?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Pneumococcal disease causes large morbidity, mortality and health care utilization and medical and non-medical costs, which can all be reduced by effective infant universal routine immunization programs with pneumococcal conjugate vaccines (PCV). We evaluated the clinical and economic benefits of such programs with either 10- or 13-valent PCVs in Malaysia and Hong Kong by using an age-stratified Markov cohort model with many country-specific inputs. The incremental cost per quality-adjusted life year (QALY) was calculated to compare PCV10 or PCV13 against no vaccination and PCV13 against PCV10 over a 10-year birth cohort's vaccination. Both payer and societal perspectives were used. PCV13 had better public health and economic outcomes than a PCV10 program across all scenarios considered. For example, in the base case scenario in Malaysia, PCV13 would reduce more cases of IPD (+2,296), pneumonia (+705,281), and acute otitis media (+376,967) and save more lives (+6,122) than PCV10. Similarly, in Hong Kong, PCV13 would reduce more cases of IPD cases (+529), pneumonia (+172,185), and acute otitis media (+37,727) and save more lives (+2,688) than PCV10. During the same time horizon, PCV13 would gain over 74,000 and 21,600 additional QALYs than PCV10 in Malaysia and Hong Kong, respectively. PCV13 would be cost saving when compared against similar program with PCV10, under both payer and societal perspective in both countries. PCV13 remained a better choice over PCV10 in multiple sensitivity, scenario, and probabilistic analyses. PCV13s broader serotype coverage in its formulation and herd effect compared against PCV10 were important drivers of differences in outcomes.
Introduction
Streptococcus pneumonia (SP) causes invasive pneumococcal diseases (IPD), meningitis and bacteremia, and non-invasive diseases such as pneumonia and acute otitis media (AOM).
Of the estimated 8.8 million global annual deaths among children <5 years of age in 2008, WHO estimated that 476,000 (333,000–529,000) were caused by pneumococcal infections.Citation1 Disease rates and mortality are higher in developing than in industrialized settings, with the majority of deaths occurring in Africa and Asia.Citation2 In Malaysia, 4% of the 7,000 death among children <5 years old were estimated as due to SP, which translate into an incidence of overall pneumonia death of 10.2 out of 10,000 children aged under 5 yCitation3 The incidence rate of IPD in Hong Kong was reported to be 2.3 per 100,000 populations.Citation4 The high morbidity and mortality in infants and elderly in both countries thus are translated into high economic burden in terms of cost of management of complications.Citation5
A 7-valent pneumococcal conjugate vaccine (PCV7; Prevenar) was approved for pediatric use and recommended by Advisory Committee on Immunization Practices (ACIP) of the US for children aged up to 59 months in 2000, and use expanded around the world in the years that followed.Citation6 Routine use of PCV7 in children <5 years has markedly decreased the incidence of IPD, pneumonia, and otitis media due to vaccine serotype pneumococcus in several countries where it has been used.Citation7 A large portion of cases prevented have been attributed to herd protection in unvaccinated populations, in particular older adults. In the US, it is estimated that more cases of IPD and hospitalized pneumonia have been prevented due to the herd effects, then directly in vaccinated children.Citation8-11
Two pneumococcal conjugate vaccines have been developed which expand serotype coverage from the 7 covered by PCV7. A 10-Valent Pneumococcal Conjugate Vaccine (PCV10; Synflorix), includes the 7 serotypes in PCV7 (4, 14, 6B, 9V, 18C, 19A, 23F) plus an additional 3 serotypes (1, 5, and 7F), and is conjugated to 3 protein carriers (protein D derived from non-typeable Haemophilus influenza, tetanus toxoid, and diphtheria toxoid).Citation12 A 13-Valent Pneumococcal Conjugate Vaccine (PCV13; Prevenar 13) includes all serotypes covered by the PCV7 and PCV10 vaccines plus an additional 3 serotypes (3, 6A, and 19A).Citation13 All serotypes in PCV13 are conjugated to CRM197, the same protein carrier used in PCV7.
Hong Kong has been using PCV13 as part of their National Immunization Program (NIP) since 2013 with the vaccine penetration rate already reaching 95% among infants below 2 y of age in 2014 while Malaysia does not include any PCV into routine infant vaccination. Cost-effectiveness data is a key criterion in the evaluation of vaccines considered for inclusion in an infant vaccination program. The cost-effectiveness of PCV7 has been reported in both countries, however, the cost effectiveness of expanded valency vaccines has not yet been evaluated.Citation14,15 The aim of this study is to apply local epidemiological and economic data to evaluate the cost-effectiveness of universal infant vaccination with PCV10 and PCV13 in Malaysia and Hong Kong.
Results
Base-case results
present cumulative 10-year estimates of health economic outcomes and cost-effectiveness of different vaccination strategies in Malaysia and Hong Kong, respectively. As shown in , in Malaysia, in the absence of an infant routine immunization program, pneumococcal disease would cause over 8,790 IPD, 12 million pneumonia, 4.7 million AOM cases, and over 40,000 deaths. Pneumococcal disease would be responsible for direct medical costs of $3.1 billion and indirect costs of $2.4 billion totalizing over $5.5 billion in societal costs. A PCV13 program would reduce pneumococcal disease by over 4,000 fewer IPD cases, 768,000 fewer pneumonia cases, and over 570,000 fewer AOM cases and would save over 7,700 lives and gain over 129,000 LYs and 120,000 QALYs. Compared to PCV10, a PCV13 program would reduce over 2,396 more IPD, 705,000 more pneumonia, 376,000 more AOM cases, and save 6,000 more lives, 80,100 more LYs and 74,000 more QALYs during a 10-year period.
Table 1. Health and Economic Outcomes of Pneumococcal Vaccination Strategies in Malaysia for Base Case Scenario
Under vaccine price parity ($55/dose) and a 4-dose (3+1 schedule) assumptions, a PCV13 program would be about $226 million cheaper in net direct medical costs and $372 million cheaper than a program with PCV10. Therefore, given that PCV13 would have greater net health benefit and lower net costs than a PCV10 program, from the cost-effectiveness perspective, a PCV13 program would be cost-saving compared to PCV10 program. Compared to no vaccination, a PCV13 program would imply cumulative 10-year net direct medical costs of ∼$770 million (or $77 million annually) and net societal costs of ∼$589 million (or $59 million annually). However, a vaccine price of $23 per dose would make a PCV13 program to be cost neutral from the societal perspective (not shown in ).
