Abstract
In the last decade, several mumps outbreaks were reported in various countries despite high vaccination coverage. In most cases, young adults were affected who have acquired immunity against mumps solely by vaccination and not by previous wild-type mumps virus infection. To investigate mumps-specific antibody levels, functionality and dynamics during a mumps epidemic, blood samples were obtained longitudinally from 23 clinical mumps cases, with or without a prior history of vaccination, and from 20 healthy persons with no serological evidence of recent mumps virus infection. Blood samples from mumps cases were taken 1–2 months and 7–10 months after onset of disease. Both vaccinated and unvaccinated mumps cases had significantly higher geomean concentrations of mumps-specific IgG (resp. 13,617 RU/ml (95% CI of 9,574–19,367 RU/ml) vs. 1,552 (445–5412) RU/ml at 1–2 months; and 6,514 (5,247–8,088) RU/ml vs. 1,143 (480–2,725) RU/ml at 7–10 months) than healthy controls (169 (135–210) RU/ml) (p = 0.001). Patterns in virus-neutralizing (VN) antibody responses against the mumps vaccine virus were similar, vaccinated and unvaccinated mumps cases had significantly higher ND50 values at both time points of sampling (resp 4,695 (3,779–5,832) RU/ml vs. 1,533 (832–2,825) RU/ml at 1–2 months; 2,478 (1,968–3,122) RU/ml vs. 1,221 (1,029–1,449) RU/ml at 7–10 months) compared with (previously vaccinated) healthy controls (122 (196–76)) RU/ml) (p = 0.001) The unvaccinated mumps cases had significantly lower mumps-specific IgG and VN antibody concentrations at both sampling points compared with previously vaccinated cases, but their antibody concentrations did not differ significantly at the 2 time points. In contrast, the mumps-specific IgG and VN antibody concentrations of the previously vaccinated mumps cases were significantly higher within the first 2 months after onset of mumps and declined thereafter, characteristic for a secondary response. A moderate correlation was found between the level of mumps-specific IgG serum antibodies and VN antibodies for the mumps cases (r = 0.64; p<0.001).
Introduction
Most countries have incorporated mumps vaccination into their national immunization programs by the implementation of the combined measles, mumps and rubella (MMR) vaccine.Citation1 In the Netherlands, MMR vaccination was implemented in 1987 by offering vaccinations to children at the age of 14 months and 9 y As a consequence, the annual mumps incidence decreased dramatically as in other countries where mumps vaccination was implemented.Citation2,3 Although it was assumed that mumps vaccination induces life-long protection,Citation4 several mumps outbreaks, especially among vaccinated student populations, have been reported during the last decade in various countries where mumps vaccination has been implemented into their national immunization programs.Citation5-14 In 2009–2012, a mumps epidemic (genotype G) arose that spread across multiple locations within the Netherlands, in which also primarily vaccinated students were affected.Citation15 Waning of vaccine-induced immunity has been suggested to play a role in these outbreaks.Citation6-10,13,14 When compared to the other components in the MMR vaccine, the mumps component seemed to be the least effective in eliciting good (high avidity) antibody responses, which were shown to wane to lower levels and in avidity index 20 y after a second MMR vaccination.Citation16 Furthermore, vaccine-induced antibody concentrations measured against a (heterologous) genotype G virus were shown to be approximately one half the concentrations measured against the vaccine strain (genotype A; Jeryl Lynn).Citation17 Thus, antigenic differences between wild-type mumps viruses and the mumps vaccine strain may further lead to loss of functional mumps immunity.
Yet our understanding of the natural serologic response against the mumps virus remains incomplete. Insight is into the ranges in mumps-specific antibody concentrations, their virus-neutralization capacity, as well as in the antibody dynamics seen in time after mumps virus exposure is lacking. The recent epidemic of the Netherlands provided an opportunity to evaluate such aspects of the antibody response following a clinical mumps virus infection. The course of anti-mumps IgG and virus-neutralizing (VN) antibody concentrations in 23 clinical mumps cases was investigated, 1–2 months and 7–10 months after onset of disease. Seven of these cases were not MMR vaccinated, which made it possible to investigate the difference in the course of antibody response between MMR vaccinated and unvaccinated persons after recent mumps virus infection. In addition, a control group was included in the study, to be able to represent the lower range of anti-mumps antibody levels as can be expected in healthy (non-infected) vaccinees that were age–matched. Our study contributes to the understanding of how mumps-specific (functional) antibody levels develop dynamically in vaccinated and unvaccinated mumps cases in a mumps epidemic setting, relative to maintenance levels of healthy non-infected vaccinees.
