Abstract
Evaluate safety and immunization coverage of a new kind of recombinant Hepatitis B vaccine (HepB) in Ningbo city, China. Two groups were carried out in 2 of 11 randomly selected countries in Ningbo in 2009. All of the infants born from July 1 to December 31, 2009 were enrolled as subjects and received 3 doses of HepB at 0, 1, 6 month. Control group (N = 3452) received current HepB derived from Saccharomyces Cerevisiae Yeast (HepB made by recombinant DNA techniques in Saccharomyces Cerevisiae Yeast, HepB-SCY; 5 μg/0.5 ml per dose) and experimental group (N = 5104) received the new kind of HepB derived from Hansenula polymorpha Yeast (HepB made by recombinant DNA techniques in Hansenula polymorpha Yeast, HepB-HPY; 10 μg/0.5 ml per dose). 3-dose and timely birth dose (TBD) coverage were available and compared between 2 groups. Standard structured questionnaires were applied to record information from parents and hospitals for selecting determinants of coverage. The data were analyzed using stepwise multiple logistic regression models. After each dose, HepB-related adverse events (AEs) and recta-temperature were recorded for 7 days. 3-dose coverage in control group (89.98%) was higher than that in experimental group (χ2 = 575.1173, P < 0.0001). TBD coverage in control and experimental group were 98.41% and 98.53%, respectively. No statistically significant difference in TBD coverage was found between 2 groups (χ2 = 0.0623, P = 0.8029). A total of 9 local AEs were reported, 4 for control group and 5 for experimental group. The percentages of subjects reporting AEs were similar across the 2 vaccination groups. No serious or immediate reactions were found in this study. From logistic models, receiving 10 μg vaccine (odds ratio [OR]:0.38; 95% confidence interval [95%CI]: 0.34–0.44) and mother migrating from other cities (OR: 0.45; 95%CI: 0.42–0.47) were the determinants for non-acceptance of 3 doses of HepB; infants born from low grade hospitals and native mothers contributed to administrate the TBD.
Abbreviations
| HepB | = | Hepatitis B vaccine |
| TBD | = | timely birth dose |
| Aes | = | HepB-related adverse events |
| OR | = | odds ratio |
| HBV | = | Hepatitis b virus |
| HCC | = | hepatocellular carcinoma |
| HbsAg | = | hepatitis b surface antigen |
| HBIG | = | Hepatitis B immunoglobulin |
| CDC | = | center for disease control and prevention |
| anti-HBs | = | hepatitis B surface antibody |
| anti-HBc | = | hepatitis B core antibody |
| HbeAg | = | hepatitis B e antigen |
| anti-Hbe | = | hepatitis B e antibody |
| EIIS | = | Electronic Immunization Information System |
| 95%CI | = | 95% Confidence Interval |
Introduction
Hepatitis b virus (HBV) infection is a world health problem, approximately 30% of the world's population presents serological evidence of current or past infection,Citation1 which contributes to chronic hepatitis, cirrhosis and hepatocellular carcinoma (HCC), with roughly 1 million deaths each year.Citation2,3 Universal hepatitis B immunization, recommended to integrated into the Expanded Program on Immunization by World Health Organization in 1992, is confirmed the most effective method to protect people from HBV infection, HBV related complications and death as well.Citation4-6 As of 2012, more than 180 countries had introduced hepatitis B vaccine (HepB)into their national immunization programs.Citation7
Many studies showed that the age at acquisition of hepatitis B infection influenced the risk of chronicity and the natural history of disease.Citation8-11 Vertical transmission from hepatitis B surface antigen (HBsAg) positive mothers to unimmunized neonates in early childhood was the major routes of infection in China.Citation12-14 Thus, effective, extensive as well as timely vaccination is critical in implementing the universal HepB program. In 1992, HepB immunization was regarded as a part of Children Immunization Programs by the Chinese Health Ministry, which encouraged infants, especially those whose mothers with the hepatitis B virus, to receive 3 doses of HepB at 0, 1, 6 month or 0, 1, 2 month with self-paid.Citation15 However, a decade later, the policy was adjusted to free-charge. During the 10 years, HepB coverage speedily increases from less than 40% to more than 90%.Citation16,17In China, plasma-derived hepatitis B vaccine was used from 1992 to 1996 and then shifted to recombinant vaccine with 5μg until 2008.Since 2009, a new kind of HepB with 10μg derived from Hansenula polymorpha Yeast was firstly applied in China, however, we have known little about the epidemical parameters in the population of infants. Therefore, the aim of this study was to fill the gap on safety, coverage and efficacy in the population of infants vaccinated with 10 μg-HepB. Because of not being finished the whole research, in this manuscript, we only shown the safety and coverage data and the total efficacy result would be detailed in other papers in the future. The safety data was checked after the research in 2010 and the results were taken in practice, however, we did not report it immediately and went on to take the following research. In 2015, we recognized the value of those data and found that no one similar manuscript was reported about Chinese newborns, so we re-checked the result and reported it in time.