As shown in , in Hong Kong in the absence of an infant routine immunization program, in a 10-year period, pneumococcal disease would cause over 1,830 IPD, 3.8 million pneumonia, 596,000 AOM cases, and over 36,000 deaths. Pneumococcal disease would be responsible for direct medical costs of $3.2 billion and indirect costs of $400 million totalizing about $3.6 billion in societal costs. A PCV13 program would reduce pneumococcal disease by about 650 fewer IPD cases, 196,000 fewer pneumonia cases, and over 56,000 fewer AOM cases and would save 2,700 lives and gain over 26,500 LYs and 22,000 QALYs. Compared to PCV10, a PCV13 program would reduce over 529 more IPD, over 172,000 more pneumonia, over 37,700 more AOM cases, and save 2,688 more lives, over 26,200 more LYs and over 21,600 more QALYs.
Table 2. Health and Economic Outcomes of Pneumococcal Vaccination Strategies in Hong Kong for Base Case Scenario
Under vaccine price assumptions of ($41 and $25 per dose of PCV13 and PCV10, respectively) in a 4-dose (3+1 schedule), a PCV13 program would be about $219 million cheaper in net direct medical costs and $244 million cheaper in societal costs than a program with PCV10. Therefore, given that PCV13 would have greater net health benefit and lower net costs than a PCV10 program, from the cost-effectiveness perspective, a PCV13 program would be cost-saving compared to both PCV10 program and no vaccination. When compared to no vaccination, a PCV13 program would imply cumulative 10-year net direct medical costs savings of about $198 million (or $19.8 million annual savings) and net societal costs savings of about $227 million (or $22.7 million annual savings).
Scenario analysis
presents cost-effectiveness results by various assumptions for herd effects in Malaysia and Hong Kong from both payer and societal perspectives. In Malaysia, compared to no vaccination, the implementation of PCV13 led to positive ICER (i.e. more costly and more QALYs gained) for all scenarios from both perspectives. When no herd effects were assumed, ICER increased to US$15,752 and US$14,981 from payer and societal perspectives while those 2 figures were beyond 3 times Malaysian 2014 GDP per capita which implied non-cost-effectiveness. PCV13 is cost-effective compared to no vaccination under all other scenarios. When compared to PCV10, PCV13 was cost-saving under all scenarios in both perspectives.
Table 3. Sensitivity Analysis of the Impact of Various PCV13s Herd Effect Assumptions on the Incremental Cost Effectiveness Ratios for Infant Universal Routine Vaccination with PCV13 in Malaysia and Hong Kong
In Hong Kong, compared to no vaccination, PCV13 remained cost-saving when assuming herd effects in IPD and pneumonia only. However, for the remaining 3 scenarios, PCV13 was cost-effective as ICERs were below 3 times Hong Kong 2014 GDP per capita. When compared to PCV10, the results were similar to PCV13 vs. no vaccination scenario.
One-way sensitivity analysis
The results were presented in . In Malaysia, the top 5 influential factors were vaccine coverage of PCV10, vaccine coverage of PCV13, CFR of hospitalized pneumonia, incidence of hospitalized pneumonia, and direct effectiveness against hospitalized pneumonia. In the Hong Kong analysis, the 5 most influential were direct cost of treating one episode of hospitalized pneumonia, CFR of hospitalized pneumonia, vaccine coverage of PCV13, vaccine coverage of PCV10 and incidence of hospitalized pneumonia.
Table 4. Sensitivity Analysis of the Impact of Various Parameters Assumptions on the Incremental Cost Effectiveness Ratios for Infant Universal Routine Vaccination with PCV13 compared to PCV10 in Malaysia and Hong Kong
PCV13 vaccine price and doses per course sensitivity analysis in Malaysia
summarizes the impact of vaccine price and doses per course, as part of an infant routine immunization program with PCV13, on net costs in Malaysia. For example, a vaccine price per dose of $33 (or 60% of the base case) in a 4-dose per course schedule, would imply a cumulative 10-year net cost of about $368 million and $187 million, from a payer and societal perspective, respectively. In a 3-dose schedule, the cumulative 10-year net cost would drop to $214 million from a payer perspective or $33.6 million from a societal perspective (or about $21 million and $3 million per year, respectively).
Table 5. Sensitivity Analysis of the Impact of Vaccine Cost on Total Net Cost of an Infant Routine Pneumococcal Vaccination Program with PCV13 in Malaysia
Multivariate probabilistic sensitivity analysis
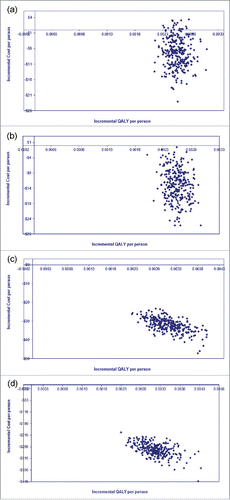
Simulation of 1,000 cohorts varying all input parameters compared PCV13 versus PCV10 from both payer and societal perspectives was shown in . In the Malaysia analysis, PCV13 improved QALYs compared to PCV10 in all simulated results from both perspectives (). The improved health outcome was accompanied with incremental cost in some occasions from payer perspective (). However, all simulated outcomes stayed below 3 times GDP line, the maximum cost-effectiveness threshold, indicating that extra effectiveness gained by PCV13 vaccination was justified by its cost for the 1,000 simulations. From societal perspective, PCV13 was cost-saving for all simulations (). In the Hong Kong analysis, all simulated outcomes stayed in the fourth (SE) quadrant of CE plane from both perspectives (), indicating that PCV10 is dominated by PCV13, i.e., less costly and more effective.
Discussion
We have populated an age-specific Markov model to evaluate cost-effectiveness of PCV13 compared to PCV10 and no vaccination over 10 y in 2 Asian countries, i.e. Malaysia and Hong Kong, which have been substantially burdened by pneumococcal diseases. The model has incorporated both direct and potential herd effect which was a vital indirect benefit due to large national vaccination program. Compared with PCV10, PCV13 was estimated to generate a greater impact on LYG, QALY gained, and pneumococcal diseases averted in both Malaysia and Hong Kong. When compared to PCV10, it was shown that PCV13 program in Malaysia was cost-saving from both societal and payer perspectives. In Hong Kong, universal infant vaccination with PCV13 is also dominant over PCV10.
