Histopathologic alterations in the lungs of mice. The mice i.p injected with 0.5 mg of rRTA (A), 0.1 mg of rRTA (B), 0.5 mg of mtRTA (C) and 0.1 mg of mtRTA (D). Both (A) and (B) showed the pathological changes, including: epithelial necrosis, partial consolidation and generalized interstitial edema. In addition, the pathological changes in (A) were more severe than that in (B). No obvious pathological changes was found in (C) and (D) as compared to the normal mouse lung histology shown in (E).
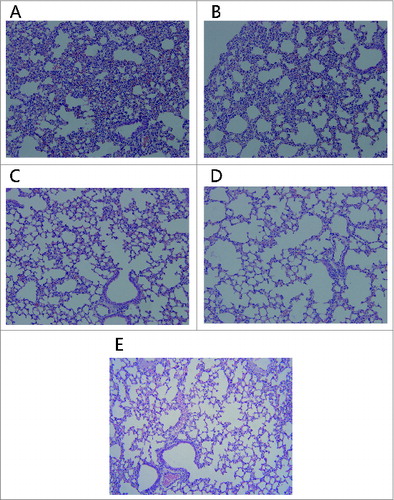
Table 1. The sequence of primers
Table 2. Toxicity assay in the mouse
Vaccination and measurement of antibody titers
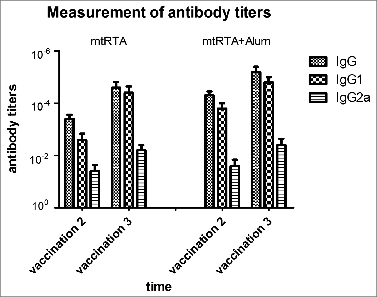
The mice were vaccinated 3 times with 15 μg of mtRTA alone or plus Alum adjuvant each time at an interval of 2 weeks. None of the mice was detected weight loss during the period of vaccination (data not shown). The ELISA results of the anti-rRTA antibody titers of sera were shown in . The results showed that the mean IgG titers were about 10−5 after the third immunization. Meanwhile, the IgG1 and IgG2a titers were also measured. It is clear that both of IgG1 and IgG2a antibodies were detected, and the mean titers of IgG1 were markedly higher than that of IgG2a.
Figure 4. Measurement of antibody titers. All immunization schedules involved mice that were s.c. immunized 3 times at 2 weeks interval and sera specimens were collected from the tail veins of mice, which were then used to measure antibody titers by ELISA one week after the second and third immunization respectively. The graphs show arithmetic mean antibody titers of 10 mice per group ± SD. The X-axis “vaccination 2, 3” represents the time point one week following the second and third immunization respectively. The Y-axis represents the antibody titers. Titer was calculated as the reciprocal of the highest dilution having an OD450 greater than 0.1 AU (absorbance unit) after correcting for background.
RT challenge
The LD50 of RT was calculated using the improved Karber's method, and the value of LD50 was 7.496 μg/kg (when RT delivered by i.p. injection) or 6.162 μg/kg (when RT delivered by intratracheal spraying). The challenge results of the mice were shown in (i.p. injection) and (intratracheal spraying), the survival rates were recorded at the time point of 10 d post challenge. The showed that more mice in the group 2 (mtRTA + alum) survived after the RT challenge than that in the group 1(mtRTA). All the vaccinated mice in the group 1 or group 2 survived after as high as 20 or 40 × LD50 of RT i.p. challenge, and the survival rates were 0 or 80% when the RT challenge doses increased to 50 × LD50. The mice vaccinated with “mRTA (15 μg) + Alum adjuvant” were conducted as the group of control 1, in which all mice survived after as high as 10 × LD50 of RT i.p. challenge. All mice in the group of control 2 were dead between the second and fourth day. There was significant difference (P < 0.01) between “mtRTA” group and “mtRTA + alum” group by cox proportional hazards model. The antigen plus Alum was better than the antigen alone in the experiment. Therefore, the “mtRTA + Alum” was selected as the antigen formulation in the experiment of , which showed that mice s.c. vaccinated with “mtRTA + Alum” completely resisted 20 × LD50 of RT challenged by intratracheal spraying. When the RT dose increased to 30 × LD50, the survival rate decreased to 60%. All mice in the control group were dead between the the third and fourth day. There was signigicant difference (P < 0.01) between the vaccinated group and the control group by Fisher's exact test.