Results
A total of 23 mumps cases and 20 healthy controls participated in the study. The demographic characteristics, clinical symptoms of mumps cases, and mumps-specific antibody levels (IgG concentration and the serum dilution titers resulting in 50% reduction of virus plaques (ND50)) are listed in . Four cases of orchitis occurred among the 23 mumps cases; 2 out of the 7 unvaccinated mumps cases had orchitis, and the other 2 cases of orchitis occurred among the 16 vaccinated mumps cases. Three mumps cases had complaints that persisted 7–10 months after onset of disease; 2 of these cases had not been previously vaccinated.
Table 1. Demographics of 23 mumps cases, mumps-specific antibody response and clinical symptoms, including summarized data of mumps cases and healthy controls
All participating patients had mumps-specific IgG concentrations far above 45 RU/ml, previously validated as criterion for seroprevalence for the fluorescent bead-based multiplex IgG immunoassay (MIA).Citation18 Both vaccinated and unvaccinated mumps cases had significantly higher geometric mean concentrations (GMC) of mumps-specific IgG at both time points of sampling (resp 13,617 RU/ml (95% CI of 9,574–19,367 RU/ml) and 1,552 (445–5412) RU/ml at 1–2 months; 6,514 (5,247–8,088) RU/ml and 1,143 (480–2,725) RU/ml at 7–10 months) than healthy controls (169 (135–210) RU/ml) (p = 0.001) (, ). The mumps-specific IgG concentrations of the previously vaccinated mumps cases were significantly higher within 2 months after onset of disease (13,617 (9,574–19,367) RU/ml) than at 7–10 months (6,514 (5,247–8,088) RU/ml), whereas the IgG concentrations of the unvaccinated cases were similar at both time points (resp 1,552 (445–5412) RU/ml at 1–2 months, and 1,143 (480–2,725) RU/ml at 7–10 months) (, ). The unvaccinated mumps cases had significantly lower IgG antibody concentrations at both sampling points as compared with cases who had received 2 doses of the MMR vaccine (). Two out of the 7 unvaccinated mumps cases had a considerably lower IgG antibody response 1–2 months than 7–10 months after onset of disease (, ).
Figure 1. Anti-mumps IgG concentrations (A) and ND50 values (B) of the various groups of mumps cases (vaccinated vs. unvaccinated; 1–2 months and 7–10 months after onset of disease) and control group. Dynamics of the anti-mumps IgG concentrations (C) and ND50 values (D) of the vaccinated mumps cases (closed dots) vs. unvaccinated (open dots) mumps cases at the different time points, i.e. One–2 months and 7–10 months, after onset of mumps. Correlation (Spearman's rank analysis) between IgG concentrations and ND50 values of mumps cases (E) Samples from both time points are included in this analysis; closed symbols represent vaccinated persons and open symbols represent unvaccinated patients; circles represent samples taken at 1–2 months and triangles represent samples taken at 7–10 months after onset of disease.
