Abstract
The aim of this study was to assess the level of humoral immunity against diphtheria and pertussis by measuring IgG to diphtheria toxoid (DT) and pertussis toxin (PT) in general population of Beijing. A total of 2147 subjects aged 0–74 y were selected with a random sample of resident population in Beijing. The information of socio-demographic characteristics, vaccination history, disease history of diphtheria and pertussis were collected for each subject by questionnaire. Serum samples were tested for IgG antibodies to DT and PT by using commercial ELISA kits. The overall positivity rate of anti-DT IgG was 66.28% with the mean concentration of 2.169 IU/ml. Age stratified data showed that the highest positivity rate of 97.63% was observed in 1–4 y and the rates decreased with age. The positivity rates were only around 50% or below since 25 y old. The positivity rate of anti-PT IgG was 12.34% with the mean concentration of 15.163 IU/ml. The highest level of positivity rate (22.23%) and antibody level (23.101 IU/ml) was seen in <1 year old. In subjects older than 10 y old, the anti-PT IgG positivity rate was 10.19%–13.51% and concentration was 13.295 IU/ml −16.353 IU/ml, with no significant differences between these groups (χ2 = 1.664, P = 0.948; F = 0.369, P = 0.899). The subjects with anti-PT IgG ≥100 IU/ml were observed in nearly all the groups older than 5 y except for 10–14 age group. The estimated incidences of pertussis infection were higher than 6000/100000 in these age groups. A sharp increase of immunity level of diphtheria was observed at 1 y and 6 y respectively, which was consistent with the current immunization schedule. But there was no significant increase of immunity to pertussis observed after booster immunization at 18–24 months, but the proportions of undetectable were lowest in <1, 1, 2 years in children <14 years. As shown in the present study, the adult population was generally lack of protective antibody against diphtheria and all the age groups showed a low immunity to pertussis indicating the potential risk of transmission and outbreaks of the 2 diseases in Beijing.
Keywords:
Introduction
Diphtheria and pertussis, as the respiratory infectious diseases caused by bacteria, are both the vaccine preventable diseases. Pertussis vaccine was introduced in Beijing since 1952, and diphtheria toxoid vaccine was used since 1955. However, the vaccination was only offered during certain months in a year as there was no standard place specified for vaccination at that time, in addition there was even no consolidated recommendation for the age of vaccination. In 1960s, combined diphtheria, tetanus, whole-cell pertussis vaccine (DTwP) replaced the monovalent vaccines and the standard immunization schedule was recommended including 3 doses of primary vaccination from 3 months of age, followed by the booster doses at the age of 18 months and 4 y, and one dose of adsorbent diphtheria vaccine at 6 y of age. Combined diphtheria, tetanus, acellular pertussis vaccine (DTaP) replaced DTwP since 2006 and the immunization schedule has been further adjusted including 3 doses of DTaP at 3, 4, 5 months, booster dose at 18–24 months, and one dose of absorbed tetanus and reduced diphtheria combined vaccine (Td) at 6 y and 15 y (students at grade 3 of middle schools), which schedule has been used until now in Beijing. DTP and Td vaccines included in Beijing EPI program are all domestic vaccines. The pertussis composition of DTaP is prepared by co-purification technology, and PT and FHA are the main components.
In the 1950s and early 1960s, the diphtheria and pertussis diseases were not well controlled although the related vaccines had been used. In 1962, the reported number of diphtheria cases was 379 with the incidence rate of 5.25/100000 while the pertussis was 35700 and the incidence rate reached 494.24/100000. The immunization schedule was standardized since 1966 and consequently the incidence of diphtheria and pertussis decreased significantly. In 1970s, the reported incidence of diphtheria had been reduced to 0.1/100000 and pertussis to 10/100000. The reported coverage rate of DTP4 has been above 99% since 1990 in Beijing and the investigated coverage rates were higher than 95% among the resident children of Beijing in the past 5 y.Citation1,2 The high vaccination coverage has resulted in the continued decrease in the incidence of the diseases as the reported cases of pertussis has remained low as 3–15 cases per year since 1990s and no diphtheria case has been reported since 1996 in Beijing.