Input parameters used in the model were considered as the best data available at the time of the analysis in which incidence, mortality, serotype coverage, and costs were country-specific. In case of lack of local data, parameters were extrapolated from international published studies where available. This methodology is consistent with recommendations from WHO that data from epidemiologically similar populations may be used to approximate the incidence of preventable pneumococcal diseases in countries where local estimates are unavailable.Citation16 It is noteworthy that we have used incidence data in the Hong Kong model before PCV13 was included in NIP. It has been shown in previous studiesCitation9,17 that nationwide vaccination with high coverage is expected to lead to substantial reduction of burden of pneumococcal diseases. To account for this impact, we have varied the base-case disease incidences in 1-way sensitivity analysis and it was shown that this uncertainty has only moderate impact on cost per QALY gained.
There are a few cost-effectiveness studies of the 2 newer vaccines in South East Asian countries. The results from our study were consistent with a previous study in Singapore which evaluated cost-effectiveness of PCV7, PC10, and PCV13.Citation18 It was shown that PCV13 was cost-effective compared to PCV10 and PCV7 in Singapore. However, the Thailand study showed that at current prices, PCV10 and PCV13 are not cost-effective even with the inclusion of vaccine herd effects.Citation19 However, there have been similarities of 3 studies in that all have utilized state-transition Markov model, and applied country-specific serotype data to adjust for vaccine effectiveness on vaccinated and unvaccinated populations. Contrary to our study results, a prior study in Malaysia concluded that a PCV10 program was cost-effective compared with PCV13.Citation20 However, the study relied on a number of questionable assumptions favoring PCV10 that lacked scientific validation. While there is no head to head comparison between PCV10 and PCV13, it is still plausible to assume that both PCV vaccines have similar effectiveness against pneumococcal diseases (IPD, AOM, and pneumonia) for the 10-common serotypes in the respective vaccine formulations, despite that PCV10 is less immunogenic compared with PCV13.Citation21 However, the prior Malaysian study's assumptions included: 1) PCV10 also had effectiveness against 2 additional serotypes, 6A and 19A, both contained in PCV13 but not in PCV10; 2) PCV13 lacked effectiveness against serotype 3 (the third additional serotype in PCV13 formulation missing in PCV10); 3) PCV10 also conferred protection against “IPD” and AOM caused by NTHi. First, surveillance from PCV10-only countries have failed to show any sustained reduction in 19A invasive disease, as previously discussed.Citation22-25 Second, there have not been any conclusive evidence of the lack of PCV13 effectiveness against serotype 3. Finally the assumption that PCV10 has effectiveness against NTHi disease has been widely and repeatedly challenged.Citation22-24,26-32 In addition, of note, by definition, IPD is a severe condition caused by streptococcus pnemoniae, not caused by NTHi. The assumption that PCV10 provided protection against invasive NTHi is not supported by any scientific evidence.Citation33 Furthermore, even if that was the hypothesis used by the authors, invasive NTHi, while rare, it occurs mainly during the first 6 weeks of life well before any benefit of pneumococcal vaccination could be achieved. Considering these shortcomings of the input parameters, the resulting output from the prior Malaysian study is likely very limited.
Although the overall conclusions derived from Malaysia and Hong Kong were similar, i.e., an infant routine pneumococcal vaccination with PCV13 would provide a greater public health impact and be cheaper when accounting disease prevented, some differences between 2 countries were observed. Albeit a much smaller population of Hong Kong (7.24 million in 2014) compared to Malaysia (around 30.07 million in 2014), more favorable cost-effectiveness results (larger negative ICERs) were observed. This might be due to a number of reasons. First, the study used lower vaccine prices for Hong Kong than for Malaysia because Malaysia only has vaccine price available for the private market. Still PCV13 was always cost-saving when compared against PCV10. When comparing PCV13 against no vaccination a $23/dose would make the program cost neutral from a societal perspective. Second, the serotype coverage of PCV13 across all age groups in Hong Kong was higher than in Malaysia especially in the infant and the elderly ones. Third, compared to Malaysia, the average treatment costs of pneumococcal meningitis, pneumococcal bacteraemia and inpatient pneumonia were far higher in Hong Kong when compared in 2012 USD, ranging from 2 to 7 times of those in Malaysia. Therefore, the interplay of both higher adjusted vaccine effectiveness and higher disease treatment cost could possibly explain why a more favorable cost-effectiveness result was observed in Hong Kong analysis. However, given higher case-fatality rates of pneumococcal diseases and much larger population size of Malaysia (3.8 times of Hong Kong), it was reasonable to see more health benefits as LYG and QALY saved in Malaysia than in Hong Kong. The most important parameter influencing cost-effectiveness ratios (other than vaccine cost), the incidence and mortality of hospitalized pneumonia in children, were both sourced from country-specific sources.
Cost-effectiveness models comparing PCVs are strongly influenced by assumptions of vaccine direct and herd effects.Citation22 Our model assumes that the direct effects of PCVs vs. all-cause pneumonia and otitis media are proportional to serotype coverage of the vaccine. Surveillance data following the substitution of PCV7 by PCV13 in the US and UK support PCV13s effectiveness in reduction of IPD caused by additional serotypes.Citation34-37 A recent clinical trial of PCV10 confirms efficacy versus covered serotypes is similar to that observed with PCV7 when applied in a 2+1 schedule.Citation29
Some models of pneumococcal vaccination have assumed additional impact of PCV10 on otitis media due to inclusion of a carrier protein derived from non-typable Haemophilus influenza (NTHi), with reference by trial results from an investigational pre-cursor to the current formulation.Citation27 However, 2 recent clinical trials comparing PCV10 to placebo report all-cause otitis media report reductions in a range expected of a vaccine specific to pneumococcal disease;Citation30,31 one of the 2 specified bacterial outcomes and reported no effectiveness vs. AOM caused by NTHi.Citation27 Further, a recently published randomized study comparing PCV7 and PCV10 on nasopharyngeal carriage found no impact of PCV10 on NTHi carriage compared to PCV7.Citation26 Therefore, there is no evidence to support an impact of PCV10 on otitis media greater than that expected from the pneumococcal serotypes contained in the vaccine.