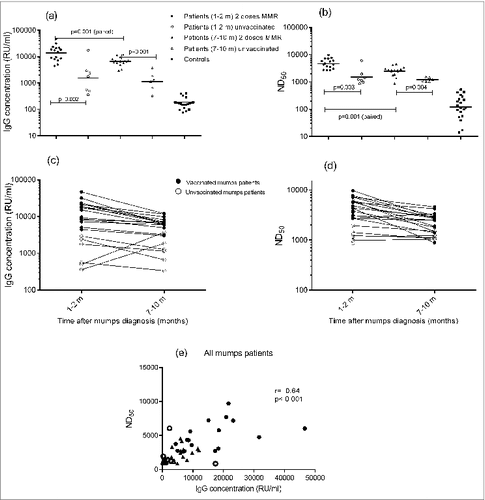
Patterns in virus-neutralizing (VN) antibody responses against mumps virus were similar to those observed in total IgG concentrations, although the variation within the patient group was less prominent. In agreement with IgG levels, vaccinated and unvaccinated mumps cases had significantly higher ND50 values at both time points of sampling (resp. Four, 695 (3,779–5,832) RU/ml and 1,533 (832–2,825) RU/ml at 1–2 months; 2,478 (1,968–3,122) RU/ml and 1,221 (1,029–1,449) RU/ml at 7–10 months) compared with healthy controls (122 (196–76)) RU/ml) (p = 0.001) (). In addition, the anti-mumps ND50 values of the previously vaccinated mumps cases were higher within 2 months after onset of disease (4,695 (3,779–5,832) RU/ml) than at 7–10 months (2,478 (1,968–3,122) RU/ml), whereas the ND50 values of the unvaccinated cases were similar at both time points (1,533 (832–2,825) RU/ml at 1–2 months, and 1,221 (1,029–1,449) RU/ml at 7–10 months) (). Unvaccinated mumps cases had significantly lower ND50 values at both sampling points than cases who had received 2 doses of the MMR vaccine (). Whereas the variation of the ND50 values among the groups of mumps cases (vaccinated and unvaccinated groups; sampling time 1–2 and 7–10 months after onset of disease) was smaller than the variation in IgG antibody concentrations, the heterogeneity of mumps-specific ND50 values among healthy controls was higher than the IgG concentrations (). Nevertheless, it must be taken into consideration that healthy controls were selected based on mumps-specific IgG levels < 500 RU/ml to minimize the risk of recruiting individuals that had recently been exposed to mumps. The observed mumps-specific IgG levels of the healthy controls (169 (135–210) RU/ml), appeared to be within the expected range for this age group as based on other Dutch studies.Citation19,20 The relationship between the antibody levels measured by multiplex immunoassay (MIA) and the virus neutralization assay appeared to be moderate (r = 0.64; p<0.001) when considering the samples obtained from the mumps cases exclusively (). For the samples of the healthy controls, no significant relationship (r = 0.34; p = 0.14) was observed. In the overall data set (n = 64 samples; including all samples from controls and mumps cases taken at different time points) a strong correlation between mumps-specific IgG concentrations and ND50 values was found (r = 0.86; p<0.001). With respect to the mumps cases, no aberrant antibody response (or trend for lower response) was observed in cases with orchitis of with persistent complaints (longer than 7–10 months). Due to the limited number of these severe cases, statistical analysis was not considered relevant.
Discussion
In the Netherlands, a high overall MMR vaccination coverage of 96% and 93% for respectively the first and second dose at 14 months- and 9 years-aged children has been reported.Citation21 In a large cross-sectional cohort (n=7900) of the Dutch population (2006–2007), it was demonstrated that mumps seroprevalence appeared to be 91%, thereby reaching the herd immunity threshold of 86–92% (i.e. threshold percentages of mumps herd immunity combined from 2 studies, 86–88%Citation22 and 88–92%Citation23). A moderate reduction in seroprevalence, i.e., below or approaching the herd immunity threshold, was observed in several age groups, including the (vaccinated) age group of 15–21 yCitation19 The relatively low mumps-specific serum antibody levels in 15–21 year-aged persons confirm the vulnerability of this group with respect to mumps virus infection, and may explain the occurrence of the recent epidemic in the Netherlands (2009–2012), 5 y after this seroprevalence study. This epidemic has been described to count a total of 1,254 laboratory-confirmed mumps cases. The majority of the mumps cases was male (59%), university student (47%), 18–25 y of age (68%), and vaccinated twice with the MMR vaccine (68%).Citation24,25 In the present study, 2 clear response patterns in IgG and VN antibody levels against the mumps vaccine strain could be detected in consecutive blood samples obtained from mumps virus infected persons during this epidemic. In previously vaccinated mumps cases, specific IgG concentrations as well as the ND50 values were significantly higher shortly (1–2 months) after onset of disease than at 7–10 months. This pattern in antibody response characterizes a secondary response, i.e. rapid production of antibodies upon subsequent encounter with the same antigen. Alternatively, unvaccinated mumps cases also mounted a seroresponse, but of generally lower IgG antibody concentrations and ND50 values at both sampling points than cases who had received 2 MMR vaccine doses. The overall IgG concentrations or ND50 values of the unvaccinated cases did not differ significantly between