However, the majority of reported pertussis cases were clinically diagnosed in Beijing. Laboratory confirmed case is defined as positive for culture or a fourfold change in IgG and PCR is not included in the national guideline for case definition. Before 2004, all the samples collected from suspected pertussis cases were sent to Beijing CDC for laboratory testing. However, it is very hard to isolate B. pertussis and also difficult to collect acute serum samples. Laboratory testing for pertussis has not been done since 2004 due to reagents issue in Beijing. Pertussis might be underreporting due to the lack of laboratory confirmation especially among adolescents or adults with atypical symptoms. Laboratory confirmation for diphtheria is also done at Beijing CDC, including culture and serology testing. Although several suspected cases of diphtheria have been reported every year, all the cases were negative for laboratory tests.
Outbreaks of diphtheria or pertussis among adults or adolescents were reported in other countries even though the coverage of vaccination maintained high. In 1990s, the epidemic of diphtheria reemerged in countries of the former Soviet Union with the reported cases of 157000 and 38%–82% of cases occurred in adults.Citation3 The coverage of childhood immunization was very high in these countries including a booster dose of diphtheria vaccine for children aged 14–16 y.Citation3 Outbreaks of pertussis were also reported in the US,Citation4 AustraliaCitation5 and Japan,Citation6 indicating the childhood vaccination of DTP with high coverage rate could not assure the persistent protection against pertussis in the population.Citation3
In 2012, a seroepidemiological study was conducted by Beijing CDC. A total of 2147 serum samples were collected and tested for pertussis, diphtheria and other vaccine preventable diseases. In 2015, we published the preliminary results of pertussis in “Chinese journal of vaccines and immunization.”Citation7 The article published in Chinese journal concluded that the population in Beijing is generally susceptible to pertussis. Although the association between vaccination and antibody levels was analyzed in children ≤14 years who are the target of EPI program in China, but the data were stratified by a relatively large interval of age and the number of doses for vaccination status, which might have not clearly revealed the impact of booster dose on the immunity level against pertussis. The present article included the data of diphtheria and pertussis as well, and provided much more detailed data for children ≤14 years old. By comparing the data of pertussis with diphtheria which are the 2 components of the same vaccine, it could help us better understand the changing trend of immunity level against 2 diseases in the population, and would further help healthcare policy makers decide whether to revise the current immunization strategy.
Results
Characteristics of study population
A total of 2147 subjects were enrolled in the study with the age range of 3 months to 74 y. The ratio of male to female was 1:1.03 (1058:1089) and the ratio of local residents and migrant residents was 1.02:1 (1082:1065). Among subjects ≤14 years old, the rate of vaccination with at least one dose of DTP is 99% and more than 93% have the confirmed history of DTP vaccination with at least 3 doses. There were no unvaccinated subjects identified in age groups of 1–4 y, 5–9 y and 10–14 y. 78.34% and 77.87% of subjects ≥15 y old failed to recall whether they had been vaccinated with diphtheria and pertussis respectively, and 91.90% of subjects older than 30 y old were unknown for the history of vaccination against diphtheria or pertussis. There is no subject with history of diphtheria disease and 4 subjects with pertussis disease history. The 4 subjects were adults with the age ranging from 23 y to 36 y and were diagnosed when they were younger than 6 y old.
Humoral immunity to diphtheria
As shown in the , the overall positivity rate of anti-DT IgG was 66.28% with the mean concentration of 2.169 IU/ml. The mean concentration of antibody against diphtheria was higher in males than that in females. Local residents have higher levels than migrant residents in terms of both positivity rate and mean concentration. Age stratified data showed that the highest positivity rate of 97.63% was observed in children aged 1–4 y and then the rates decreased with age. Although there was a booster dose at 15 y old, there was no increase of positivity rate observed for 15–19 y, instead the rates continually decreased to 73.64% from 83.80% of 10–14 y. The positivity rates were only around 50% or below since 25 y old. The lowest level of 34.11% was observed in subjects older than 40 y. The concentration of antibody against diphtheria was highest in children aged 5–9 y (6.262 IU/ml) and gradually decreased to the lowest level in group aged 35–39 y (0.233 IU/ml). showed the antibody level of diphtheria stratified by one year in children aged 0–14 y. A sharp increase of immunity level was observed at 1 y and 6 y respectively, which was consistent with the current immunization schedule in Beijing.
