Abstract
In addition to its well-known effects on bone metabolism, vitamin D is an immunomodulating hormone. Serum vitamin D levels in males 18–25 years were measured at baseline, and HPV antibody titers were measured one month following the third quadrivalent HPV vaccine dose. Vitamin D levels were ≥30 ng/ml (normal) in 60 males and <30 ng/ml (low) in 113 males. Reverse cumulative distribution curves and scatter plots showed higher antibody titers with low vitamin D for all vaccine strains (P < 0.05). In linear regression analyses, antibody titers for all HPV strains were significantly higher among those with lower vitamin D levels and among younger participants (P < 0.05). These relationships add to the body of knowledge of the complex role of vitamin D in immunoregulation.
Introduction
Vitamin D has multiple immunologic effects beyond its well-known role in bone metabolism. For instance, vitamin D insufficiency has been linked to inflammatory diseases, such as multiple sclerosis and inflammatory bowel disease.Citation1 In multiple sclerosis (MS), low vitamin D levels are associated with increases in MS activity and in the number of gadolinium-enhancing lesions.Citation2
Research on vitamin D and immunological response to vaccination shows mixed results. Studies of children and adolescents, and of adults >50 y of age have found no association between vitamin D deficiency and response to influenza vaccine.Citation3,4 In patients with chronic kidney disease, vitamin D deficiency is associated with a poor response to hepatitis B immunization.Citation5 Vitamin D supplementation resulted in higher antibody titers after tetanus toxoid boosters.Citation6 A systematic review of vitamin D for the treatment of infectious diseases was inconclusive but stressed the need for further research on the topic.Citation7 This study, conducted in the context of a trial of different HPV vaccine schedules in college-aged males, sought to determine the association between pre-vaccination serum vitamin D levels and post HPV vaccination antibody titers.
Methods
Participants
Men 18–25 y of age were recruited from October 2010 through May 2011 for a trial testing their immune response to the quadrivalent HPV vaccine, comparing administration of the third dose at 6 months (recommended standard schedule) versus 12 months. Methods used in this study have been published.Citation8 Potential participants were excluded if they had: more than 4 lifetime sexual partners, health problems that would interfere with the immune response or their ability to complete the study, hospitalization during the past year, hypersensitivity to yeast or HPV vaccine components, inability to complete the scheduled appointments, previously received HPV vaccine or if they were taking any immunosuppressive medications. Young women were not included in the clinical trial; therefore no vitamin D samples were available for women in this study. Participants completed a survey at baseline in which they were asked about vitamin D supplementation and other demographic and health characteristics. Height and weight were measured at the dose 3 visit. Vitamin D levels were measured at baseline only, so that all samples would be collected at a consistent time relative to vaccine administration. This study was approved by the University of Pittsburgh Institutional Review Board (PRO10070407).
Sample processing, immunogenicity and vitamin D assays
Vaccine storage and delivery followed standard procedures. Blood samples were drawn immediately prior to the first dose and 2–6 weeks after the third dose into serum separator tubes. Samples were spun at 3200 rpm for 10–15 minutes and serum was transferred to labeled nunc cryovials which were stored at −70°C. Frozen nunc tubes were shipped on dry ice to the laboratory by an express carrier. Serology testing for each of the 4 HPV types was performed at PPD Vaccines and Biologics Laboratory (Wayne, PA) using a competitive Luminex immunoassay (cLIA) that measures type-specific antibodies to neutralizing epitopes on the virus-like particles (VLPs) as described in Dias et al.Citation9 Participants who had anti-HPV serum cLIA levels >20 milliMerck units/mL (mM/mL) for HPV types 6 and 16, >16 mM/mL for type 11, and >24 mM/mL for type 18 were considered to be seropositive at baseline and were excluded from analyses only for the type(s) for which they were seropositive.