the 2 time points, and 2 out of the 7 unvaccinated mumps cases had a lower IgG antibody response 1–2 months compared with 7–10 months after onset of disease. This antibody pattern is illustrative of a primary response. It is striking that 4 out of the 7 unvaccinated mumps cases were aged ≥ 40 years, and it is likely, although not certain, that these persons had encountered wild-type mumps virus earlier in life. However, although natural infection with mumps is thought to confer lifelong protection, incidentally cases of reinfections have been described,Citation26–28 and with the absence of circulating wild-type mumps virus also naturally-acquired immunity against mumps may diminish. In addition, it must be kept in mind that in our assays antibodies were measured against the mumps vaccine strain, which may result in a relatively lower response in persons with naturally-acquired immunity induced by other mumps virus strain(s). One of the 2 persons who showed a rise in mumps-specific IgG antibody level in time, suggestive of a primary response, was aged >50 years, but the observed virus-neutralizing (VN) antibody response of this particular person was not typical for a primary response. VN antibody concentrations, can be considered a more reliable measure for protection than total IgG concentrations, as it demonstrates exclusively the functional part of mumps-specific antibodies. However, VN antibody assays are more labor-and time-intensive. In the present study, a moderate correlation was found between the level of IgG antibodies determined by multiplex immunoassay (MIA) and VN (functional) antibody levels in the plasma samples derived from the mumps cases (Pearson's r = 0.64) (). No statistical correlation was observed between the IgG levels and ND50 values in control samples of healthy persons (Pearson's r = 0.34). Our results are in agreement with another study among MMR vaccinated university students and staff, where a similar correlation was found between IgG and VN antibody levels (Pearson's r = 0.45).Citation29 In a recent report, the IgG and VN antibody levels (against whole-mumps virus) also showed a correlation similar to our results (r = 0.37 and r = 0.66; for samples obtained from infants at respectively 2 to 4 months after the second MMR dose and 2 y after the first MMR dose).Citation30 In our study, the correlation appeared to be stronger over a broad range of antibody levels when taking all samples from control subjects and mumps cases together (Pearson's r = 0.86).
The study design, i.e., observational study with limited sample size, did not allow determination of why mumps cases became infected despite vaccination. Recently, Gouma et al calculated a provisional cutoff level for protection of mumps-specific IgG of 243 RU/mL to discriminate between the pre-outbreak IgG concentrations from infected and non-infected persons in a large longitudinal serological database of students over the years 2009–2012.Citation31 Vaccinated mumps cases in our study might have had pre-exposure levels below this cutoff value, based on the IgG antibody concentrations of the control group. Notwithstanding its limited sample size, our present study describes how mumps-specific antibody concentrations and their virus-neutralization capacity develop in time after mumps virus infection; the antibody response was significantly higher in the vaccinated mumps cases compared to the unvaccinated cases at both sampling times, suggestive of a better and more prolonged immunity against mumps. Although the humoral response certainly plays a role in protection against mumps, it should be considered that in vitro measured VN antibody concentrations, but also IgG concentrations, may not be fully predictive of immunological antibody activity in vivo, given that Fc-mediated phagocytosis, antibody-dependent cell-mediated cytotoxicity, and other processes that occur in the host are not reflected in the corresponding assays.Citation32 Additionally, other immune mechanisms, such as cellular immunity, are likely involved in the protection against mumps disease as well as in the viral clearance. The cellular immunity against mumps has only been scarcely explored and deserves more attention.
Summarizing, mumps patients developed high levels of both mumps-specific IgG concentrations and mumps VN antibodies; vaccinated patients had higher antibody levels than unvaccinated patients. Antibody dynamics of vaccinated vs. unvaccinated mumps cases differed, i.e. vaccinated mumps cases had higher antibody levels 1–2 months after onset of disease that declined at 7–10 months, which is characteristic of a secondary response. Previous MMR vaccination resulted in higher (functional) antibody levels in the mumps cases, probably by pre-existing B cell memory, although it was not effective enough to prevent mumps virus infection.