Regarding cross-protection of PCV10 against 19A, one effectiveness study suggests a positive and significant point estimate for PCV10 effect against disease caused by 19A,Citation38 however in this case control study very few discordant pairs supported their findings. Although the study design was robust, the results are inconsistent with the national surveillance system in Brazil, which shows an increase in the incidence of serotype 19A invasive pneumococcal disease between 2006 and 2011 in children younger than 5 y of age.Citation39,40 Domingues and colleagues concluded that “Validation of this finding in other settings is important because the point estimate of effectiveness against serotype 19A disease is higher than what might be expected based on immunogenicity data, and the 95% CI was wide. Additionally, PCV10 has not reduced 19A nasopharyngeal carriage in Kenya, where it was introduced in early 2011.” As another example, in Finland, where PCV10 has been used extensively, the incidence of serotype 19A invasive pneumococcal disease continues to rise, driven mostly by disease in older groups.Citation41 Despite an initial case control study in the United States that demonstrated a reduction in serotype 19A disease,Citation42 real world experience confirms that PCV7 does not provide cross-protection against serotype 19A.Citation9,43 Combined with the lack of confirmation of any 19A cross-protection in countries where PCV10 is used in a national program,Citation44,45 it is inappropriate to use cross-protection against 19A as a base case assumption.
Herd effects of vaccination have a significant impact on cost-effectiveness models of PCVs. The evidence supporting herd effects with PCV7 is very strong, with research supporting significant impact on carriage, impact on reduced transmission to contacts, changes in serotypes causing invasive disease in several countries,Citation46 and large impacts on pneumonia in ecological studies in multiple settings.Citation10,47 Initial evidence in nasopharyngeal carriage and invasive disease with PCV13 supports a similar effect will be observed with additional serotypes as was observed with PCV7.Citation34,35,48 The real-world herd effects of PCV13 on IPD and pneumonia have become available in many countries, e.g. USA, Nicaragua, Spain, Denmark, France, Ontario, Israel, South Africa, and UK when high vaccine coverage was achieved.Citation49-56 However, in settings with vastly different environmental risk factors, mixing patterns, family structures, and culture such as Malaysia and Hong Kong, it is uncertain the extent to which similar effects will be observed. In the absence of country-specific estimates of herd effects, it was assumed that herd effects observed in these 2 countries would be very similar to that observed in the US post-PCV7 era. To address this uncertainty, we explored several alternative assumptions of herd effects for PCV13. In Hong Kong, by assuming direct effects alone, PCV13 appeared to be cost-effective with ICERs below 1 time HK GDP when compared to no vaccination or PCV10. In Malaysia, PCV13 was also cost-effective compared to no vaccination and PCV10 based on direct protection alone with ICER slightly above 1 time Malaysian GDP. To date, there is no evidence of significant impact on pneumococcal disease in adults in countries where PCV10 has been used.
Our model is limited to the extent that it addresses the dynamic nature of herd effects. So far, there is only one study in Asia-Pacific region which utilized transmission dynamic model to evaluate dynamics of pneumococcal diseases.Citation57 However, that study only provided cost-effectiveness of PCV13 compared to no vaccination. A universal infant vaccination program raises concerns about the potential for emerging serotypes from more prevalent non-vaccine serotype disease as they face less competition from vaccine types and could offset the benefits of a new vaccine, a phenomenon known as serotype replacement. Proportionally, IPD from emerging serotypes since the introduction of PCV7 has been small compared to the dramatic effects of PCV7 on serotype disease. Based on the US PCV7 experiences, since the potential for emerging serotype disease with PCV13 is unknown, and the inclusion of emerging serotype disease estimates would likely not have a large impact on the results, therefore, we did not explicitly model its impact. Although our model does not explicitly consider it, emerging serotype disease would largely impact herd effects, and we have taken an indirect approach by showing that even when herd effects are reduced from the model, vaccination with PCV13 could still produce favorable cost-effectiveness results.
Given the substantial case fatality rates and high incidence of pneumococcal diseases among infants and the elderly above 65 in both countries, infant universal routine PCV13 vaccination is potentially cost-effective with the inclusion of herd effects based on WHO-recommended threshold. Health authorities of Malaysia and Hong Kong may consider infant universal routine PCV13 vaccination for all newly born infants in order to decrease pneumococcal disease burden.
Methods
Local data on disease epidemiology, serotype coverage, and cost, together with international data on PCV efficacy and effectiveness, were applied to a 10-year Markov model to estimate the local impact of PCV10 and PCV13 on pneumococcal disease burden and associated cost. Details of the Markov model as applied to the United States are published elsewhere.Citation58 The analysis takes both payer and the societal perspectives, and the primary outcome for each country is the incremental cost per life year and QALY gained for PCV10 and PCV13 compared to no vaccination and PCV13 compared to PCV10. Future costs and outcomes were discounted at 3% per annum. A major benefit of vaccination is herd effect which is the indirect protection of vaccination program on the unvaccinated population. It has been shown in the US that herd effect after universal infant vaccination with PCV7 was accrued gradually and achieved maximum effect at the 7th year after the start of vaccination.Citation59 Thus, the simulation period was set to be long enough to fully account for this accumulating indirect effect.
Model structure
We adapted a 10-year Markov model of pneumococcal disease to the settings of Malaysia and Hong Kong. The model simulates vaccination and outcomes among 10 successive birth cohorts of children, and estimates accumulating herd effects across the unvaccinated population over the 10 y horizon. Life years and QALYs lost due to events in the model period are applied for lifetime based on discounted life expectancy. The starting point of the model is the choice of vaccination strategy, including no vaccination, vaccination with PCV10, or vaccination with PCV13. Event probabilities for persons aged <5 years are estimated for each year of life, and for those ≥5 years are stratified into 5 age groups (5–17, 18–34, 35–49, 50–64, and ≥65).
The Markov model allocates a portion of the population to states of unvaccinated, vaccinated, vaccinated (waning efficacy), and dead. Within each annual cycle, the cohort is subject to pneumococcal disease, as a function of incidence rates and vaccine effects, including IPD (pneumococcal meningitis or bacteremia), all-cause pneumonia (hospitalized or non-hospitalized), and all-cause AOM (complex [requiring ≥3 visits and/or tympanostomy tube placement] or simple). During each model cycle, persons may survive, die from pneumococcal disease, or die from other causes. Persons with meningitis may develop long-term sequelae of deafness or disability. Persons who survive return to the appropriate Markov state, depending on vaccination status and time since vaccination, advancing one year in age each cycle. At the beginning of each model cycle, an incoming birth cohort enters the model and a new cohort of children is vaccinated, with the probability of vaccination depending on vaccination strategy and assumptions regarding vaccine coverage.