Vitamin D assays were performed with a Waters ultra-performance liquid chromatogram detector and tandem mass spectrometer that revealed molecular weight for 25-hydroxy vitamin D2 and D3. The first quadruple mass analyzer was tuned for the parent ions. The second mass analyzer was tuned for specific daughter ions; these are detected by the photomultiplier system. The machine's coefficient of variation was 10 for both vitamin D2 and D3. The results of vitamin D2 and D3 were combined for a total vitamin D level for analysis; the range of D2 values was 4 to 12; the range of D3 values was 4 to 60. Normal Vitamin D was set at ≥30 ng/ml and low Vitamin D was set at <30 ng/ml based on Endocrine Society guidelines for insufficiency.Citation10 One participant who did not have vitamin D values and one whose vitamin D value was an outlier, were excluded.
Statistical analyses
Descriptive analyses of participants' demographic characteristics were performed at baseline, for all participants combined and comparing those with low and normal baseline Vitamin D levels. Further, we assessed differences in antibody titers by age, race and sexual orientation of participants and timing of dose 3.
Because antibody titers were skewed, the data were natural log-transformed and used to calculate HPV type-specific mean titers for each group. Potential confounders in the relationship between vitamin D and HPV antibody titers were eliminated by comparing the demographic variables related to vitamin D by Chi square tests and those related to mean log transformed HPV antibody titers using ANOVA. Age, race, sexual orientation, dosing group and vitamin D levels (as both categorical and continuous variables) were included in linear regression analyses to determine their relationships to HPV titers. Reverse cumulative distribution curves (RCDs) and scatter plots were created for each HPV vaccine strain combining 6 and 12 month dosing groups, showing distributions of log transformed titers by vitamin D levels. Log rank (Mantel-Cox) tests for equality of distributions for log2 HPV antibody titers between participants with baseline normal and low vitamin D levels were conducted. Statistical significance was set at P < 0.05.
Results
Baseline characteristics
Of 311 men who were screened, 91 did not meet inclusion criteria, leaving 220 enrollees; 173 received all 3 doses and completed the post vaccination blood draw within the respective study windows and were included in these analyses. Eighty-six participants received dose 3 at 6 months and 87 received dose 3 at 12 months. Their mean age was 21.5 y; 18.5% were non-white; 13.3% were homo-/bisexual; 6.9% were smokers; 8.1% were not students. The body mass index averaged 25.2 with a standard deviation of 5.6 (Table 1). There were no demographic variables significantly related to both vitamin D and HPV antibody titers, indicating no confounding.
Reported vitamin D supplementation
Thirty-three percent (57/173) of the participants reported taking a vitamin D supplement of some form (i.e., multivitamin or vitamin D supplement); the percentage of those who took supplements did not vary between the 6 month (38%) and 12 month groups (28%, P = 0.13). There was no significant difference in the proportion of participants with low serum vitamin D levels between those taking a vitamin D supplement (57%), and those not taking a vitamin D supplement (69%, P = 0.14).
Vitamin D levels and HPV antibody titers
In the 6 month group, the minimum vitamin D level was 4 ng/ml, the mean was 26.7 ng/ml, and the maximum was 60 ng/ml. In the 12 month group the minimum, mean and maximum vitamin D levels were 8 ng/ml, 27.8 ng/ml, and 57 ng/ml, respectively, P = 0.483. Overall, the vitamin D levels were normal in 60 males and low (<30 ng/ml) in 113 males. In the 6 month group, the percentage of participants with vitamin D levels <30 ng/ml was 64% versus 67% in the 12 month group (P = 0.415).
Log transformed HPV antibody titers and serum vitamin D levels
Reverse cumulative distribution curves and scatter plots were created for each HPV vaccine strain combining 6 and 12 month dosing groups, showing that the normal vitamin D level was associated with a lower titer for each strain (, respectively). The Mantel-Cox log rank test indicated that these values of log transformed titers differed significantly across vitamin D levels (P < 0.05).