Patients and Methods
Subjects and blood sampling
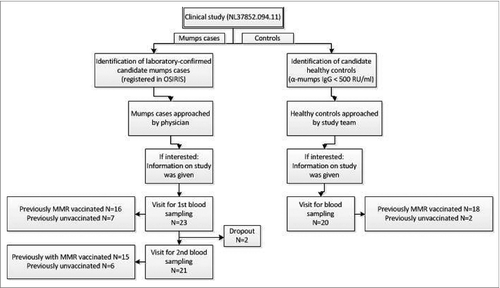
The present observational clinical study was performed between November 2011 and May 2013 according to EU Good Clinical Practice guidelines and the principles outlined in the Helsinki Declaration. Ethical committee approval was obtained (clinical study number NL37852.094.11) and informed, written consent was obtained from all participants. Laboratory-confirmed mumps cases (>18 years) were identified through the web-based system OSIRIS for national registration of compulsory notifiable diseases of the Netherlands. Uninfected age-matched controls (>18 years) were recruited from contacts of cases, but without symptoms and serological evidence of mumps disease (mumps-specific IgG levels <500 RU/ml). Selected mumps cases and healthy controls were approached by telephone and were informed about the nature of the study before a visit was planned (see for flow chart). After giving informed consent, blood samples were taken, and a small questionnaire was conducted providing information with regard to basic demographics, vaccination status, and clinical symptoms of mumps disease. If the vaccination status was indefinite, the status was verified in the nationwide vaccination registration system (Praeventis). Blood samples, obtained by venipuncture, were taken from 23 subjects 26 yrs (CI 95% 23–30 yrs) with mumps at 2 time points after onset of disease. The first samples were taken at 1.3 months (CI 95% 1.2–1.5 months) after the first day of disease. Convalescent blood samples from these subjects were taken with an average of 8.8 months (8.3–9.4 months) after the first day of illness. Seven out of the 23 mumps cases were unvaccinated and 16 cases were previously vaccinated with 2 doses of the MMR vaccine. In addition, 20 healthy control persons (25 yrs (22–29 yrs; 35% male) were included who had no symptoms of mumps or evidence of recent mumps virus infection based on serologic data. Two out of the 20 healthy controls (aged 55 and 57 years) were unvaccinated, and 18 controls were previously vaccinated with 2 doses of the MMR vaccine.
Laboratory procedures
Blood samples, collected in sodium heparin-containing tubes, were processed within 20 hafter venipuncture. Peripheral blood mononuclear cells and plasma were separated by density gradient centrifugation according to manufacturer's instruction, and plasma was stored frozen at −20°C until antibody analysis was performed.
Mumps-specific IgG concentrations were analyzed using the mumps vaccine strain (Jeryl Lynn (JL)) as antigen. The fluorescent bead-based multiplex immunoassay (MIA) using Luminex technology was performed as described before.Citation33 Briefly, plasma samples were diluted 1/200 and 1/4,000 in phosphate buffered saline containing 0.1% Tween 20 and 3% bovine serum albumin. On each plate, controls, blanks and the WHO International Standard Anti Rubella serum RUBI-1–94 (NIBSC) were included. The fluorescent intensity of the samples was interpolated onto the standard curve of the reference serum (RUBI-1–94) to obtain antibody concentrations, which were expressed in RIVM units per milliliter (RU/ml) for mumps. An antibody concentration of ≥45 RU/ml was used as a criterion for seroprevalence, as previously described.Citation18 The RIVM units for mumps used in that assay were standardized against other mumps standards. RUBI-1–94 has a mumps-specific IgG concentration of 4384.512 RU/ml and was selected as alternative (internationally-available) standard for mumps, thus enabling comparison and bridging of our serological data, expressed in RU/ml, to other studies.Citation31
Mumps virus-neutralizing (VN) antibodies were detected by focus reduction neutralization test (FRNT), partly based on the protocol described by Vaidya et al.Citation34 Mumps vaccine virus (JL strain; stored at −80°C) was thawed and mixed with heat-inactivated (45 min 56°C) plasma samples (both 37.5 µl) to be incubated for 2 hat 37°C. Culture medium (Dulbecco's modified eagle medium (Life Technologies) supplemented with 5% fetal calf serum, penicillin, streptomycin, and L-glutamine) was removed from Vero cells (2 × 104 cells/mL) and 50 µl of virus/plasma mixture was added to each well of a 96 wells plate (i.e., > 20 plaques mumps virus per well). Plates were incubated for 4 hat 36°C, wells were emptied and 200 µl of 0.8% carboxymethylcellulose medium was added to each well. Plates were incubated for 40 hat 36°C with 5% CO2, before they were washed with PBS and subsequently fixed with a mixture of aceton and methanol (2:3). After 10 min, plates were washed with ice-cold PBS, and incubated with block buffer (PBS containing 1% BSA) for 30 min at 36°C. Anti-mumps nucleoprotein antibody (Abcam) was in block buffer (1:3000) and 100 µl was added to each well. After incubation for 1 hat 36°C, plates were washed with PBS containing 0.1% Tween-20 (PBST). Subsequently, 100 µl of goat-anti-mouse IgG-HRP (DAKO) in block buffer (1:2000) was added to each well and plates were incubated for one hour at 36°C. Plates were washed with PBST and wells were stained with 50 µl of True Blue peroxidase substrate (KPL, Inc.). The numbers of plaques were counted and the 50% VN antibody dose (ND50) of each sample was calculated. The WHO international standard RubI-1–94 (NIBSC) was used as positive control in each assay run and to calculate relative ND50 value in order to adjust for inter-assay differences.