Results
Study subjects
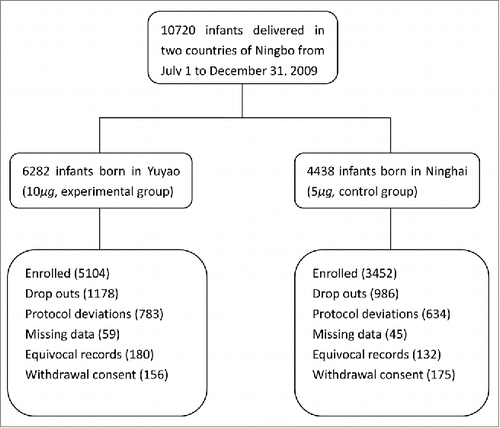
Among 10720 new babies born in Yuyao and Ninghai between the third and fourth seasons of 2009, a total of 8556 subjects were enrolled at the time of this study. The remaining infants were excluded because of withdrawal consent, missing data, equivocal records or protocol deviations (). All the infants who responded to the study invitation comprised 4617 boys and 3939 girls. And 657 of them were born to HBsAg-positive mothers, with the HBsAg-positive rate of 7.68% [657/8556, 95% Confidence Interval (95%CI): 7.11%˜8.24%]. Subjects across 2 groups were similar with respect to gender, but statistically significant difference in mother's HBsAg status ().
Table 1. Statistical analysis for the association between sample characteristics and immunization with 3 doses of HepB/timely birth dose HepB within 24hours after birth
Table 2. Distribution of baby gender, mother's HBsAg status, immunization and infants followed at first, second and third doses of HepB between control and case groups
Vaccination coverage
A total of 6557 infants finished the entire 3-dose series, with the overall 3-dose HepB coverage of 76.64% (6557 of 8556, 95%CI: 75.74%˜77.53%); this means that 1999 subjects missed at least one dose of HepB. 3-dose HepB coverage was much higher in infants who received 5 μg-HepB than in those who received 10 μg-HepB: 89.98% (3106/3452, 95%CI: 88.98%˜90.98%) vs. 67.61% (3451/5104, 95%CI: 66.33%˜68.90%) (χ2 = 575.1173, P < 0.0001). All subjects received the birth dose of HepB, and 8428 of them was timely with the overall TBD coverage of 98.50% (8428 of 8556; 95% CI: 98.25%˜98.76%). Statistically significant difference in TBD coverage was not found between control and experimental groups: 98.41% (3397/3452, 95%CI: 97.99%˜98.82%) vs.98.53% (5029/5104, 95%CI: 98.20%–98.86%) (χ2 = 0.0623, P = 0.8029).
Logistic regression analyses were performed to estimate the odds ratios and 95% CI of potential impacts associated with acceptance HepB. The results were presented in . Model 1 presents that factors significantly associated with HepB coverage were ‘vaccine dose’ and ‘mother's city’. The result of model 2 indicates that timely birth dose coverage of HepB was higher among participants who were born to low level hospital grade and whose mothers living in Ningbo.
Table 3. Multiple stepwise logistic regression derived odds ratio (and 95% Confidence Intervals) for the model 1 (determinants for 3 doses of HepB) and model 2 (determinants for timely birth dose HepB within 24 hours after birth)
In this work, we also analyzed the application of HBIG for infants born to HBsAg-positive mothers. Of the 657 infants, 544 had received self-paid HBIG by parental choice and consent, with the HBIG coverage of 82.80% (544/657, 95%CI: 79.91%˜85.69%); it was much higher in control group than that in experimental group: 94.18% (275/292; 95%CI: 91.49%˜96.86%) vs.73.70% (269/365; 95%CI: 69.18%˜78.22%) (χ2 = 47.7750, P < .0001).