Table 3 Recombinant mtRTA immunogen protect mice from different doses of RT i.p. injection
Table 4. Recombinant mtRTA immunogen protect mice from different doses of RT intratracheal spraying
Toxin neutralization assay in vitro and in vivo
To determine whether the anti-mtRTA antibodies could neutralize RT, sera from the vaccinated mice were pooled and tested for their ability to protect both cells and mice from RT. Negative sera from unvaccinated mice plus RT and only RT were both used as controls. Cells and mice were monitored for death and the results were listed in and .
Figure 5. Toxin neutralization assay in BEAS-2B cell line model. 50 μl RT of different concentrations (triple serial diluted) were incubated with the same volume (50 μl) of the immune sera (•
 ) or no serum (▴
) or no serum (▴ ) at 37°C for 1 h, and then added to the cells. The X-axis represents the concentration of RT, the Y-axis represents the cell viability. “*” represents P < 0.05.
) at 37°C for 1 h, and then added to the cells. The X-axis represents the concentration of RT, the Y-axis represents the cell viability. “*” represents P < 0.05.
Table 5. Assay of passive protection against RT challenge in mice
Toxin neutralization assay in BEAS-2B cells
As shown in , the viability of cells in different groups varied. As expected, the RT neutralization effects were only obtained with the sera from the vaccinated mice. The cell viabilities in this group were significant higher than that of the other 2 control groups (P < 0.05), and there was no significant difference in cell viability between the 2 control groups. The results indicated that the mtRTA could induce specific neutralization antibodies against RT.
Passive protection of mice against RT challenge
The immune sera or non-immune sera were mixed with an equal volume (100 μl) of different doses of RT (10, 15, 20, 25 × LD50) and i.p injected to the mice respectively. Then the survival rates of the mice were recorded each day for 10 d and the results were shown in . The results of passive protection experiment showed that the anti-mtRTA antibodies specifically protected the mice from RT challenge. There was significant difference (P < 0.01) between “immune sera” group and “non-immune sera” control group by Fisher's exact test.
Discussion
As a biothreat agent, the threat of RT has been concerned for years. For example, weapons-grade RT was manufactured and tested in the late 1980s in Iraq,Citation7 and letters which addressed to the US. President, a senator and a judge were intercepted and detected RT white powder by the FBI in 2013. Studies for RT vaccines have also been developed for years and a consensus viewpoint for vaccine design strategy has been formed that the reduction of protein subunits to a minimum essential domain containing neutralizing epitopes should be tested on antigens.Citation23 Previous studies showed that the A-chain was more immunogenic than the B-chainCitation24-25 and discovered some key amino acid residues in RTA, which include the enzymatically active site (Y80, Y123, E177, R180, N209 and W211)Citation26 and the pulmonary vascular leak (PVL) residues (L74, D75 and V76).Citation27 A reasonable strategy for developing a safe vaccine was to introduce some mutations into these sites to eliminate the toxicity of RTA.Citation28 Previous researches have also demonstrated that the major immune dominant linear B cell and T cell epitopes involve regions nearby or inside these active residues of RTA (161–185 and 124–140).Citation29-30 Mutations of the RTA sequences within or near these regions may change its immunogenicity. So it would be better if the mutation sites were chosen located far away from these active residues. Furthermore, researchers have found that the C-terminal domain of RTA did not play an important role in its immunogenicity because the neutralizing epitopes rarely localized in the C-terminal domain (199–267).Citation31 Meanwhile, deleting the C-terminal domain could also abolish the toxic N-glycosidase activity of RTA and make contribution to the increased solubility and stability.Citation23,31 For all these reasons, we made mutations in 3 sites (D75A V76M Y80A) and removed the C-terminal domain (199–267) of RTA to develop a novel derivative of RTA (mtRTA), which may contain both merits of RiVax and RVEc. We speculated that this alteration in RTA would not impinge on immunogenicity, and probably increase its solubility and stability. Actually, this was confirmed in our experiment.