Statistics
Age, intervals between sampling and disease, and antibody concentrations were described as geometric mean with lower and upper 95% confidence intervals (95% CI). IgG concentrations and ND50 values of healthy controls vs. mumps cases, and vaccinated vs. unvaccinated mumps cases at both sampling time points were compared with the nonparametric Mann Whitney U test (for independent samples). IgG concentrations and ND50 values of vaccinated mumps cases determined from samples taken at 1–2 months vs. Seven–10 months after disease onset were analyzed with the nonparametric Wilcoxon signed rank test for related samples. Correlation between IgG concentrations and ND50 values of different sampling groups, as well as for all samples together, was performed with the nonparametric Spearman's rank analysis. For all statistical analysis, -values p-values <0.05 were considered statistically significant. Data were analyzed with IBM SPSS Statistics version 19.0.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are grateful to Lidian Izeboud (Julius Center for Health Sciences and Primary Care at the UMC, Utrecht, the Netherlands) and Anneke Westerhof (RIVM) who performed the study visits, including venous blood sampling, and Nynke Rots, Marianne van der Sande and Susan Hahné for respectively supervision and coordination of the clinical study. Furthermore, we would like to thank the Municipal Health Services, Tessa Schurink-van‘t Klooster and Olga Ophorst for participant recruitment, Martien Poelen and Kina Helm for blood sample handling, Gaby Smits for providing instructions for the multiplex IgG immunoassay (MIA), and Mirjam Knol for assistance with statistical analysis. Finally, we would like to express our gratitude to all participants.
Funding
The study was funded by ZonMw and the Dutch Ministry of Health, Welfare and Sport (VWS).
References
- Mumps virus vaccines. Weekly epidemiological record (WHO); 2007; 7:51-60.
- Karagiannis I, van Lier A, van Binnendijk RS, Ruijs H, Fanoy E, Conyn-Van Spaendonck MA, de Melker H, Hahné S. Mumps in a community with low vaccination coverage in the Netherlands. Eurosurveillance (Weekly) 2008; 13; PMID:18761946.
- Plotkin SA, Rubin SA. Mumps vaccines (Chapter 20). Saunders (Elsevier Inc.); 2008.
- Dowdle WR, Orenstein WA. Quest for life-long protection by vaccination. Proc Natl Acad Sci U S A 1994; 91:2464-8; PMID:8146140; http://dx.doi.org/10.1073/pnas.91.7.2464.
- Vandermeulen C, Roelants M, Vermoere M, Roseeuw K, Goubau P, Hoppenbrouwers K. Outbreak of mumps in a vaccinated child population: a question of vaccine failure? Vaccine 2004; 22:2713-6; PMID:15246601; http://dx.doi.org/10.1016/j.vaccine.2004.02.001.
- Cohen C, White JM, Savage EJ, Glynn JR, Choi Y, Andrews N, Brown D, Ramsay ME. Vaccine effectiveness estimates, 2004–2005 mumps outbreak, England. Emerg Infect Dis 2007; 13:12-7; PMID:17370510.
- Dayan GH, Quinlisk MP, Parker AA, Barskey AE, Harris ML, Schwartz JM, Hunt K, Finley CG, Leschinsky DP, O'Keefe AL, et al. Recent resurgence of mumps in the United States. N Engl J Med 2008; 358:1580-9; PMID:18403766; http://dx.doi.org/10.1056/NEJMoa0706589.
- Peltola H, Kulkarni PS, Kapre SV, Paunio M, Jadhav SS, Dhere RM. Mumps outbreaks in Canada and the United States: time for new thinking on mumps vaccines. Clin Infect Dis 2007; 45:459-66; PMID:17638194; http://dx.doi.org/10.1086/520028.