Safety
Out of 23046 doses administrated, approximately 2.25% (518/23046) of them was lost to follow-up with following reasons: missing communication or moving away, refusing to record or wrong record, receiving other vaccines within 7 days after HepB vaccination. The distribution of immunization and follow-up at different doses between control and case groups were both statistically significant ().
None of participants dropped out of the study, nor were any immediate relations noted after each HepB dose. Among 22528 doses followed, 9 AEs were verified to be relation with HepB by physicians, with the AEs rate of 39.95 per 100,000 doses (9/22528). No statistically significant was found between 5 μg and 10 μg groups: 40.51 per 100,000 doses (4/9875) vs.39.52 per 100,000 doses (5/12653) (P = 1.00, fisher exact test).
9 reports of AEs came from 8 infants, appearing within the first 24 hours after vaccination. 7 of them resolved spontaneously over the next 2 days; however, the remaining one with diarrhea received medical care for 5 days. One AE was not strikingly ascertained the relationship with HepB, for receiving one dose of HBIG at the same day of HepB ().
Table 4. Number of subjects with at least one type of reaction
Discussion
Although many studies researching safety and coverage of HepB-derived from Saccharomyces Cerevisiae Yeast or hamster ovary cell have been reported,Citation15,18,19 this is the first time to perform the safety of 10 μg HepB-HPY (HepB derived from Hansenula polymorpha Yeast) in Chinese newborns. Sun reported the efficacy of HepB-HPY in the population aged greater than or equal to 16 years in China in 2010, which demonstrated that the safety of 10 μg HepB-HPY had been supported in adults.Citation20 However, as we know, the response to the same kind of HepB in adults is remarkable different with newborns, we do not copy the safety results with HepB-HPY in adults directly to the newborns without any epidemical research. According to the whole study designed prior, safety and coverage researches are a part of it; so, in this article, we only solve 2 questions: how is the safety of 10ug-HepB-HPY administrated to newborns and what is the coverage about the new dosage of HepB and related factors. Immune response results are very important. In our research, the positive rates of hepatitis B surface antibody (anti-HBs) are 97.86%, 92.09% and 89.45% at 1 month, 1 year and 3 years after 3-dose series, respectively (unpublished data). More specifically and extensively data will not be published until the whole study finished with the year of 2016.
In this field investigation, TBD coverage of 10 μg-HepB reaches to 98.53%, which is consistent with previous observations demonstrating that TBD coverage fluctuates from 81.7% to 99.4% in eastern provinces of China.Citation21 There, is no meaningful difference between 5 μg-HepB and 10-μg HepB groups with respect to TBD coverage; however, 3-dose coverage is remarkably discrepant. we find that the coverage of 3-doses-10 μg-HepB (67.61%) was much lower than that of 5 μg-HepB (89.98%); obviously, it is also lower than other reports.Citation16,22,23 From the logistic regressions, ‘vaccine dose’ and ‘mother's city’ are 2 factors affecting HepB coverage displayed by model 1, meaning that, with the same migration status, the higher HepB dose are provided, the less probability of being administrated the whole vaccination schedule are. For the further interpretation, another survey with the question ‘why your baby not getting all the HepB’ was carried out on the basis of the original investigation by telephone. 10% mothers per group were chosen randomly, of the population whose infants did not finish 3 doses of HepB () and the statistical result is significant (χ2 = 22.389, P < .0001). We re-checked the immunization Information of infants in Ninghai who missed at least one dose HepB-HPY on the standard vaccination schedule from EIIS and found that 86.93% infants achieved the 3-dose HepB (1437/1653). Among 1437 infants, 103 received 10 μg HepB-HPY, 1236 received HepB imported from foreign manufacturer with self-paid and the remaining 98 still received 5 μg HepB-SCY. Of 1653 infants, 1143 born in native families with the 3-dose coverage of 90.55% (1035/1143) and it was much higher than that in non-native families: 78.82% (402/510) (χ2 = 40.3890, P < .0001). Results summarized from all above, we could achieve 2 important implications. First, public education for immunization is necessary. ‘Knowing little about the new kind of HepB’ is the predominant reason for preventing parents from vaccinating for their babies in case group. Most of them thought that it was adventurous for infants to vaccinate a new kind of HepB launched in Ningbo in the first year; on the contrast, the foreign vaccines were popular. Although adequate immunization advertisement and healthy speech, we thought, had been done, in fact, it was far away from sufficiency. Second, vaccination for infants whose families move frequently is still a non-neglectful problem, which is also described in Hu's survey. Citation24 Factors such as mothers' age, mothers' education presented in other surveys were not found in our study. Citation24,25 Those disparity may be due to the differences in sample characteristics, cultural and socioeconomic environments between China and other countries.