In our study, mtRTA was successfully produced in a soluble form by E. coli strain and it was identified as being effective and almost non-toxic. Additionally, mtRTA antigen induced preeminent protection against 40 × LD50 of RT i.p. challenge or 20 × LD50 of RT intratracheal challenge in mouse model. As the i.p. challenge results shown in , mice s.c. immunized with “mtRTA + Alum” acquired a 100% or 80% protection against a dose of 40 or 50 × LD50 of RT i.p. injection respectively. Without Alum, the mtRTA also provided a full protection against 20 × LD50 of RT i.p. injection. This result also consists with the conclusion of antibody titers in . We also considered that RT is likely to be disseminated as an aerosol, so another challenge experiment was carried out by RT intratracheal spraying. In addition, the Alum adjuvant markedly improved the immunogenicity of the mtRTA antigen in the vaccination experiment. Therefore, the “mtRTA + Alum” was selected to vaccinate the mice and the inrtatracheal spraying challenge results were shown in , which displayed that the vaccinated mice completely resisted 20 × LD50 of RT intratracheal challenge. The results in and demonstrated that “mtRTA + Alum” s.c. immunized in mice provided both systematic and mucosal protection, and the systematic protection was the dominant. Moreover, if the antigen was vaccinated in mice by mucosal route, the protective efficacy would be improved when RT was sprayed in the intratracheal, and this will be considered in later study.
In order to further confirm whether the sera from “mtRTA + Alum” immunized mice could neutralize the toxin in mice, 2 passive protection experiments were carried out both in vitro and in vivo. The showed that the vaccinated sera played an important role in neutralizing RT, while the non-immune sera failed to protect the cells in the same conditions. The showed that the 100 μl of sera from mtRTA immunized mice provided a complete protection against an equal volume of 25 × LD50 of RT i.p. challenge. The two passive protection experiments indicated that the specific anti-mtRTA antibodies in the immune sera could neutralize RT. The protective efficacy provided by the neutralizing antibody in the immune sera demonstrated that the antibodies induced by mtRTA is the dominant protective factor. And this was also confirmed by the IgG subtype assay in . The IgG subtype results showed that the mice developed both IgG1 and IgG2a antibody titers, and IgG1 antibody titer was markedly higher than that of IgG2a. The results supported the dominant expression of a Th2-mediated immune response, which is associated with humoral immunity. This is consistent with the previous investigation that the majority of RTA-specific IgG mAbs were of the IgG1 subclass.Citation25,32
In conclusion, the as-prepared mtRTA is non-toxic both in cells and mice. When mixed with Alum adjuvant, the mtRTA antigen can induce enough titers of anti-RTA specific antibodies to protect mice completely from a dose of 40 × LD50 of RT i.p. injection or 20 × LD50 of RT intratracheal spraying, which is superior to the 10 × LD50 of RT challenge induced by mRTA, a RiVax-like vaccine candidate prepared by our laboratory. Taken together, all these results in current study suggest that mtRTA is a potentially promising vaccine candidate for RT and deserves further development.
Materials and Methods
Construction of the mtRTA expression plasmid
The pET-His plasmid with RTA gene fragment (GenBank accession number: X03179) was used for site-directed mutagenesis and truncation. Firstly, the 3 site mutations (D75AV76MY80A) were introduced into the RTA sequence using the QuickChange® Lightning Site-Directed Mutagenesis Kit (Stratagene, 210519). The mutagenic primers (primer 1) with 3 site mutations were used in this step; Secondly, the fragment of mRTA (D75AV76MY80A) and another pair of primers (primer 2) incorporating the 5′ and 3′ restriction sites of EcoRІ and NheІ respectively, were used to amplify the fragment of mtRTA by polymerase chain reaction (PCR). The process of the mtRTA constructing was described in Figure 1, and the 2 pairs of primers were listed in . The amplified mtRTA gene was then ligated into the pET-His vector to generate the novel recombinant expression vector, pET-His-mtRTA.
Protein expression, purification and confirmation
The recombinant plasmid was further transformed into E.coli strain BL21(DE3)pLysS (TransGen Biotech, CD701) and grown in 5 ml LB medium overnight containing 100 μg/ml of ampicillin at 37°C with 200 rpm shaking. The overnight grown culture was inoculated in 500 ml of LB medium and was grown at 37°C in a shaker until it reached an A600 of 0.6. The culture was then induced with 0.4 mM IPTG (Merck, C9H1805S) and the cells were incubated for another 12 h at 20°C for expression of protein containing 6 × His tag at the N-terminal. The induced bacterial cells were harvested and lysed by sonication and centrifuging to separate the supernatant and cell debris. The protein in the supernatant was purified by nickel-nitrilotriacetic affinity chromatography (GE Healthcare, 17–5248) and the concentration was measured by a BCA assay kit (Novagen, 71285–3). As a positive control, the recombinant wild type RTA (rRTA) was prepared and purified using the same methods as the mtRTA, except for the protein inducing condition (medium: TB, inducing temperature: 30°C, IPTG concentration: 0.4 mM, inducing time: 4 h).