Figure 1. Reverse cumulative distribution curves for antibody concentrations after quadrivalent HPV vaccine administration, by HPV type and by total serum vitamin D level (<30 versus ≥30 ng/mL). Legend: The percentage of participants achieving a specified log-transformed concentration was plotted, comparing ≥30 vs. <30 ng/mL of total serum vitamin D levels. Using the Mantel-Cox log rank test, these values differ significantly (P < 0.05).
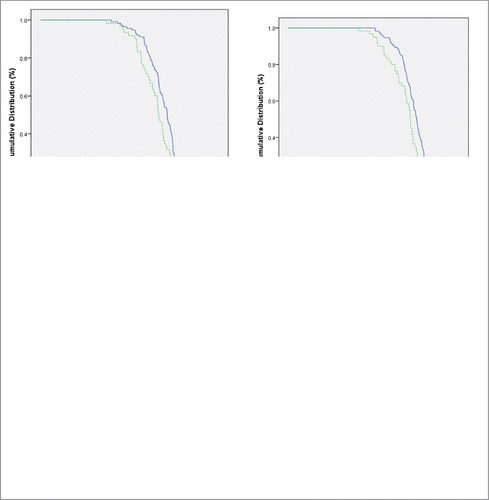
Figure 2. Distribution of log transformed HPV antibody levels (mMerck unit/mL) associated with total serum vitamin D levels (ng/mL).
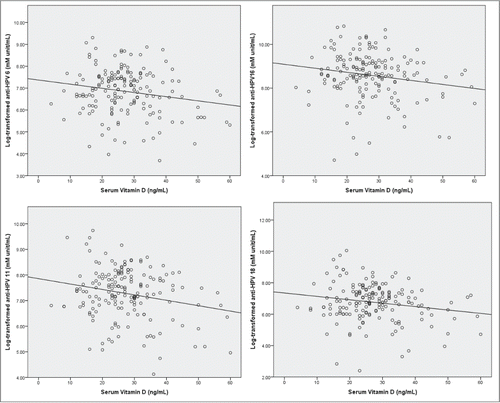
In linear regression analyses that included demographic variables and vitamin D levels, mean log antibody titers to HPV strains 6 and 16 were significantly and negatively related to age and vitamin D whether as a continuous or categorical variable (P < 0.05; ). For HPV 11, age and vitamin D levels were significantly, negatively related to mean log antibody titer. In addition, dosing schedule and race were significantly related to mean log titer, with the 12 month dosing group significantly higher than the 6 month dosing group, and white participants significantly higher than non-white participants. For HPV 18, younger age and vitamin D as a continuous variable were significantly, negatively related to mean log titer. Thus, for all 4 HPV strains, vitamin D and age were negatively associated with log transformed HPV antibody titers.
Table 1. Demographics by vitamin D level (top) and by log transformed HPV antibody titers (bottom)
Table 2. Association of HPV geometric mean titer (GMT) by HPV type with age, race, sexual orientation and vitamin D level (≥ 30 vs. < 30 ng/mL; top) and by overall vitamin D level (bottom) using linear regression; N = 173
Discussion
We found that low vitamin D levels at baseline were associated with significantly higher antibody titers in response to 3 doses of HPV vaccine when analyzed either using RCD curves or adjusting for demographic variables using mean log titers. Together, these data suggest that vitamin D has an immunomodulating effect on HPV vaccine response. However, the impact of vitamin D, while statistically significant, does not affect the titers sufficiently to change any anticipated clinical outcomes; indeed, all the titers were higher than those produced by natural infection. Similar to our findings by age group, significant differences in elicited antibody responses to HPV vaccine have been observed in racial, age and sexual orientation groups of men, yet all have been deemed to be sufficient for protection against HPV infection.Citation11 In fact, HPV antibody titers were higher in the present study than those previously reported.Citation11
This inverse relationship is not inconsistent with the exaggerated inflammatory responses seen in conditions such as multiple sclerosis and inflammatory bowel disease that have been associated with low vitamin D levels.Citation1 We speculate that as an immunoregulatory hormone, vitamin D tempers the response to HPV vaccination to an appropriate yet robust level; whereas insufficient vitamin D leads to a higher immune response. A similar relationship between season of vaccination and antibody response to rubella vaccination has been reported. Children who received rubella vaccine in the winter, with lower levels of vitamin D-stimulating ultraviolet radiation, had significantly higher GMTs than those vaccinated in the summer.Citation12 Such associations provide the impetus for further study of the role of vitamin D in the immunology of vaccine response. A recent review of the role of vitamin D in the immune response to vaccination found mixed results and concluded that the current state of knowledge is insufficient about the true relationship between vitamin D and immunogenicity; indeed, the authors suggested that either an increased or decreased response might occur.Citation13