Figure 1. Mean concentration (95% CI) and distribution of (A) diphtheria IgG antibodies and (B) anti-PT IgG in children aged 0–14 y.
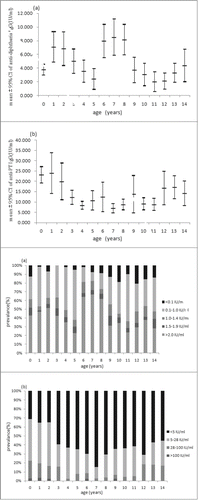
Table 1. Immunity to diphtheria and pertussis in subjects by different groups
Humoral immunity to pertussis
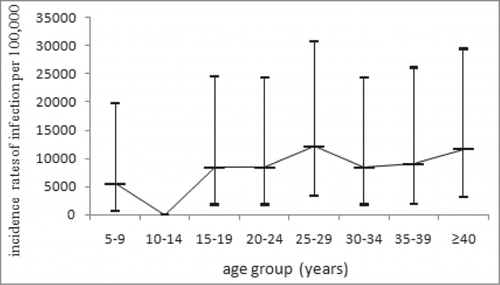
The positivity rate of anti-PT IgG was 12.34% with the mean concentration of 15.163 IU/ml. The positivity rate and antibody level was higher in male than that in female (). The highest level of positivity rate (22.23%) and antibody level (23.101 IU/ml) was seen in the group of <1 year old. Then both of them significantly decreased and the lowest level was seen in group of 5–9 y, with the positivity rate of 5.75% and the antibody concentration of 10.532 IU/ml. There were no significant differences observed between groups older than 10 y old (χ2 = 1.664, P = 0.948; F = 0.369, P = 0.899). There was no significant increase of immunity observed after booster immunization at 18–24 months, but the proportions of undetectable were lowest in <1, 1, 2 y groups in children <14 years (). In addition, there is no significant difference in the antibody levels between subjects with and without a history of pertussis disease. The subjects with the anti-PT IgG ≥100 IU/ml were observed in nearly all the groups except for 10–14 y old and there were no significant differences in the proportions of subjects ≥100 IU/ml among these age groups(χ2 = 5.908, P = 0.749). The estimated incidences of pertussis infection were higher than 6000/100000 in age groups older than 5 y where the subjects with ≥100 IU/ml were identified (Fig. 2).
DTaP has been offered and completely replaced DTwP from 1st January 2006 in Beijing. Among 451 subjects ≤14 years old with the history of vaccination with 4 doses of DTP and the information for the types of vaccines documented, 241 subjects were vaccinated with 4 doses of DTaP and all of them were aged ≤6 years, while 160 subjects had the history of full-course vaccination of DTwP and were all aged ≥7 years old. Only 50 subjects had a mixed vaccination with the age range of 6–11 y, including 47 with 3 wP +1aP, 1 with 2 wP +2aP and 2 with 1 wP +3aP. There was no significant difference in antibody level of pertussis between those with 4aP, 4wP and mixed vaccination in 6–11 y old.
Discussion
As shown in the present study, the antibody levels of diphtheria and pertussis were low in the resident population of Beijing in 2012. The adult population was generally lack of protective antibody against diphtheria and all the age groups showed a low immunity to pertussis indicating the potential risk of transmission and outbreaks of the 2 diseases in Beijing. The different patterns of immunity level observed for the 2 components in one combined vaccine regardless of its high vaccination coverage could be related to various factors including the current schedule, immunogenicity of vaccines and the circulation of bacteria in the population.
The results of diphtheria immunity in the study showed that high levels of antibody were generated after diphtheria vaccination. Due to the 1st booster at 18 months, there was a significant increase of antibodies level observed in 1 y group. In the following 4 y, the concentration of antibody decreased rapidly but the positivity rates still remained high levels. Hence no significant increase of positivity rate was observed after the 2nd booster immunization at 6 y, but the proportion of high concentration (>2 IU/ml) increased significantly in this age.