Population and vaccination rates
Estimates of population by age were applied from the Department of Statistics at Ministry of Health in Malaysia and Hong Kong Census and Statistics Department, respectively.Citation60,61 With reference to internal data on the overall number of PCV13 doses utilized for the NIP of Hong Kong, the vaccination rates already reached 95% among the target population, i.e. infants of 0–2 y of age in 2013, the year of implanting PCV13 into NIP. In Malaysia, based on previous experiences of other vaccines, the DTP dose 1 vaccination rate was 98–99% between 2002 to 2013, DTP dose 3 vaccination rates was 94–97%, and Hep B dose 3 rate was 94–97%.Citation62 Therefore infant pneumococcal vaccination rates of 90% was assumed valid and used for both countries. In addition, infant pneumococcal vaccination was assumed to consist of 4 doses per course (3+1 schedule).
Epidemiological data
Disease incidence rates were calculated by age-specific episodes divided to population size. Due to the low coverage rate of PCV in both countries at the time of data applied to each model, the incidence rates represent disease burden without PCV use. Parameters were derived from local studies where available and were extrapolated using regional or international data sources where local data were unavailable. The use of extrapolated data to populate a pharmacoeconomic model with the absence of local data is well justified.Citation63
Epidemiologic and costs inputs from Malaysia and Hong Kong are presented in . The rates of IPD in Malaysia were extrapolated from the Taiwan study of Hsieh et al.Citation64 The rates of all-cause hospitalized pneumonia were estimated from a previous Malaysian PCV7 cost-effectiveness study by Aljunid et al.Citation14 As there are no known nationwide epidemiological data on all-cause outpatient pneumonia in Malaysia, the incidence was estimated from the 2012 HIMS(Health Information Management System) unpublished data from the Health Informatics publication from Ministry of Health in Malaysia. The total outpatient attendances for year 2012 were 49,602,486 where the breakdown attendances are 7,828,297, 7,151,109, 24,196,003 and 10,427,095 for various age groups <10 y, 10–19y, 20–59y and >60 y, respectively. Furthermore, 6,068,345 (45.2%) attendances for public health facilities were due to diseases for respiratory system for year 2012. Based on a simple survey in March 2014 at one large outpatient clinic, 40 out of 800 (5%) monthly attendees has pneumonia that required antibiotic prescription. All the above information was thus used to estimate the age-stratified incidence of outpatient all-cause pneumonia. The rates of complex AOM were based on hospitalized AOM from Malaysia reported in the Aljunid et al study. The rates of simple AOM were also Malaysia-specific.Citation65
Incidences of IPD in Hong Kong were estimated based on Hong Kong surveillance data.Citation57 The incidences of all-cause hospitalized pneumonia was applied from a previous report of PCV7 cost-effectiveness where data were derived from the Hong Kong Hospital Authority.Citation15 The rates of all-cause outpatient pneumonia were extrapolated from Taiwan study by Wu et al.Citation66 The rates of complex AOM were based on Lee et al's study. The rates of simple AOM were estimated from Sung et al.Citation67
In Malaysia, the case fatality rate (CFR) of pneumococcal meningitis was extrapolated from 2 Taiwan studies by Wu et al and Lin et al respectively.Citation66,68 The CFR of pneumococcal bacteremia was estimated using Singapore and Thailand studiesCitation69,70 considering the geographical and population similarity between these countries. The CFR of all-cause hospitalized pneumonia was estimated using Taiwan study by Wu et al and World Health Organization report (WHO).Citation66,71 In Hong Kong, the CFRs of pneumococcal meningitis, pneumococcal bacteremia, and hospitalized pneumonia (<=10 y of age) were based on a previously published Hong Kong study.Citation15 The CFRs (>10 y of age) of all 3 pneumococcal diseases were another Hong Kong study.Citation57
Economic data
Direct medical costs, including hospitalizations and outpatient care related to pneumococcal disease as well as the discounted lifetime cost of meningitis sequelae were included in the payer perspective. Indirect costs related to productivity loss (of parents if children with pneumococcal disease) were estimated to add to the societal perspective.
The direct medical costs of treating pneumococcal meningitis, pneumococcal bacteremia, hospitalized pneumonia, and complex AOM in Malaysia were applied from the previously published cost-effectiveness study of PCV7 in Malaysia.Citation14 The costs of outpatient pneumonia and simple AOM were obtained from an economic burden study in Malaysia.Citation72 The life-time medical cost for deafness and disability due to pneumococcal meningitis were estimated using the following formula: Local lifetime medical cost = (US lifetime medical costs) × (Total expenditure on health per capita in Malaysia / Total expenditure on health per capita in the US) × (Currency ratio of Malaysia to the US).Citation73,74
With the lack of local research related to work loss due to pneumococcal diseases in Malaysia, we extrapolated indirect cost among children under year of 5 from an international study by Nakamura et alCitation75 which reported cost effectiveness of child pneumococcal conjugate vaccination in middle-income countries. The indirect cost for those above 5 y of age was estimated using the local unpublished questionnaire on patients' average length of hospitalization stay multiplied by the median average daily salary from Malaysian government website.Citation76 Outpatient pneumonia was assumed to incur only 0.5 day loss of productivity.
The average direct medical costs of treating per episode of pneumococcal meningitis, pneumococcal bacteremia, hospitalized pneumonia, and complex AOM in Hong Kong were estimated from a previous study by Lee at alCitation15 from the perspective of Hong Kong healthcare system. The lifetime medical cost for deafness and disability was also calculated similar to Malaysian setting.
The indirect costs (<20 years of age) of pneumococcal meningitis, pneumococcal bacteremia, hospitalized pneumonia, and complex AOM were applied from the previous Hong Kong Lee' et. al. study.Citation15 Half-day loss of productivity for general consultation was assumed for non-hospitalized pneumonia where median monthly wage was derived from Hong Kong government website.Citation77
All cost parameters are presented in . All costs were inflated to common 2014 y costs (the point of model simulation), using the 2014 Consumer Price Index (CPI) value of both Malaysia and Hong KongCitation78,79 divided by the CPI value of the year when the costs were estimated. Costs were converted to USD based on exchange rates of 3.33 MYR:USD and 7.75 HKG:USD as of December, 2014.
Table 6. Epidemiological Parameters of Pneumococcal Diseases in Malaysia and Hong Kong
Table 7. Cost parameters of pneumococcal diseases in Malaysia and Hong
Regarding vaccine cost, as there is no public price in Malaysia due to the fact that PCVs are not included in NIP, we assumed price parity for PCV10 and PCV13 with a base case of RM183 (US$ 55.08) per dose for based on private market prices, plus the administration fee of RM10 (US$3.00) per dose. Sensitivity analysis of price included vaccine cost of half and one third of base case. In Hong Kong, PCV10 and PCV13 prices per dose were HKD195 (US$25.16) and HKD318 (US$41.03) respectively. The administration fee of HKD12 (US$1.55) per dose for each of the 2 vaccines is assumed.