Table 5. Reasons for mothers in refusing the 3-dose HepB
From Model 2, timely vaccination of first-dose HepB is mostly relation with the co-effect of hospital and family. ‘Lower grade hospitals’ and ‘mothers'city’ contribute to fulfill the first-dose HepB for infants within 24 hours after their birth (). ‘Hospital grade’ could be regarded as a phenomenon of ‘hospital-chosen’. Namely, senior hospitals are more prone to draw the high-risk puerperant, whose infants may have much chance to owning adverse symptoms so that they got the first-dose HepB beyond 24 hours. On the other hand, mothers who migrate from other cities, especially from cities outside of Zhejiang province, are a population with a high percentage of unhealthy puerperant living in such a situation in China: low household income, poor education, as well as terrible medical treatment. Smith's research has shown that parents are prone to intentionally delay vaccine administration for their babies when they own a bad living situation.Citation26 In the pre-survey, we found that infants born at home were rarely in Ningbo; what's more, ‘home birth’ has been confirmed as one of the important determinants affecting TBD coverage.Citation27 So, based on the reasons above, we exclude those infants in this study.
The vaccines are safety and tolerated. We find that AEs due to HepB vaccination are notified to occur rarely and AEs rate in 10 μg-HepB group is not higher than that in the other group. No serious AEs are attributed to HepB, whatever 10 μg-HepB or 5 μg-HepB, which is similar to those types of adverse events illustrated by Kojouharova et al.Citation18 Our survey showed that AEs rate of infants in 10 μg-HepB vaccination was lower than Bulgarian and India,Citation18,19,28 which could be considered the difference in methods, technology of vaccine-manufacture and sample size. A group of data about HepB AEs summarized through a large database of reported adverse events following immunization (AEFI) during 10 years in Rondonia (a state of Brazil) is nearly to us.Citation29
Practical significance in this study can be regarded as the dominant aim. 10 μg HepB-HPY as a new vaccine for infants was firstly applied in Ningbo, China and also as a unique kind of HepB is supported by government which would be administrated to about 80,000 newborns every year. Thus, the safety and coverage of the vaccine is necessary to be verified in the target population. Sun' report with a small sample researched the efficacy in the whole population, not specific to infants.Citation20 Secondly, our study presents a larger sample size than other reports.Citation30,31 As we known, the larger sample size we have, the more scientific results we get. Thirdly, in method, we take measures to avoid AEs restricted to target vaccines being confused by other vaccines, which was not mentioned in other papers.Citation18,19 However the shortcomings are existent in the present study. Although the similar articles about Chinese infants are not searched, the timeliness of our manuscript is limited with the usage of safety and coverage results in practice since 2010. Moreover, this time we do not report entire efficacy results of HepB-HPY, which maybe decrease the meaning of this manuscript. What's more, the factors related coverage detected in this study is common with other vaccines and not restricted to the HepB-HPY, so the novelty is weakened. However, on the other hand, this is a signal that the thinking of our study design is correct and the 2 factors are dominant and general for vaccines coverage. In the future, the more detailed and multicenter studies are needed. Finally, BCG (Bacillus Calmett-Guérin), DTP (diphtheria-tetanus-pertussis) and polio vaccines are necessary for infants aged from birth to 6 months in China. Although a lag of 14 ‘clean-up’ days is taken, bias are inescapable and AEs result from HepB only cannot be distinguished so clearly. For infants who successfully catch one dose HepB, 7-day's surveillance for AEs is not enough, which might ignore the long-term potential AEs and overestimate the safety of HepB. In process of this study, we were cautious to judge vaccine-related adverse events and, consequently, the false-negative is possible.