According to the current schedule in Beijing, a second booster dose of Td vaccine is recommended for children at grade 3 of middle schools, about 15 y old, but no significant increase of positivity rate and concentration of antibody to diphtheria were observed in 15–19 y. The vaccination history of subjects ≥15 years old were based on their own recall with only the number of doses collected. As the date of immunization was not collected, it is not able to analyze the association between antibody levels and the time since booster immunization. In addition, most subjects ≥15 years old were not sure about the history of vaccination, so the actual rate of vaccination with Td recommended for 15 y old was unknown, which made it difficult to analyze the potential reasons why there is no obvious increase in the immunity against diphtheria. The estimated immunization coverage rate of this booster dose is 70%–80% in Beijing. As recommended by WHO in European region, a minimum coverage rate of 90% in children and 75% of adults is required to achieve elimination of diphtheria.Citation8 Coverage rate in children at middle schools should be improved in Beijing, which is important to protect adolescents and adults against diphtheria.
Although the positivity rates of anti-DT IgG remained above 70% among <20 years old, it declined to 59.01% among 20–24 y old and further less than 50% among ≥25 years old. Despite the high coverage of childhood immunization, immunity to diphtheria showed an obvious trend of decrease with age, indicating lack of protection among adult population and the potential risk of adult outbreaks of diphtheria in Beijing. Similarly, studies conducted in both developed and developing countries also showed the same trend of changing with age and increased susceptibility in adult population.Citation9-11
There has been no diphtheria cases ever reported in the past 18 y in Beijing and no cases reported in recent 7 y in China,Citation12 which means natural boosting by circulating toxigenic C. diphtheria is less likely to occur in Beijing, and the significant decrease of protective immunity with age is the result of natural waning after vaccination. As shown in the previous studies in Beijing, high level of antibody against diphtheria could last for 5–10 y after vaccination and then decreased significantly.Citation13 In other studies, the protective antibody could last for 8–10 y after vaccination.Citation14 WHO recommended booster doses at about 10-year intervals in addition to the childhood immunization for low-epidemic or non-epidemic areas, therefore adult immunization with diphtheria vaccine should be considered in Beijing.
Quite different from diphtheria, the antibody levels of pertussis maintained low level throughout all age groups, and even no immune responses were observed after the boost dose. There were 62 subjects in 1 age group, of which only 20 subjects had completed DTaP booster vaccination. The average level of pertussis antibody in these 20 subjects was higher than that of 42 subjects who had not yet received the booster vaccination and also higher than that of subjects <1 year. But the increase for pertussis was not as significant as diphtheria, so a significant increase was observed for pertussis in 1 age group. The duration of antibody to pertussis is limited after vaccination,Citation3 with a rapid decline especially in the following first year.Citation15 As shown in the study conducted in Tianjin, the positive rate of anti-PT IgG was highest 2 months after vaccination (92%), but declined rapidly to 35% after 4–5 months and 25% after 5–11 months.Citation16 This could be the main reason why there was still no significant increase in the antibody to pertussis observed in the subjects aged 2 y. Because of the low level of anti-PT IgG, it is also hard to observe the changing trend with age in adolescents and adults. The results of the study indicated that vaccination has limited contribution to the immunity level of pertussis in the population.
A cross-sectional study on anti-PT lgG levels in young children of Australia showed that, at the population level the vaccination signature may be more apparent through a reduction in the prevalence of undetectable antibody levels than an increase in the prevalence of high levels.Citation5 As shown in , the proportion of subjects with undetectable level (<5 IU/ml) of anti-PT IgG increased significantly during the first 7 y after vaccination with the peak of 84.62% in 7 y old, indicating that another booster dose should be required before 7 y old.
The reported incidence of pertussis in Beijing is extremely low as 10 cases per year or <0.1/100000 in recent years. All of the reported cases were under 1 y old. However, the estimated incidence of pertussis infection in this study was 8287.34/100000 among children ≥10 years old and pertussis infections were identified in nearly all the age groups investigated. Many studies have already found evidences that asymptomatic infections of pertussis were very common in adolescents and adults, which became the important source of childhood infections.Citation17–19 In some developed countries with high coverage of childhood immunization, the incidence of infection or disease showed a trend of increase in adolescents.Citation3 Although there were no significant differences in the incidences of infection among age groups in this study, attention should be paid to those born after 2005 because these population were vaccinated with DTaP. As shown in other studies, the protective rates of DTaP in adolescents were lower than that of DTwP.Citation20,21
The antibody levels of diphtheria and pertussis in males were higher than that of females. It could be caused by sampling error as there were no differences between genders were observed in each age group. There were differences in the antibody levels of diphtheria between different types of residents. The reason could be the different coverage of vaccination.