Antigenicity of the protein
The purified mtRTA and rRTA were used to run SDS-PAGE electrophoresis, and then the proteins were transferred onto a nitrocellulose membrane (Pall, 66485) to confirm the antigenicity of mtRTA by Western blotting. The transferred nitrocellulose membrane was blocked with 3% BSA in 0.01 M PBST (PBS + 0.05% Tween-20) for 1 h and then incubated in 0.01 M PBST with rabbit anti-ricin polyclonal antibody (made by our laboratory) for 1 h at room temperature. Finally, the membrane was incubated with HRP-coupled goat anti-rabbit IgG (abcam, ab6721) at room temperature for another 1 h, followed by incubation with SuperSignal Substrate Working Solution (Thermo, 34075) for 5 minutes, and exposure in AE-1000 cool CCD image analyzer (Beijing BGI-GBI Biotech Co., Ltd).
Cytotoxicity assay of mtRTA, rRTA and RT
The human bronchial epithelial cell line, BEAS-2B, was selected to test the cytotoxicity of mtRTA, rRTA and RT by using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega, G3580). The BEAS-2B cells were cultured in RPMI 1640 (Hyclone, SH30809.01B) supplemented with 10% FBS. About 104 cells in 100 μl medium per well were seeded into a 96-well plate, and the 96-well plate was incubated at 37°C with 5% CO2 for 24 h. Then 100 μl of each concentration of serial triple diluted mtRTA, rRTA and RT protein in the medium were added to the wells respectively and then incubated at the same condition for another 24 h. Finally, the used medium was abandoned and a mixture of 100 μl medium plus 20 μl reagents (from the Cell Proliferation Assay) were added in each well of the plate. The absorbance of the plate was read at a wavelength of 490 nm after 2 h incubation, and the data obtained were directly proportional to the number of living cells in the well. 100% viability was assessed with cells without any protein in the medium.
Toxicity assay in the mouse
Twelve BALB/c mice (about 6 weeks old) were equally divided into 4 groups (3 mice/group). Two different doses (0.5 and 0.1 mg/mouse) of mtRTA or rRTA (control) were i.p injected to mice as a single injection. Survival rates were recorded daily for 10 d and then the representative mice were euthanized to do histopathology analysis. Vital organs (heart, liver and lung) were chosen for histopathological studies.
LD50 determination of RT i.p. injection and intratracheal spraying
BALB/c mice (about 20 g) were randomly divided into 5 groups, 8 mice each group, and they were injected i.p with different doses of RT respectively. The same number of BALB/c mice were anaesthetized by i.p injecting 120 μl Pentobarbital sodium salt (Merck, 24898448) solution (1%, dissolved in PBS), and then different doses of RT dissolved in 50 μl PBS were immediately sprayed into the trachea of these mice respectively by using MicroSprayer® Aerosolizer – Model IA-1C, FMJ-250 High Pressure Syringe and Model LS-2 Small Animal Laryngoscope (Penn-Century™, 843–6540). The survival of the mice was recorded daily for 10 d The LD50 was calculated using the improved Karber's method according to the formula below:
Xm: log value of the according concentration of maximum mortality, I: difference between the log value of the gradient concentrations, ∑P: sums of the mortality rates in various groups.
Vaccination and RT challenge
Six weeks old BALB/c mice were randomly divided into 12 groups and 10 mice (5 male, 5 female) in each group. Mice in 5 groups were vaccinated s.c. with mtRTA (15 μg per mouse) in 200 μl PBS, and another 5 groups of mice were vaccinated s.c. with mtRTA (15 μg per mouse) in 130 μl PBS plus 70 μl Alum adjuvant (Thermo, 77161). As the control 1, 10 mice were vaccinated s.c. with “mRTA + Alum adjuvant,”and the rest 10 mice vaccinated s.c. with PBS alone or PBS plus Alum were conducted as the group of control 2. Vaccine was administered on days 0, 14, and 28. Different groups of mice were challenged with their matching doses of RT (10, 20, 30, 40, 50, 60 × LD50) by i.p. injection one week after the third vaccination.