The benefit and necessity of HBIG for children born to HBsAg-carrier mothers has been an issue of controversy for a long time.Citation32,33 HBIG immunizations, in China, for infants born to HBsAg-positive mothers is not obligatory but chosen by parental will. Because of inadequate information and unmatched questionnaire in this study, determinants of HBIG are not analyzed. But, according to a recent survey carried out in the same city, ‘mothers' city’ is the important one.Citation34
Vaccines as a preventable strategy against infectious diseases for newborns remain the principle tool. Vaccination, in today, is not only the issue in immunology, but the event in society. At present, our data shows that the safety of 10 μg-HepB is comparable to 5 μg-HepB; however 3-dose coverage of 10 μg-HepB is inadequate. ‘Lack of enough knowledge about the new vaccine for parents’ and ‘without effective administration for infants from migrant mothers’ are 2 major reasons leading to the low HepB coverage; hence, a mass awareness program for the public and several appropriate policies being put into effect in administrating the population of migration-mothers are needed.
Material and methods
Field background
Ningbo, the second biggest city in Zhejiang province, is located in South-eastern China. As one of the national HBV serological investigation fields, 2 large-scale investigations had been conducted here in 1992 and 2002, respectively. According to the results of the whole nation investigations, during 1992–2002, HBsAg carrier rate decreased by 2.57% (from 9.75% to 7.18%) in the population aged less than 60 years old in China.Citation34 Of note is that the HBsAg prevalence reduced remarkably among children aged < 15 years, especially among those aged < 5 years in whom the rate was less than 1%.Citation35
Study design and population
This research was a prospective field trial, and 2 countries (Yuyao and Ninghai) were randomly selected from 11 countries of Ningbo as the research sites. The treatment assignment applying a randomized schedule (using the random number table) as following: Infants born in Yuyao regard as the experimental group, received HepB-HPY (HepB made by recombinant DNA techniques in Hansenula polymorpha Yeast and produced in China, HepB-HPY; 10 μg/0.5ml per dose), the new kind vaccine; and Infants born in Ninghai regard as the control group, received HepB-SCY (HepB made by recombinant DNA techniques in Saccharomyces Cerevisiae Yeast and produced in China, HepB-SCY; 5 μg/0.5ml per dose), the current vaccine. All subjects received 3 doses vaccines with free at 0, 1, and 6 months of age by intramuscular injection in the upper arm deltoid, and one dose of Hepatitis B immunoglobulin (HBIG) (100IU/0.5ml or 200IU/0.5ml) was given to infants of HBsAg-positive mothers according to parental need with self-paid within 24 hours after birth in the other arm. Test methods of HBV-markers in different hospitals checked prior to this study were same.
This study was designed to have 85% power to detect AEs rate of 0.4899Citation36 and 0.4516Citation36 in infants with 5 ug and 10 ug HepB, respectively, with a 2-sided α of 0.05. The smallest sample size was 3049 infants per group. Normally, in half a year, the amount of newborns in Yuyao country and Ninghai country were approximately 5500 and 4000, respectively. So, in this research, considering having sufficient funds and pursuing more objective results, we invited all infants born in hospitals with obstetrical department from July 1 to December 31, 2009 in Yuyao country and Ninghai country to join this study. However, the followings were excluded: infants born at home, participating in other trials during the course of this study or whose parents/legal guardians refusing to sign the informed consent.
Vaccination coverage
Infants' HepB immunization information including dates of each dose of HBIG and vaccines was transcribed from Electronic Immunization Information System (EIIS) administrated by Ningbo center for disease control and prevention (CDC) and was confirmed from at least 1 of 2 sources: hospital records and official immunization booklets held by parents. Influencing factors comprised birth information of infants and sociodemographic characters of mothers; the former was abstracted from hospital records including baby gender, mother's HBsAg status, pregnant weeks, baby weight, baby Apgar score, delivery way, hospital grade and hospital classification; the latter was obtained by interviewing parents with a standardized questionnaire at the second time of HepB vaccination including vaccine dose, mother's city, mother's age, mother's education years and household income per year. Two doctors were designated as the researcher in every clinic, one for information recording, and the other for information checking. If any missing data appeared, going back to interview again to fill up the blank information. Parents whose infants did not fulfill the 3-dose series before the deadline would be informed by short message, telephone or interview. Several vaccination rates were defined prior:
3-dose HepB coverage: the percentage of infants received 3 doses of HepB at 0, 1, and 6 months of age after born.