There are some limitations in this study. The first could be the limited sample size. When the data were stratified by one year for children under 14 y old, the sample size was only about 45 (36–62) for each age group which might have weakened the power of data. The trend of antibody level with age for children <14 years old needs to be further investigated with more sufficient sample size. The second limitation could be the cut-off of antibody level for pertussis infection used in the study. There is no definition specified for Chinese population so far. This could be the reason why the estimated incidence of pertussis infection was found to be 0 in subjects aged 10–14 y. There are various cut-offs for recent infection used in other studies, such as 30 IU/mlCitation,22 80IU/mlCitation,23 100 IU/ml, 125 IU/ml.Citation5,24 In the present study, we decided to use the cut-off defined in Dutch population. However, it is unknown whether the definition is suitable for Chinese population given the distinct level of disease prevalence and immunization status compared to other countries. Further efforts should be made to develop the local definition for serum antibody of pertussis and validate the current ELISA methods available in China. Another limitation could be that most subjects ≥15 years old were unknown for the history of vaccination, which made it difficult to evaluate the effect of second booster dose of Td recommended for 15 y old.
The present study showed that antibody levels of diphtheria in adults were not enough to provide persistent protection after childhood immunization and most importantly the prevalence of pertussis might have been underestimated in Beijing. There is an urgent need to reevaluate the current immunization schedule to eliminate the potential risk of diphtheria disease imposed on adult population and to improve the protective immunity against pertussis in older children, adolescents and adults.
Materials & Methods
Study population
We selected 9/18 districts of Beijing using multi-stage stratified sampling method based on the geographical location and demographic characteristics. 10 villages were selected from each district using systematic sampling method. Individuals who had resided for at least 6 months in each village were divided into 10 age groups (<1,1–4, 5–9, 10–14, 15–19, 20–24, 25–29, 30–34, 35–39, ≥40 years) and invited into the study according to the pre-defined sample size. The calculation of sample size was based on the formula: n = u2a × p × q/δ2/.The positivity rates of antibody were assumed at 50% for each age group in both genders. A minimum sample size of 192 was required for each age group, with confidence level of 95% and an absolute precision of 10% (α = 5%, δ = 10%, p = 50%,q = 1−p). Therefore, more than 200 subjects were enrolled for each age group resulted in a total of 2147 subjects.
Vaccination history
History of vaccination against diphtheria and pertussis was obtained from recall for subjects older than 14 y. Information for children less than 14 y old was extracted from the Beijing Immunization Program Management Information System.
Disease history
History of diphtheria and pertussis disease was collected using questionnaire by asking whether the subjects had been diagnosed with diphtheria and pertussis.
Laboratory methods
Serum samples were collected from each subject and stored at −20°C. The samples were tested at the laboratory of Beijing CDC. IgG antibodies against PT of B. pertussis were measured quantitatively by a commercial ELISA kit (Euoimmun Medizinische Labordiagnostika AG). According to the kit instruction, serum antibody ≥28 IU/ml was defined as seropositive and ≥ 100 IU/ml indicated for an acute infection or recent vaccination.
IgG against toxoid of C. diphtheriae were tested quantitatively with a commercial ELISA kit (Virion/Serion GmbH, Würzburg, Germany). According to the instruction, anti-diphtheria antibody concentration <0.1 IU/ml was defined as no immune protection or seronegativity and susceptibility, a value of ≥0.1 IU/ml was given for a secure individual protection and seropositivity. The following vaccination and booster-vaccination are recommended in the instruction: <0.1 IU/ml as need of primary vaccination, 0.1–1.0 IU/ml as need of booster vaccination, 1.0–1.4 IU/ml as need of booster after 5 y, 1.5–1.9 IU/ml as need of booster after 7 y, ≥2.0 IU/ml as need of booster after 10 y.