Another twenty-5 mice were equally divided into 5 groups. Twenty mice in 4 groups were vaccinated s.c. with mtRTA plus Alum and the rest 5 mice in the control group were vaccinated s.c. with PBS plus Alum alone. The vaccination program was the same as before. Four groups of immunized mice were challenged with their matching doses of RT (5, 10, 20, 30 × LD50) by intratracheal aerosol spraying one week after the third vaccination and the mice in control group were challenged with 5 × LD50 of RT in the same way.
The weights and deaths of the mice were recorded daily for 10 days, a period of time sufficient for all mice to regain their initial weight. Blood samples were collected from tail vein of these mice one week post the second and third vaccination to determine the titers of specific anti-RTA antibody IgG, IgG1 and IgG2a by using ELISA method. Briefly, the rRTA protein was used as coating antigen, and goat anti-mouse antibody (HRP) serotypes IgG, IgG1, and IgG2a (Southern biotech, 98609, 1073, 1080) were used to detect the specific titers. The details of the ELISA were performed according to our laboratory's previously published protocol.Citation33
Toxin neutralization assay in vitro and in vivo
Toxin neutralization assay in BEAS-2B cells
BEAS-2B cell line (human bronchial epithelia cell) was selected to test the ability of the sera from vaccinated mice to neutralize RT. The concentration of RT was triple serial diluted and different concentrations of RT (50 μl) were incubated with the same volume (50 μl) of the sera at 37°C for 1 h. Then the 100 μl mixtures of RT and sera were added to the well containing about 104 cells in 100 μl RPMI 1640 medium. The non-immune sera were conducted as a negative control. The following procedures were the same as described in 4.4.
Passive protection of mice against RT challenge
Serum samples were collected from the other 15 vaccinated mice and tested for the ability to protect mice against RT challenge. Every 100 μl of the immune sera or non-immune sera (from non-vaccinated mice as control) were mixed with an equal volume of different doses of RT (10, 15, 20 and 25 × LD50) respectively and incubated at 37°C for 1 h. Thirty untreated mice were equally divided into 6 groups: mice in 4 groups were i.p injected with 200 μl of the different immune sera mixed solution (10, 15, 20 and 25 × LD50) respectively; as control group, mice in the rest 2 groups were injected with the non-immune sera mixed solution (5 and 10 × LD50) respecitvely, the injection volume was 200 μl per mouse. The survival status of mice was recorded for 10 d
Statistical analysis
Statistical analysis was carried out with GraphPad Prism 5 and SAS. All data were shown as the arithmetic mean ± SD. Fisher's exact test, cox proportional hazards model and analysis of variance (ANOVA) were used to compare group difference. *P < 0.05 indicated statistical significance between the 2 groups.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Animal Ethics
All animal experiments were carried out according to the Guideline for Animal Experiment of the Academy of Military Medical Sciences (Beijing, China), and were approved by the Animal Ethics Committee of the Academy of Military Medical Sciences.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website
Supplemental_Figures.zip
Download Zip (369.4 KB)References
- Lord JM, Roberts LM, Robertus JD. Ricin: structure, mode of action, and some current applications. FASEB J 1994; 8:201-8; PMID:8119491
- Endo Y, Mitsui K, Motizuki M, Tsurugi K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J Biol Chem 1987; 262:5908-12; PMID:3571242
- Zhou XX, Ji F, Zhao JL, Cheng LF, Xu CF. Anti-cancer activity of anti-p185HER-2 ricin A chain immunotoxin on gastric cancer cells. J Gastroenterol Hepatol 2010; 25:1266-75; PMID:20594254; http://dx.doi.org/10.1111/j.1440-1746.2010.06287.x