Vaccine effectiveness
Direct effects of PCV10 and PCV13 for reduction of IPD is assumed to be 94% versus covered serotypes, based on clinical trial data for PCV7.Citation80 Direct effectiveness of PCV10 and PCV13 for prevention of all-cause pneumonia and acute otitis media in vaccinated children are derived from observed effectiveness of PCV7 and adjusted for in proportion to current serotype coverage locally for each country,Citation81,82 as compared the US PCV7 coverage at the time of the efficacy studies. In general, this may be expressed as:
For example, by 2011, given the serotype frequency distribution of IPD and the serotype coverage based on PCV13 and PCV10 vaccine formulations, it was assumed that PCV13 and PCV10 could prevent up to 91.4% and 68.6% of the IPD disease, respectively, in Hong Kong and up to 78.0% and 56.0%, respectively, in Malaysia.
For hospitalized pneumonia, clinical trial data were used to estimate the baseline efficacy of PCV7 against radiographically confirmed pneumonia.Citation83 Similar estimates for non-hospitalized pneumonia reflect PCV7 effectiveness reported for clinical pneumonia, and all-cause AOM reflect PCV7 effectiveness at preventing all-cause AOM visits.Citation80,84
As infants receive no direct benefit from the vaccine before their first dose at age 2 months, and the immune response in children <4 months is unknown, we assumed a reduction in direct benefits of one-third in the first year of life. Consistent with previously published economic studies of PCV7, the direct protection from PCVs is assumed to wane to 91% of the original benefit 2 y after the initial dose.Citation85,86 No direct protection is assumed after 5 y of age.
Herd effects against IPD, pneumonia, and AOM in infants and children were estimated using effectiveness data on PCV7, which was adjusted in proportion to local serotype coverage, similar to the approach taken for estimation of direct effects. The herd effect data are derived from all-cause estimates, and therefore represent a net effect of herd protection and potential serotype replacement. The estimated herd effects for PCV7 in vaccinated children were derived by combining direct effects and ecologic data. The midpoint of the direct effect from the PCV7 trialsCitation80,83,84 and reductions in disease from observational studies were assumed to represent the total effect (direct and herd) of PCV7 on hospitalized pneumonia and AOM incidence.Citation87,88 This derived herd effect was then adapted to PCV13 in proportion to local serotype coverage.
The herd effects of PCV13 against pneumonia in adults were similarly derived from ecologic data on reductions in pneumonia admissions after the introduction of PCV7.Citation89 We assumed that effects from PCV7 in the unvaccinated were equal to 50% of the estimated reduction in pneumonia reported by Grijalva et al.Citation89 Similar to above, the age-specific herd effects for PCV7 were adapted to PCV10 and PCV13 by the proportional differences in age-specific local serotype coverage. The herd effect on non-hospitalized pneumonia was estimated by adjusting the herd effect for hospitalized pneumonia by the proportion of non-hospitalized pneumonia that is confirmed with a chest radiograph.
Herd protection for PCV10 was assumed to be zero in the base case due to limited and inconclusive data regarding the ability of PCV10 to impact nasopharyngeal carriage. The likelihood of herd protection cannot be assumed the same given formulation differences and uncertain carriage impact.Citation27,90 By contrast, PCV13 has been shown to impact nasopharyngeal carriage for additional serotypes similarly to PCV7,Citation48 and impact on invasive disease due to additional serotypes has been reported from US and UK surveillance data.Citation34,35
The Markov model estimates herd protection as a reduction in disease incidence across all age groups that increases each year to a maximum effect at 7 years, reflecting the long term trends observed in the US and UK.
In summary, base case scenario assumed that PCV13 would provide both direct and indirect protection against IPD and pneumococcal pneumonia and AOM, while PCV10 would provide only direct protection among infant vaccinated.
Model outcomes
Pneumococcal events, pneumococcal-related deaths, and vaccination and medical costs were directly estimated from the model calculations. Life years gained (LYG) are estimated by applying discounted life years lost to each fatal event based on country-specific WHO life tables. Quality-adjusted life years (QALY) are estimated by applying utility decrements to acute events and a lifetime QALY penalty to the life years of meningitis survivors with long-term sequelae. Utility values, preference-based measures of quality-of-life ranging from 0 to 1 used to calculate QALYs, were adapted from the literature.Citation91 The following utility decrements were applied to acute events: 0.0232 for meningitis, 0.0079 for bacteremia, 0.004 for non-hospitalized pneumonia, 0.006 for hospitalized pneumonia, and 0.005 for acute AOM.
Disability from long-term sequelae is incorporated in the model to a proportion of meningitis survivors. It is assumed that 13% of meningitis survivors will suffer from deafness, and 7% from long-term disability.Citation85,92 Utilities for chronic health states (deafness and disability) are applied over remaining life expectancy and were estimated from retrospective studies of meningococcal complications as 0.73 for deafness and 0.68 for disability and applied to the remaining life expectancy.Citation93,94
The incremental cost per LYG and QALY gained was estimated by ordering interventions from least to greatest LYG and QALY, then calculating cost-effectiveness for each successive program per country. Since there are no specified cost-effectiveness thresholds in Malaysia and Hong Kong, WHO guidelines were used: an intervention is “cost-effective” if the incremental cost-effectiveness ration (ICER) is less than 3 times the country's gross domestic product (GDP) per capita, and an intervention is “very cost-effective” if the ICER is less than a country's GDP per capita.Citation95 Three times Malaysia and Kong Kong GDPCitation96 per capita 2012 were US$9,843 and US$110,099, respectively.
Scenario analysis
Alternative assumptions regarding the application of herd effects were applied in scenario analyses to examine the impact of herd protection on cost-effectiveness outcomes: (1) no herd effect; (2) herd effect against IPD only; (3) herd effect against IPD and hospitalized and non-hospitalized pneumonia; (4) herd effect for children <5 years only.