Timely birth dose (TBD) coverage: the percentage of infants with the administration of the first dose of HepB no later than 24 hours after birth.
HBIG coverage: the percentage of infants born to HBsAg-positive mothers with the administration of HBIG.
Safety
All subjects were forbidden leaving vaccination clinics within 30 min after each dose for observing appearance of immediate reactions. Adverse events (AEs) card with detail descriptions and strict definitions of all kinds of symptoms was used to record any local and systemic AEs daily for a period of 7 days following each vaccination by Parents/legal guardians. Additionally, study staff actively contacted with all parents/legal guardians by phone daily to ask whether AEs occurred or not on that day. Physician visits were necessary, if the AEs reports were positive, to make sure the relationship between occurrence of AEs and HepB vaccination. Body temperature was measured by parents/legal guardians, with fever defined as recta-temperature ≥38°C. In order to illuminate AEs only deriving from HepB vaccination and avoid being confused by other vaccines, an interval of 7 days before and after each HepB vaccination was held (BCG and HBIG was still given on the birth day).
Ethics
This study was reviewed and approved by the ethics committee of Ningbo CDC. The objectives and procedure of the study and the potential risk and benefits of participation were given during recruitment of study subjects samples. Written informed consents were obtained from all parents/legal guardians whose babies participating in the survey.
Data analysis
A database was established using Epidata3.1, and statistical analysis was performed by SAS9.1 and Excel 2007. Continuous data were transformed into ordinal data which were compared using Mantel–Haenszel test. Categorical data and outcome were contrasted between 2 groups using Chi-square test or Fisher exact test. The determinants on acceptance of 3 doses of HepB and first dose of HepB within 24 hours after birth were analyzed with the aid of 2 multiple stepwise logistic regression (selection=backward) named model 1 and model 2, respectively. Gender and variables with a P-value of <0.2 on univariate analysis were entered into the models (). The equation for sample size referred publicationCitation37 written by Chow, et al. A P-value of ≤0.05 was considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are thankful to all the infants and parents or legal guardians who participated in this study, to the physicians as well as nurses working at clinics and to the doctors in Ningbo, Yuyao and Ninghai CDC.
Funding
This project was funded by grant number 2012ZX10002001 from Chinese Ministry of Science and Technology Program for Important Infectious Diseases Control and Prevention and by grant number TQGB20120046 from TianQing Liver Disease Research Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Trepo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet 2014;9938:2053-63; http://dx.doi.org/10.1016/S0140-6736(14)60220-8
- Ganem D, Prince AM. Hepatitis B virus infection–natural history and clinical consequences. N Engl J Med 2004; 350:1118-29; PMID:15014185; http://dx.doi.org/10.1056/NEJMra031087
- Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infect Dis 2002; 2:395-403; PMID:12127351; http://dx.doi.org/10.1016/S1473-3099(02)00315-8
- Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Shau WY, Liang DC Chen DC. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med 1997;336:1855-9; PMID:9197213; http://dx.doi.org/10.1056/NEJM199706263362602
- Kao JH, Hsu HM, Shau WY, Chang MH, Chen DS. Universal hepatitis B vaccination and the decreased mortality from fulminant hepatitis in infants in Taiwan. J Pediatr 2001; 139:349-52; PMID:11562612; http://dx.doi.org/10.1067/mpd.2001.116277
- Hung HF, Chen TH. Probabilistic cost-effectiveness analysis of the long-term effect of universal hepatitis B vaccination: an experience from Taiwan with high hepatitis B virus infection and Hepatitis B e Antigen positive prevalence. Vaccine 2009;27:6770-6; PMID:19735755; http://dx.doi.org/10.1016/j.vaccine.2009.08.082
- World Health Organization. Hepatitis B.; Updated 28 2014 July 28;Cited 2014 Oct 19. Available from: www.who.int/mediacentre/factsheets/fs204/en/.