Strengths and limitations
To our knowledge, this is the only study to assess serum vitamin D levels and HPV vaccine antibody titers. In contrast to earlier reports,Citation14 the competitive Luminex immunoassay has been found to correlate well with 2 other common HPV antibody assays.Citation15 Several limitations should be noted. Much debate occurs in the literature about the cut-points for adequate vitamin D serum levels; we used the Endocrine Society insufficiency cut-points.Citation10 Too few participants had vitamin D levels below 20 ng/mL to allow for meaningful analyses by subgroup, such as race or sexual orientation. However the results were similar when including vitamin D level as a continuous variable. Young women, whose antibody responses to HPV vaccine in an identical clinical trialCitation16 were higher than young men's in this trial, were not included in this study, because vitamin D levels were not measured in that study. A similar study including females is warranted. Vitamin D was only measured at baseline when the first dose of HPV virus was administered which avoids the potential variability that may have resulted from collecting vitamin D samples at 2 different times since dose 1, i.e., 6 and 12 months. However this method did not allow for determining the effect of vitamin D levels at the time that the subsequent doses were administered. Finally, vitamin D levels were not used in randomization; therefore, causality cannot be established, although temporal relationships were present.
Conclusion
The relationship of vitamin D to the immune system's response to vaccine antigens is complexCitation13 and should be further investigated in larger datasets across different vaccines and across various infectious diseases.Citation7 In addition, host-response and genetic translation pathways should be elucidated.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank the laboratory of Ted Ross, PhD for specimen handling and the Special Chemistry and Reference Laboratory of UPMC for vitamin D assays.
Funding
The authors and this work (NCT01184079) were supported in part by a research grant from the Investigator-Initiated Studies Program of Merck & Co., Inc., manufacturer of Gardasil® human papillomavirus vaccine. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck & Co., Inc. The project described was also supported by the National Institutes of Health through Grant Numbers UL1 RR024153 and UL1TR000005.
References
- Cantorna MT. Vitamin D and its role in immunology: multiple sclerosis, and inflammatory bowel disease. Prog Biophys Mol Biol Sep 2006; 92(1):60-4; PMID:16563470; http://dx.doi.org/10.1016/j.pbiomolbio.2006.02.020
- Munger KL, Kochert K, Simon KC, Kappos L, Polman CH, Freedman MS, Hartung HP, Miller DH, Montalbán X, Edan G, et al. Molecular mechanism underlying the impact of vitamin D on disease activity of MS. Ann Clin Transl Neurol Aug 2014; 1(8):605-17; PMID:25285313; http://dx.doi.org/10.1002/acn3.91