- Schwarz NG, Bernard H, Melnic A, Bucov V, Caterinciuc N, an der Heiden M, Andrews N, Pebody R, Aidyralieva C, Hahné S. Mumps outbreak in the Republic of Moldova, 2007–2008. Pediatr Infect Dis J 2010; 29:703-6; PMID:20308934; http://dx.doi.org/10.1097/INF.0b013e3181d743df.
- Hassan J, Dean J, Moss E, Carr MJ, Hall WW, Connell J. Seroepidemiology of the recent mumps virus outbreaks in Ireland. J Clin Virol 2012; 53:320-4; PMID:22269391; http://dx.doi.org/10.1016/j.jcv.2011.12.022.
- Brockhoff HJ, Mollema L, Sonder GJ, Postema CA, van Binnendijk RS, Kohl RH, de Melker HE, Hahné SJ. Mumps outbreak in a highly vaccinated student population, The Netherlands, 2004. Vaccine 2010; 28:2932-6; PMID:20188683; http://dx.doi.org/10.1016/j.vaccine.2010.02.020.
- Karagiannis I, van Lier A, van Binnendijk R, Ruijs H, Fanoy E, Conyn-Van Spaendonck MA, de Melker H, Hahné S. Mumps in a community with low vaccination coverage in the Netherlands. Euro Surveill 2008; 13; PMID:18761946.
- Kaaijk P, van der Zeijst B, Boog M, Hoitink C. Increased mumps incidence in the Netherlands: review on the possible role of vaccine strain and genotype. Euro Surveill 2008; 13; PMID:18761918.
- Braeye T, Linina I, De Roy R, Hutse V, Wauters M, Cox P, Mak R. Mumps increase in Flanders, Belgium, 2012–2013: results from temporary mandatory notification and a cohort study among university students. Vaccine 2014; 32:4393-8; PMID:24973734; http://dx.doi.org/10.1016/j.vaccine.2014.06.069.
- Greenland K, Whelan J, Fanoy E, Borgert M, Hulshof K, Yap KB, Swaan C, Donker T, van Binnendijk R, de Melker H, et al. Mumps outbreak among vaccinated university students associated with a large party, the Netherlands, 2010. Vaccine 2012; 30:4676-80; PMID:22579874; http://dx.doi.org/10.1016/j.vaccine.2012.04.083.
- Kontio M, Jokinen S, Paunio M, Peltola H, Davidkin I. Waning antibody levels and avidity: implications for MMR vaccine-induced protection. J Infect Dis 2012; 206:1542-8; PMID:22966129; http://dx.doi.org/10.1093/infdis/jis568.
- Rubin SA, Qi L, Audet SA, Sullivan B, Carbone KM, Bellini WJ, Rota PA, Sirota L, Beeler J. Antibody induced by immunization with the Jeryl Lynn mumps vaccine strain effectively neutralizes a heterologous wild-type mumps virus associated with a large outbreak. J Infect Dis 2008; 198:508-15; PMID:18558869; http://dx.doi.org/10.1086/590115.
- Andrews N, Pebody RG, Berbers G, Blondeau C, Crovari P, Davidkin I, Farrington P, Fievet-Groyne F, Gabutti G, Gerike E, et al. The European Sero-Epidemiology Network: standardizing the enzyme immunoassay results for measles, mumps and rubella. Epidemiol Infect 2000; 125:127-41; PMID:11057968; http://dx.doi.org/10.1017/S0950268899004173.
- Smits G, Mollema L, Hahne S, de Melker H, Tcherniaeva I, Waaijenborg S, van Binnendijk R, van der Klis F, Berbers G. Seroprevalence of mumps in The Netherlands: dynamics over a decade with high vaccination coverage and recent outbreaks. PLoS One 2013; 8:e58234; PMID:23520497; http://dx.doi.org/10.1371/journal.pone.0058234.
- Gouma S, Schurink-van't Klooster TM, de Melker HE, Kerkhof J, Smits GP, Hahné SJM, et al. Mumps serum antibody levels before and after an outbreak to assess infection and immunity in vaccinated students. Open Forum Infect Dis 2014; 1(3):ofu101; PMID:25734169; http://dx.doi.org/10.1093/ofid/ofu101; Accepted for publication.
- van Lier E, Oomen PJ, Mulder M, Conyn-van Spaendonck MA, Drijfhout IH, de Hoogh PAAM, et al. RIVM Rapport 150202001/2013 Immunisation coverage National Immunisation Programme in the Netherlands; Year of report 2013. 2013 [in Dutch; summary in English].