Statistical analysis
The database was set up by Epidata 3.1 software. Data analysis was performed using SPSS 17.0 software. Means and 95% confidence intervals (CI) by different groups were calculated for the seropositivity rates and concentrations of anti-PT IgG and anti-diphtheria IgG. The differences in the level of IgG antibodies between different groups were tested by one-way ANOVA. The chi-square test was used to compare the seropositivity rates by different groups. A p value <0.05 was considered statistically significant. For the purpose of mean calculation, the value of anti-PT IgG level <5 IU/ml was set at 5 IU/ml and >174 IU/ml was set at 174 IU/ml. For diphtheria antibody, <0.05 IU/ml was counted as 0.05 IU/ml and > 2.0 IU/ml as 2.0 IU/ml.
The incidence of pertussis infection was estimated using the method described by de Melker et al.Citation25 It was shown that after pertussis infection, PT IgG will be declined to 100 IU/ml after the average time of 58.6 d. With the cut-off of 100 IU/ml, the incidence of infection can be calculated using the formula: 365.2/58.6×proportion of subjects with PT IgG ≥100 IU/ml. To exclude potential interference of vaccination on the antibody level, only subjects ≥5 years old were included in the analysis on incidence of pertussis infection.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank all the staff of district centers for disease control and prevention in Beijing involved in this study, including Xicheng District CDC, Dongcheng District CDC, Fengtai District CDC, Shijingshan District CDC, Mentougou District CDC, Shunyi District CDC, Fangshan District CDC, Pinggu District CDC, and Miyun District CDC.
References
- Wen-Yan J, Wei-Xiang L, Liang M, Rui Y, Dong-Lei L, Jiang W. Vaccination coverage survey among local children in Beijing. Chin Prev Med 2012; 11:858-63
- Liu Da-wei, Sun Mei-ping, Liu Wei-xiang, Fan Chen-yang, Lu Li, Liu Dong-lei, Zeng Yang, Wang Ling-yun, Zhang Xue-chun. Comparative study on immunization coverage rates of nine vaccines between local and floating children. Chinese J Vaccine Immunization 2007; 02:165-9
- Pertussis vaccines: WHO position paper. Wkly Epidemiol Rec 2010; 85(40):385-400; PMID:20939150
- Bell GC, Foster SL. pertussis outbreak and updated Tdap recommendations. J Am Pharm Assoc 2010; (2003) 2011; 51(1):118-20
- Campbell P, Mcintyre P, Quinn H, Hueston L, Gilbert GL, McVernon J. Increased population prevalence of low pertussis toxin antibody levels in young children preceding a record pertussis epidemic in Australia. PLoS One 2012; 7(4):e35874; PMID:22558249; http://dx.doi.org/10.1371/journal.pone.0035874
- Kamano H, Mori T, Maeta H, Taminato T, Ishida T, Kishimoto N, Katami T, Sato M, Kamachi K, Mochida Y. Analysis of Bordetella pertussis agglutinin titers during an outbreak of pertussis at a university in Japan. Jpn J Infect Dis 2010:63(2):108-12; PMID:20332572
- LI X-M, Zhang T-G, Zeng Y, Li Juan, Sun Mu, Sun Hao, Wang Zhong-zhan, Guo Fang-ru, Zhang Yi-hua, Wang Feng-shuang, et al. Survey of antibodies to pertussis in resident population of Beijing in 2012. Chinese J Vaccine Immunization 2014; 20(6):542-6
- Begg N. Manual for the Management and Control of Diphtheria in the European Region. The Expanded Programme on Immunization in the European Region of WHO. Copenhagen. World Health Organisation, 1994.
- Kurugol Z, Midyat L, Turkoglu E, Işler A. Immunity against diphtheria among children and adults in Izmir, Turkey. Vaccine 2011; 29(26):4341-4; PMID:21510994; http://dx.doi.org/10.1016/j.vaccine.2011.04.016
- Zhang Q, Han F, Nie Q, Ren H, Zhang B, Liu Q, He Q, Shao Z. Seroprevalence of antibodies to pertussis and diphtheria among healthy adults in China. J Infect 2011; 63(6):441-6; PMID:21864569; http://dx.doi.org/10.1016/j.jinf.2011.07.018
- Lee HF, Tseng LR, Yueh YY, Wu YC. Immunity against diphtheria in Taiwan. J Microbiol Immunol Infect 1999; 32(3):206-12; PMID:10637720
- World Health Organisation, Immunization, Vaccines and Biologicals: data, statistics and graphics-disease incidence. http://www.who.int/immunization/monitoring_surveillance/data/en/.