- Zhang XJ, Ke LM, Yang J, Lin LW, Xue ES, Wang Y, Yu LY, Chen ZK. Development, characterization and anti-tumor effect of a sequential sustained-release preparation containing ricin and cobra venom cytotoxin. Pharmazie 2012; 67:618-21; PMID:22888519
- Liao P, Liu W, Li H, Gao H, Wang H, Li N, Sun Y. Morphological changes of ricin toxin-induced apoptosis in human cervical cancer cells. Toxicol Ind Health 2012; 28:439-48; PMID:21937530; http://dx.doi.org/10.1177/0748233711414608
- Lord MJ, Jolliffe NA, Marsden CJ, Pateman CS, Smith DC, Spooner RA, Watson PD, Roberts LM. Ricin. Mechanisms of cytotoxicity. Toxicol Rev 2003; 22:53-64; PMID:14579547; http://dx.doi.org/10.2165/00139709-200322010-00006
- Audi J, Belson M, Patel M, Schier J, Osterloh J. Ricin poisoning: a comprehensive review. JAMA 2005; 294:2342-51; PMID:16278363; http://dx.doi.org/10.1001/jama.294.18.2342
- Kende M, Yan C, Hewetson J, Frick MA, Rill WL, Tammariello R. Oral immunization of mice with ricin toxoid vaccine encapsulated in polymeric microspheres against aerosol challenge. Vaccine 2002; 20:1681-91; PMID:11858879; http://dx.doi.org/10.1016/S0264-410X(01)00484-4
- Yan C, Rill WL, Malli R, Hewetson J, Naseem H, Tammariello R, Kende M. Intranasal stimulation of long-lasting immunity against aerosol ricin challenge with ricin toxoid vaccine encapsulated in polymeric microspheres. Vaccine 1996; 14:1031-8; PMID:8879098; http://dx.doi.org/10.1016/0264-410X(96)00063-1
- Griffiths GD, Bailey SC, Hambrook JL, Keyte MP. Local and systemic responses against ricin toxin promoted by toxoid or peptide vaccines alone or in liposomal formulations. Vaccine 1998; 16:530-5; PMID:9491508; http://dx.doi.org/10.1016/S0264-410X(97)80007-2
- O'Hara JM, Yermakova A, Mantis NJ. Immunity to ricin: fundamental insights into toxin-antibody interactions. Curr Top Microbiol Immunol 2012; 357:209-41; PMID:22113742
- Smallshaw JE, Vitetta ES. Ricin vaccine development. Curr Top Microbiol Immunol 2012; 357:259-72; PMID:21805396
- Smallshaw JE, Richardson JA, Pincus S, Schindler J, Vitetta ES. Preclinical toxicity and efficacy testing of RiVax, a recombinant protein vaccine against ricin. Vaccine 2005; 23:4775-84; PMID:15961194; http://dx.doi.org/10.1016/j.vaccine.2005.04.037
- Smallshaw JE, Richardson JA, Vitetta ES. RiVax, a recombinant ricin subunit vaccine, protects mice against ricin delivered by gavage or aerosol. Vaccine 2007; 25:7459-69; PMID:17875350; http://dx.doi.org/10.1016/j.vaccine.2007.08.018
- Marconescu PS, Smallshaw JE, Pop LM, Ruback SL, Vitetta ES. Intradermal administration of RiVax protects mice from mucosal and systemic ricin intoxication. Vaccine 2010; 28:5315-22; PMID:20562013; http://dx.doi.org/10.1016/j.vaccine.2010.05.045
- Smallshaw JE, Firan A, Fulmer JR, Ruback SL, Ghetie V, Vitetta ES. A novel recombinant vaccine which protects mice against ricin intoxication. Vaccine 2002; 20:3422-7; PMID:12213413; http://dx.doi.org/10.1016/S0264-410X(02)00312-2
- Vitetta ES, Smallshaw JE, Coleman E, Jafri H, Foster C, Munford R, Schindler J. A pilot clinical trial of a recombinant ricin vaccine in normal humans. Proc Natl Acad Sci U S A 2006; 103:2268-73; PMID:16461456; http://dx.doi.org/10.1073/pnas.0510893103
- Vitetta ES, Smallshaw JE, Schindler J. Pilot phase IB clinical trial of an alhydrogel-adsorbed recombinant ricin vaccine. Clin Vaccine Immunol 2012; 19:1697-9; PMID:22914366; http://dx.doi.org/10.1128/CVI.00381-12
- McLain DE, Lewis BS, Chapman JL, Wannemacher RW, Lindsey CY, Smith LA. Protective effect of two recombinant ricin subunit vaccines in the New Zealand white rabbit subjected to a lethal aerosolized ricin challenge: survival, immunological response, and histopathological findings. Toxicol Sci 2012; 126:72-83; PMID:21987460; http://dx.doi.org/10.1093/toxsci/kfr274