- Anderson RM, May RM. Vaccination and herd immunity to infectious diseases. Nature 1985; 318:323-9; PMID:3906406; http://dx.doi.org/10.1038/318323a0.
- Wallinga J, Teunis P, Kretzschmar M. Using data on social contacts to estimate age-specific transmission parameters for respiratory-spread infectious agents. Am J Epidemiol 2006; 164:936-44; PMID:16968863; http://dx.doi.org/10.1093/aje/kwj317.
- Sane J, Gouma S, Koopmans M, de Melker H, Swaan C, van Binnendijk R, Hahné S. Epidemic of mumps among vaccinated persons, The Netherlands, 2009–2012. Emerg Infect Dis 2014; 20:643-8; PMID:24655811; http://dx.doi.org/10.3201/eid2004.131681.
- Gouma S, Sane J, Gijselaar D, Cremer J, Hahne S, Koopmans M, van van Binnendijk R. Two major mumps genotype G variants dominated recent mumps outbreaks in the Netherlands (2009–2012). J Gen Virol 2014; 95:1074-82; PMID:24603524; http://dx.doi.org/10.1099/vir.0.062943-0.
- Gut JP, Lablache C, Behr S, Kirn A. Symptomatic mumps virus reinfections. J Med Virol 1995; 45:17-23; PMID:7714488; http://dx.doi.org/10.1002/jmv.1890450104.
- Crowley B, Afzal MA. Mumps virus reinfection–clinical findings and serological vagaries. Commun Dis Public Health 2002; 5:311-3; PMID:12564247.
- Yoshida N, Fujino M, Miyata A, Nagai T, Kamada M, Sakiyama H, Ihara T, Kumagai T, Okafuji T, Okafuji T, et al. Mumps virus reinfection is not a rare event confirmed by reverse transcription loop-mediated isothermal amplification. J Med Virol 2008; 80:517-23; PMID:18205215; http://dx.doi.org/10.1002/jmv.21106.
- Date AA, Kyaw MH, Rue AM, Klahn J, Obrecht L, Krohn T, Rowland J, Rubin S, Safranek TJ, Bellini WJ, et al. Long-term persistence of mumps antibody after receipt of 2 measles-mumps-rubella (MMR) vaccinations and antibody response after a third MMR vaccination among a university population. J Infect Dis 2008; 197:1662-8; PMID:18419346; http://dx.doi.org/10.1086/588197.
- Latner DR, McGrew M, Williams NJ, Sowers SB, Bellini WJ, Hickman CJ. Estimates of mumps seroprevalence may be influenced by antibody specificity and serologic method. Clin Vaccine Immunol 2014; 21:286-97; PMID:24371258; http://dx.doi.org/10.1128/CVI.00621-13.
- Gouma S, Schurink-Van't Klooster TM, de Melker HE, Kerkhof J, Smits GP, Hahne SJ, van Els CA, Boland GJ, Vossen AC, Goswami PR, et al. Mumps serum antibody levels before and after an outbreak to assess infection and immunity in vaccinated students. Open Forum Infect Dis 2014; 1:ofu101; PMID:25734169.
- Cortese MM, Barskey AE, Tegtmeier GE, Zhang C, Ngo L, Kyaw MH, Baughman AL, Menitove JE, Hickman CJ, Bellini WJ, et al. Mumps antibody levels among students before a mumps outbreak: in search of a correlate of immunity. J Infect Dis 2011; 204:1413-22; PMID:21933874; http://dx.doi.org/10.1093/infdis/jir526.
- Smits GP, van Gageldonk PG, Schouls LM, van der Klis FR, Berbers GA. Development of a bead-based multiplex immunoassay for simultaneous quantitative detection of IgG serum antibodies against measles, mumps, rubella, and varicella-zoster virus. Clin Vaccine Immunol 2012; 19:396-400; PMID:22237896; http://dx.doi.org/10.1128/CVI.05537-11.
- Vaidya SR, Brown DW, Jin L, Samuel D, Andrews N, Brown KE. Development of a focus reduction neutralization test (FRNT) for detection of mumps virus neutralizing antibodies. J Virol Methods 2010; 163:153-6; PMID:19761798; http://dx.doi.org/10.1016/j.jviromet.2009.09.006.