Sensitivity analyses
One-way sensitivity analysis was applied to evaluate infant routine vaccination with PCV13 compared against vaccination with PCV10. All input parameters, e.g., disease incidence, vaccine efficacy, vaccine coverage, decay of vaccine efficacy, direct and indirect costs were varied incidence, vaccine efficacy, vaccine coverage, decay cost per QALY gained from societal perspective. As Malaysia, unlike Hong Kong, has not implemented an infant routine pneumococcal vaccination program yet, we reviewed the impact of vaccine price and doses per course on net cost from both payer and societal perspectives. Finally, multivariate probabilistic sensitivity analysis based on 1,000 Monte Carlo simulations from both perspectives was also performed to examine simultaneous impact of multiple parameters on cost-effectiveness outcomes.
Disclosure of Potential Conflicts of Interest
DBCW and KKCL received funding from Pfizer Inc. to conduct this study. KKT has received honorarium for speaking at Pfizer CME. CR and LWH are employees and shareholders in Pfizer Inc. The rest of authors reported no conflict of interest.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Supplemental_Material.zip
Download Zip (27.6 KB)Acknowledgments
The authors are grateful to Jose A. Suaya, MD (Pfizer) for manuscript review and edits.
References
- Estimated Hib and pneumococcal deaths for children under 5 years of age, 2008. http://www.who.int/immunization/monitoring_surveillance/burden/estimates/Pneumo_hib/en/ Accessed 17 May 2015
- O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009; 374:893-902; PMID:19748398; http://dx.doi.org/10.1016/S0140-6736(09)61204-6
- Le CF, Jefferies JM, Yusof MY, Sekaran SD, Clarke SC. The epidemiology of pneumococcal carriage and infections in Malaysia. Expert Rev Anti Infect Ther 2012; 106:707-19; PMID:22734960; http://dx.doi.org/10.1586/eri.12.54
- Centre for Health Protection. Frequently asked questions on pneumoccocal infection and pneumoccocal vaccines. http://www.chp.gov.hk/files/pdf/faqs_on_pneumoccocal_infection_and_pneumoccocal_vaccines.pdf Accessed 2 May 2015
- Bravo LC, Asian strategic alliance for pneumococcal disease prevention working G. Overview of the disease burden of invasive pneumococcal disease in Asia. Vaccine 2009; 27:7282-91; PMID:19393708; http://dx.doi.org/10.1016/j.vaccine.2009.04.046
- Advisory Committee on Immunization Practices (ACIP). Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2000; 49(RR9):1-35
- Fitzwater SP, Chandran A, Santosham M, Johnson HL. The worldwide impact of the seven-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 2012; 31:501-8; PMID:22327872; http://dx.doi.org/10.1097/INF.0b013e31824de9f6
- CDC. Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease–United States, 1998-2003. MMWR Morb Mortal Wkly Rep 2005; 54:893-7; PMID:16163262
- Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010; 201:32-41; PMID:19947881; http://dx.doi.org/10.1086/648593
- Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013; 369:155-63; PMID:23841730; http://dx.doi.org/10.1056/NEJMoa1209165
- Simonsen L, Taylor RJ, Young-Xu Y, Haber M, May L, Klugman KP. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. MBio 2011; 2:e00309-10; PMID:21264063; http://dx.doi.org/10.1128/mBio.00309-10
- EMA. Synflorix: Summary of product characteristics. 2009.
- Wyeth submits marketing application to FDA for its 13-valent vaccine for the prevention of pneumococcal disease in infants and toddlers. 31 March 2009. http://www.wyeth.com/news?nav=display&navTo=/wyeth_html/home/news/pressreleases/2009/1238506420199.html Accessed 2 March 2009
- Aljunid S, Abuduxike G, Ahmed Z, Sulong S, Nur AM, Goh A. Impact of routine PCV7 (Prevenar) vaccination of infants on the clinical and economic burden of pneumococcal disease in Malaysia. BMC Infect Dis 2011; 11:248; PMID:21936928; http://dx.doi.org/10.1186/1471-2334-11-248
- Lee KKC, Rinaldi F, Chan MKU, Chan STH, So TMT, Hon EKL, Lee VWY. Economic evaluation of universal infant vaccination with 7vPCV in Hong Kong. Value in Health 2009; 12:S42-S8; PMID:20586981; http://dx.doi.org/10.1111/j.1524-4733.2009.00626.x
- WHO. Pneumococcal conjugate vaccine for childhood immunization – WHO position paper. Weekly Epidemiological Record 2007; 82:93-104
- Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis 2011; 11:760-8; PMID:21621466; http://dx.doi.org/10.1016/S1473-3099(11)70090-1
- Tyo KR, Rosen MM, Zeng W, Yap M, Pwee KH, Ang LW, Shepard DS. Cost-effectiveness of conjugate pneumococcal vaccination in Singapore: comparing estimates for 7-valent, 10-valent, and 13-valent vaccines. Vaccine 2011; 29:6686-94; PMID:21745516; http://dx.doi.org/10.1016/j.vaccine.2011.06.091
- Kulpeng W, Leelahavarong P, Rattanavipapong W, Sornsrivichai V, Baggett HC, Meeyai A, Punpanich W, Teerawattananon Y. Cost-utility analysis of 10- and 13-valent pneumococcal conjugate vaccines: protection at what price in the Thai context? Vaccine 2013; 31:2839-47; PMID:23588084; http://dx.doi.org/10.1016/j.vaccine.2013.03.047
- Aljunid S, Maimaiti N, Ahmed Z, Nur AM, Isa ZM, Azmi S, Sulong S. Economic impact of pneumococcal protein-D conjugate vaccine (PHiD-CV) on the Malaysian national immunization programme. Value in Health Regional Issues 2014; 3:146-55; http://dx.doi.org/10.1016/j.vhri.2014.04.008
- van Westen E, Wijmenga-Monsuur AJ, van Dijke HH, van Gaans-van den Brink JA, Kuipers B, Knol MJ, Berbers GA, Sanders EA, Rots NY, van Els CA. Differential B cell memory around the 11-month booster in children vaccinated with a 10- or 13-valent pneumococcal conjugate vaccine. Clin Infect Dis. Forthcoming 2015
- See comment in PubMed Commons below Farkouh RA, Klok RM, Postma MJ, Roberts CS, Strutton DR. Cost-effectiveness models of pneumococcal conjugate vaccines: variability and impact of modeling assumptions. Expert Rev Vaccines 2012; 11:1235-47; PMID:23170992; http://dx.doi.org/10.1586/erv.12.99