- Ando T, Sugiyama K, Goto K, Miyake Y, Li R, Kawabe Y, Wada Y. Age at time of hepatitis Be antibody seroconversion in childhood chronic hepatitis B infection and mutant viral strain detection rates. J Pediatr Gastroenterol Nutr 1999;29:583-7; PMID:10554127; http://dx.doi.org/10.1097/00005176-199911000-00020
- Shimakawa Y, Yan HJ, Tsuchiya N, Bottomley C, Hall AJ. Association of early age at establishment of chronic hepatitis B infection with persistent viral replication, liver cirrhosis and hepatocellular carcinoma: a systematic review. PloS one 2013; 8:e69430; PMID:23894479; http://dx.doi.org/10.1371/journal.pone.0069430
- Hyams KC. Risks of chronicity following acute hepatitis B virus infection: a review. Clin Infect Dis 1995; 20:992-1000; PMID:7795104; http://dx.doi.org/10.1093/clinids/20.4.992
- Hsieh CC, Tzonou A, Zavitsanos X, Kaklamani E, Lan SJ, Trichopoulos D. Age at first establishment of chronic hepatitis B virus infection and hepatocellular carcinoma risk. A birth order study. Am J Epidemiol 1992; 136:1115-21; PMID:1334366
- Yao JL. Perinatal transmission of hepatitis B virus infection and vaccination in China. Gut 38 Suppl 1996;2:S37-8; http://dx.doi.org/10.1136/gut.38.Suppl_2.S37
- Stevens CE, Beasley RP, Tsui J, Lee WC. Vertical transmission of hepatitis B antigen in Taiwan. N Engl J Med 1975;292:771-4; PMID:1113797; http://dx.doi.org/10.1056/NEJM197504102921503
- Cui Y, Jia J. Update on epidemiology of hepatitis B and C in China. J Gastroenterol Hepatol 2013;1:28 Suppl 7-10; http://dx.doi.org/10.1111/jgh.12220
- Zhang Y, Ma JC, Qi SX, Wang F, Zhao C, Bi SL. Effectiveness of a Chinese hamster ovary cell derived hepatitis B vaccine in Chinese rural communities. Vaccine 2011;29:3905-8; PMID:21457729; http://dx.doi.org/10.1016/j.vaccine.2011.03.030
- Cui F, Liang X, Gong X, Chen Y, Wang F, Zheng H, Wu Z, Miao N, Hadler SC Hutin YJ, et al. Preventing hepatitis B though universal vaccination: reduction of inequalities through the GAVI China project. Vaccine 2013;231 Suppl 9:J29-35; http://dx.doi.org/10.1016/j.vaccine.2012.07.048
- Hadler SC, Fuqiang C, Averhoff F, Taylor T, Wang FZ, Li L, Liang XF Yang WZ. The impact of hepatitis B vaccine in China and in the China GAVI Project. Vaccine 2013;31 Suppl 9:J66-72; PMID:24331023; http://dx.doi.org/10.1016/j.vaccine.2013.03.043
- Kojouharova M, Teoharov P, Bahtchevanova T, Maeva I, Eginlian A. Safety and Immunogenicity of a Yeast-Derived Recombinant Hepatitis B Vaccine in Bulgarian Newborns. Infection 2001; 29:342-4; PMID:11787837; http://dx.doi.org/10.1007/s15010-001-1150-6
- Velu V, Nandakumar S, Shanmugam S, Jadhav SS, Kulkarni PS. Comparison of three different recombinant hepatitis B vaccines: GeneVac-B, Engerix B and Shanvac B in high risk infants born to HBsAg positive mothers in India. World J Gastroenterol 2007; 13:3084-9; PMID:17589924
- Sun J, Han Y, Wang Y, Wang P, Jia XY, et al. Serum antibody response to different doses of hepatitis B vaccine made by recombinant DNA techniques in yeast and Hansenula polymorpha yeast. Chin Prev Med 2010;16:140-2
- Cui F, Luo H, Wang F, Zheng H, Gong X, Chen Y, Wu Z, Miao N, Kane M, Hennessey K, et al. Evaluation of policies and practices to prevent mother to child transmission of hepatitis B virus in China: results from China GAVI project final evaluation. Vaccine 2013; 31 Suppl 9:J36-42; PMID:24331019; http://dx.doi.org/10.1016/j.vaccine.2012.11.061
- Esteghamati A, Keshtkar AA, Nadjafi L, Gouya MM, Salaramoliandel M, Roshandel G, Yaghini F. Hepatitis B vaccination coverage among Iranian children aged 15–26 months in 2006. East Mediterr Health J 2011;17:93-100