- Sundaram ME, Talbot HK, Zhu Y, Griffin MR, Spencer S, Shay DK, Coleman LA. Vitamin D is not associated with serologic response to influenza vaccine in adults over 50 years old. Vaccine 2013; 31(16):2057-61; PMID:23453766; http://dx.doi.org/10.1016/j.vaccine.2013.02.028
- Science M, Maguire JL, Russell ML, Smieja M, Walter SD, Loeb M. Serum 25-hydroxyvitamin D level and influenza vaccine immunogenicity in children and adolescents. PloS One 2014; 9(1):e83553; PMID:24427274; http://dx.doi.org/10.1371/journal.pone.0083553
- Zitt E, Sprenger-Mahr H, Knoll F, Neyer U, Lhotta K. Vitamin D deficiency is associated with poor response to active hepatitis B immunisation in patients with chronic kidney. Vaccine Jan 20 2012; 30(5):931-5; PMID:22142584
- Heine G, Drozdenko G, Lahl A, Unterwalder N, Mei H, Volk HD, Dörner T, Radbruch A, Worm M. Efficient tetanus toxoid immunization on vitamin D supplementation. Eur J Clin Nutr Mar 2011; 65(3):329-34; PMID:21224870; http://dx.doi.org/10.1038/ejcn.2010.276
- Yamshchikov AV, Desai NS, Blumberg HM, Ziegler TR, Tangpricha V. Vitamin D for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocr Pract Jul–Aug 2009; 15(5):438-49; PMID:19491064; http://dx.doi.org/10.4158/EP09101.ORR
- Lin CJ, Zimmerman RK, Nowalk MP, Huang HH, Raviotta JM. Randomized controlled trial of two dosing schedules for human papillomavirus vaccination among college age males. Vaccine Feb 3 2014; 32(6):693-9; PMID:24342252; http://dx.doi.org/10.1016/j.vaccine.2013.11.098
- Dias D, Van Doren J, Schlottmann S, Kelly S, Puchalski D, Ruiz W, Boerckel P, Kessler J, Antonello JM, Green T, et al. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol Aug 2005; 12(8):959-69; PMID:16085914
- Holick MF, Binkley NC, Bischoff-Ferrari HAs, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab Jul 2011; 96(7):1911-30; PMID:21646368; http://dx.doi.org/10.1210/jc.2011-0385
- Hillman RJ, Giuliano AR, Palefsky JM, Goldstone S, Moreira ED Jr, Vardas E, Aranda C, Jessen H, Ferris DG, Coutlee F, et al. Immunogenicity of the quadrivalent human papillomavirus (type 6/11/16/18) vaccine in males 16 to 26 years old. Clin Vaccine Immunol Feb 2012; 19(2):261-7; PMID:22155768; http://dx.doi.org/10.1128/CVI.05208-11
- Linder N, Abudi Y, Abdalla W, Badir M, Amitai Y, Samuels J, Mendelson E, Levy I. Effect of season of inoculation on immune response to rubella vaccine in children. J Trop Pediatr Aug 2011; 57(4):299-302; PMID:19889749; http://dx.doi.org/10.1093/tropej/fmp104
- Lang PO, Aspinall R. Can we translate vitamin D immunomodulating effect on innate and adaptive immunity to vaccine response? Nutrients Mar 2015; 7(3):2044-60; PMID:25803545; http://dx.doi.org/10.3390/nu7032044
- Schiller JT, Lowy DR. Immunogenicity testing in human papillomavirus virus-like-particle vaccine trials. J Infect Dis Jul 2009; 200(2):166-71; PMID:19519255; http://dx.doi.org/10.1086/599988
- Brown D, Muller M, Sehr P, Pawlita M, Seitz H, Rubio I, Antonello J, Radley D, Roberts C, Saah A. Concordance assessment between a multiplexed competitive Luminex immunoassay, a multiplexed IgG Luminex immunoassay, and a pseudovirion-based neutralization assay for detection of human papillomavirus types 16 and 18. Vaccine Oct 7 2014; 32(44):5880-7; PMID:25148777; http://dx.doi.org/10.1016/j.vaccine.2014.08.004
- Zimmerman RK, Nowalk MP, Lin CJ, Fox DE, Ko FS, Wettick E, Cost G, Hand L, Hayes J, Michaels M. Randomized trial of an alternate human papillomavirus vaccine administration schedule in college-aged women. J Womens Health (Larchmt) Aug 2010; 19(8):1441-7; PMID:20629576; http://dx.doi.org/10.1089/jwh.2009.1753