- Carra JH, Wannemacher RW, Tammariello RF, Lindsey CY, Dinterman RE, Schokman RD, Smith LA. Improved formulation of a recombinant ricin A-chain vaccine increases its stability and effective antigenicity. Vaccine 2007; 25:4149-58; PMID:17408819; http://dx.doi.org/10.1016/j.vaccine.2007.03.011
- Olson MA, Carra JH, Roxas-Duncan V, Wannemacher RW, Smith LA, Millard CB. Finding a new vaccine in the ricin protein fold. Protein Eng Des Sel 2004; 17:391-7; PMID:15187223; http://dx.doi.org/10.1093/protein/gzh043
- McLain DE, Horn TL, Detrisac CJ, Lindsey CY, Smith LA. Progress in biological threat agent vaccine development: a repeat-dose toxicity study of a recombinant ricin toxin A-chain (rRTA) 1-33/44-198 vaccine (RVEc) in male and female New Zealand white rabbits. Int J Toxicol 2011; 30:143-52; PMID:21378370; http://dx.doi.org/10.1177/1091581810396730
- McHugh CA, Tammariello RF, Millard CB, Carra JH. Improved stability of a protein vaccine through elimination of a partially unfolded state. Protein Sci 2004; 13:2736-43; PMID:15340172; http://dx.doi.org/10.1110/ps.04897904
- Marsden CJ, Smith DC, Roberts LM, Lord JM. Ricin: current understanding and prospects for an antiricin vaccine. Expert Rev Vaccines 2005; 4:229-37; PMID:15889996; http://dx.doi.org/10.1586/14760584.4.2.229
- Maddaloni M, Cooke C, Wilkinson R, Stout AV, Eng L, Pincus SH. Immunological characteristics associated with the protective efficacy of antibodies to ricin. J Immunol 2004; 172:6221-8; PMID:15128810; http://dx.doi.org/10.4049/jimmunol.172.10.6221
- Olson MA. Ricin A-chain structural determinant for binding substrate analogues: a molecular dynamics simulation analysis. Proteins 1997; 27:80-95; PMID:9037714; http://dx.doi.org/10.1002/(SICI)1097-0134(199701)27:1%3c80::AID-PROT9%3e3.0.CO;2-R
- Baluna R, Rizo J, Gordon BE, Ghetie V, Vitetta ES. Evidence for a structural motif in toxins and interleukin-2 that may be responsible for binding to endothelial cells and initiating vascular leak syndrome. Proc Natl Acad Sci U S A 1999; 96:3957-62; PMID:10097145; http://dx.doi.org/10.1073/pnas.96.7.3957
- Ready MP, Kim Y, Robertus JD. Site-directed mutagenesis of ricin A-chain and implications for the mechanism of action. Proteins 1991; 10:270-8; PMID:1881883; http://dx.doi.org/10.1002/prot.340100311
- Castelletti D, Fracasso G, Righetti S, Tridente G, Schnell R, Engert A, Colombatti M. A dominant linear B-cell epitope of ricin A-chain is the target of a neutralizing antibody response in Hodgkin's lymphoma patients treated with an anti-CD25 immunotoxin. Clin Exp Immunol 2004; 136:365-72; PMID:15086403; http://dx.doi.org/10.1111/j.1365-2249.2004.02442.x
- Tommasi M, Castelletti D, Pasti M, Fracasso G, Lorenzetti I, Sartoris S, Pera C, Ferrara GB, Tridente G, Colombatti M. Identification of ricin A-chain HLA class II-restricted epitopes by human T-cell clones. Clin Exp Immunol 2001; 125:391-400; PMID:11531946; http://dx.doi.org/10.1046/j.1365-2249.2001.01525.x
- O'Hara JM, Neal LM, McCarthy EA, Kasten-Jolly JA, Brey RN 3rd, Mantis NJ. Folding domains within the ricin toxin A subunit as targets of protective antibodies. Vaccine 2010; 28:7035-46; PMID:20727394; http://dx.doi.org/10.1016/j.vaccine.2010.08.020
- O'Hara JM, Kasten-Jolly JC, Reynolds CE, Mantis NJ. Localization of non-linear neutralizing B cell epitopes on ricin toxin's enzymatic subunit (RTA). Immunol Lett 2014; 158:7-13; PMID:24269767; http://dx.doi.org/10.1016/j.imlet.2013.11.009
- Li Q, Xin W, Gao S, Kang L, Wang J. A low-toxic site-directed mutant of Clostridium perfringens epsilon-toxin as a potential candidate vaccine against enterotoxemia. Hum Vaccin Immunother 2013; 9:2386-92; PMID:23835363