- Denis F, Cohen R, Martinot A, Stahl JP, Lery T, Le Danvic M, Gaudelus J. Evolution of hepatitis B vaccine coverage rates in France between 2008 and 2011. Med Mal Infect 2013; 43:272-8; PMID:23876204; http://dx.doi.org/10.1016/j.medmal.2013.06.016
- Hu Y, Chen E, Li Q, Chen Y, Qi X. Immunization coverage and its determinants among children born in 2008–2009 by questionnaire survey in Zhejiang, China. AsiaPac J Public Health 2011; 27(2):NP1132-43
- Basaleem HO, Al-Sakkaf KA, Shamsuddin K. Immunization coverage and its determinants among children 12–23 months of age in Aden, Yemen. Saudi Med J 2010;31:1221-6; PMID:21063652
- Smith PJ, Humiston SG, Parnell T, Vannice KS, Salmon DA. The association between intentional delay of vaccine administration and timely childhood vaccination coverage. Public Health Rep 2010;125:534-41; PMID:20597453
- Kane MA, Hadler SC, Lee L, Shapiro CN, Cui F, Wang X, Kumar R. The inception, achievements, and implications of the China GAVI Alliance Project on Hepatitis B Immunization. Vaccine 31 Suppl 2013; 9:J15-20; http://dx.doi.org/10.1016/j.vaccine.2013.03.045
- Sapru A, Kulkarni PS, Bhave S, Bavdekar A, Naik SS. Immunogenicity and reactogenicity of two recombinant hepatitis B vaccines in small infants: a randomized, double-blind comparative study. J Trop Pediatr 2007;53:303-7; PMID:17478542; http://dx.doi.org/10.1093/tropej/fmm016
- Cunha MP, Dorea JG, Marques RC, Leao RS. Vaccine adverse events reported during the first ten years (1998–2008) after introduction in the state of Rondonia, Brazil. BioMed Res Int 2013; 853083; PMID:23509790
- Carniel EF, Morcillo AM, Blotta MH, Da Silva MT, Mazzola TN, Antonio MA, Zanolli ML, Netto AA, Higashi HG, Raw I, et al. Immunogenicity and safety of combined intradermal recombinant Hepatitis B with BCG vaccines at birth. Vaccine. 2008; 30:647-52; http://dx.doi.org/10.1016/j.vaccine.2007.11.048
- Gupta I, Ratho RK. Immunogenicity and safety of two schedules of Hepatitis B vaccination during pregnancy. J Obstet Gynaecol Res 2003; 29:84-6; PMID:12755527; http://dx.doi.org/10.1046/j.1341-8076.2002.00076.x
- Boxall EH, A Sira J, El-Shuhkri N, Kelly DA. Long-term persistence of immunity to hepatitis B after vaccination during infancy in a country where endemicity is low. J Infect Dis 2004;190:1264-1269; PMID:15346336; http://dx.doi.org/10.1086/423818
- Yang YJ, Liu CC, Chen TJ, Lee MF, Chen SH, Shih H, Chang MH. Role of hepatitis B immunoglobulin in infants born to hepatitis B e antigen-negative carrier mothers in Taiwan. Pediatric Infect Dis J 2003;22:584-8; PMID:12867831
- Sijia Y, Hongju D. Survey on the coverage of hepatitis B immunoglobulin adminstration among infants born to hepatitis B surface antigen positive mother in Ningbo. Chin Prev Med 2013;14:413-6
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Lui J, Gong X, Chen Y, et al. Epidemiological serosurvey of hepatitis B in China–declining HBV prevalence due to hepatitis B vaccination. Vaccine 2009;27:6550-7; PMID:19729084; http://dx.doi.org/10.1016/j.vaccine.2009.08.048
- Ying Zeng, Jie Li, Xue-fang Song, Xin-hong Pan, Ju-yi Zhang, et al. Safety evaiuation of 10µg/Dose hepatitis B vaccine made by recombinant deoxyribonucleic acid techniques in saccharomyces cerevisciae yeast among newborns. Chinese Journal of Vaccine and Immunization 2011;17:490-493, 515
- Chow SC, Shao J, Wang H. Sample Size Calculations in Clinical Research. Marcel Dekker. New York. 2003