- Xiao-Mei L, Dong-Lei L, Jie Y. Analysis of diphtheria antibody levels in healthy population in Beijing municipal, 2007. Chinese J Vaccine Immunization 2010; 3(16):222-4
- Diphtheria vaccine. Wkly Epidemiol Rec 2006; 81(3):24-32; PMID:16671240
- Wirsing CH. The Immunological Basis for Immunization Series. Module 4: Pertussis. Geneva: World Health Organization 2009.
- Huang HT, Zhang Y, Liu Y, Li YC, Liu P, Ding YX, Chen W, Gao ZG, Zhang ZL. Surveillance and analysis the immunity status and risk factors of pertussis in Tianjin Municipal in 2009. Chinese J Vaccines Immunization 2010; 16:536-40
- Kathryn M, Edwards, Michael D, Decker. Pertussis Vaccines. Vaccines. fifth edition ed. Elsevier Inc 2008:467-517.
- Wiley KE, Zuo Y, Macartney KK, McIntyre PB. Sources of pertussis infection in young infants: a review of key evidence informing targeting of the cocoon strategy. Vaccine 2013; 31(4):618-25; PMID:23200883; http://dx.doi.org/10.1016/j.vaccine.2012.11.052
- de Greeff SC, Mooi FR, Westerhof A, Verbakel JM, Peeters MF, Heuvelman CJ, Notermans DW, Elvers LH, Schellekens JF, de Melker HE. Pertussis disease burden in the household: how to protect young infants. Clin Infect Dis 2010; 50(10):1339-45; PMID:20370464; http://dx.doi.org/10.1086/652281
- Witt MA, Arias L, Katz PH, Truong ET, Witt DJ. Reduced risk of pertussis among persons ever vaccinated with whole cell pertussis vaccine compared to recipients of acellular pertussis vaccines in a large US cohort. Clin Infect Dis 2013; 56(9):1248-54; PMID:23487373; http://dx.doi.org/10.1093/cid/cit046
- Klein NP, Bartlett J, Fireman B, Rowhani-Rahbar A, Baxter R. Comparative effectiveness of acellular versus whole-cell pertussis vaccines in teenagers. Pediatrics 2013; 131(6):e1716-e1722; PMID:23690518; http://dx.doi.org/10.1542/peds.2012-3836
- Zhang Q, Zheng H, Liu M, Han K, Shu J, Wu C, Xu N, He Q, Luo H. The seroepidemiology of Immunoglobulin G antibodies against pertussis toxin in China: a cross sectional study. BMC Infect Dis 2012; 12:138; PMID:22892100; http://dx.doi.org/10.1186/1471-2334-12-138
- Zhang Y, Huang H-T, Liu Y, Gao Zhi-gang, Shi Kui-yu, Chen De-rong, Wu Shu-qin. Incidence surveillance of pertussis based on community and analysis of its transmitted features in Tianjin. Chinese J Vaccine Immunization 2011; 17 (3):209-11
- Launay O, Toneatti C, Bernède C, Njamkepo E, Petitprez K, Leblond A, Larnaudie S, Goujon C, Ungeheuer MN, Ajana F, et al. Antibodies to tetanus, diphtheria and pertussis among healthy adults vaccinated according to the French vaccination recommendations. Hum Vaccines 2009; 5(5): 341-6; PMID:19221513; http://dx.doi.org/10.4161/hv.5.5.7575
- de Melker HE, Versteegh FG, Schellekens JF, Teunis PF, Kretzschmar M. The incidence of Bordetella pertussis infections estimated in the population from a combination of serological surveys. J Infect 2006; 53(2):106-13; PMID:16352342; http://dx.doi.org/10.1016/j.jinf.2005.10.020