- Farkouh RA, Hall-Murray C, Klok RM, Hilton B, Isturiz RE. Cost-effectiveness evaluation of the 10-valent pneumococcal non-typeable haemophilus influenzae protein D conjugate vaccine and 13-valent pneumococcal vaccine in Japanese children [Comment]. Infect Dis Ther 2015:1-7
- Klok RM, Lindkvist RM, Ekelund M, Farkouh RA, Strutton DR. Cost-effectiveness of a 10- versus 13-valent pneumococcal conjugate vaccine in Denmark and Sweden [Letter to the editor]. Clin Ther 2013; 35:1048-50; PMID:23806326; http://dx.doi.org/10.1016/j.clinthera.2013.05.010
- Strutton D, Hwang S, Farkouh R, Roberts C. Outcomes and costs associated with PHiD-CV, a new protein D conjugate pneumococcal vaccine, in four countries [Response]. Vaccine 2011; 29:7591-2; PMID:21406264; http://dx.doi.org/10.1016/j.vaccine.2011.02.103
- van den Bergh MR, Spijkerman J, Swinnen KM, Francois NA, Pascal TG, Borys D, Schuerman L, Ijzerman EP, Bruin JP, van der Ende A, et al. Effects of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D-conjugate vaccine on nasopharyngeal bacterial colonization in young children: a randomized controlled trial. Clin Infect Dis 2013; 56:e30-9; PMID:23118268; http://dx.doi.org/10.1093/cid/cis922
- Prymula R, Irena H, Miroslav S, Kriz P, Motlova J, Lebedova V, Lommel P, Kaliskova E, Pascal T, Borys D, et al. Impact of the 10-valent pneumococcal non-typeable Haemophilus influenzae Protein D conjugate vaccine (PHiD-CV) on bacterial nasopharyngeal carriage. Vaccine 2011; 29:1959-67; PMID:21215830; http://dx.doi.org/10.1016/j.vaccine.2010.12.086
- Prymula R, Habib A, François N, Borys D, Schuerman L. Immunological memory and nasopharyngeal carriage in 4-year-old children previously primed and boosted with 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) with or without concomitant prophylactic paracetamol. Vaccine 2013; 31:2080-8; PMID:23391599; http://dx.doi.org/10.1016/j.vaccine.2013.01.044
- Palmu AA, Jokinen J, Borys D, Nieminen H, Ruokokoski E, Siira L, Puumalainen T, Lommel P, Hezareh M, Moreira M, et al. Effectiveness of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against invasive pneumococcal disease: a cluster randomised trial. Lancet 2013; 381:214-22; PMID:23158882; http://dx.doi.org/10.1016/S0140-6736(12)61854-6
- Vesikari T, Forstén A, Seppä I, Puumalainen T, Soininen A, Lommel P, Hezareh M, Moreira M, Borys D, Schuerman L. Effectiveness of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine against acute otitis media. ICAAC 2012 (Abstract). San Francisco, CA, September 9–12, 2012.
- Sáez-Llorens X, Castrejon M, Rowley S, Wong D, Calvo A, Rodriguez M, Troitino M, Lommel P, Hausdorff W, Borys D. Efficacy of 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) against acute otitis media in children in Panama. Poster presentation. The 9th International Symposium on Antimicrobial Agents and Resistance (ISAAR) March 13–15, Kuala Lumpur, Malaysia.
- Arguedas A, Soley C. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study [Comment]. Lancet 2006; 367:1898; PMID:16765750; http://dx.doi.org/10.1016/S0140-6736(06)68833-8
- GlaxoSmithKline. Evaluation of effectiveness of GSK Biologicals' pneumococcal conjugate vaccine GSK1024850A against invasive disease. Study No.: 111442 (10PN-PD-DIT-043) 2013. Unpublished report.
- Moore M, Link-Gelles R, Farley MM, Schaffner W, Thomas A, Reingold A, Harrison L, Lexau C, Zansky S, Petit S, Gershman K, et al. Impact of 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease, U.S, 2010-11. ID Week 2012. https://idsa.confex.com/idsa/2012/webprogram/Paper36569.html Accessed 20 January 2013
- Health Protection Agency. Cumulative weekly number of reports of Invasive Pneumococcal Disease due to any of the six serotypes in Prevenar13™ but not in PCV7: persons aged >5 Years in England and Wales by Epidemiological Year: July-June (2005 – To Date). http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/Pneumococcal/EpidemiologicalDataPneumococcal/CurrentEpidemiologyPneumococcal/InPrevenar13NotInPrevenarPCV7/pneumo09Cummulativeweekly5IN13NOTIN7vacc/ Accessed 15 July 2013
- Kaplan SL, Barson WJ, Lin PL, Romero JR, Bradley JS, Tan TQ, Hoffman JA, Givner LB, Mason EO Jr. Early trends for invasive pneumococcal infections in children after the introduction of the 13-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 2013; 32:203-7; PMID:23558320; http://dx.doi.org/10.1097/INF.0b013e318275614b
- Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Effectiveness of the new serotypes in the 13-valent pneumococcal conjugate vaccine. Vaccine 2011; 29:9127-31; PMID:21983361; http://dx.doi.org/10.1016/j.vaccine.2011.09.112
- Domingues CM, Verani JR, Montenegro Renoiner EI, de Cunto Brandileone MC, Flannery B, de Oliveira LH, Santos JB, de Moraes JC; Brazilian Pneumococcal Conjugate Vaccine Effectiveness Study Group. Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: a matched case-control study. Lancet Respir Med 2014; 2:464-71; PMID:24726406; http://dx.doi.org/10.1016/S2213-2600(14)70060-8
- Pan American Health Organization. Informe Regional de SIREVA II, 2006: Datos por país y por grupos de edad sobre las características de los aislamientos de Streptococcus pneumoniae, Haemophilus influenzae y Neisseria meningitidis, en procesos invasores. Washington DC: Pan American Health Organization; 2008.
- Pan American Health Organization. Informe Regional de SIREVA II, 2011: Datos por país y por grupos de edad sobre las características de los aislamientos de Streptococcus pneumoniae, Haemophilus influenzae y Neisseria meningitidis, en procesos invasores. Washington DC: Pan American Health Organization; 2012.
- National Institute for Health and Welfare. Incidence of invasive pneumococcal disease in Finland. https://www.thl.fi/fi/web/thlfi-en/topics/information-packages/incidence-of-invasive-pneumococcal-disease-in-finland Accessed 27 January 2015
- Whitney CG, Pilishvili T, Farley MM, Schaffner W, Craig AS, Lynfield R, Nyquist AC, Gershman KA, Vazquez M, Bennett NM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet 2006; 368:1495-502; PMID:17071283; http://dx.doi.org/10.1016/S0140-6736(06)69637-2
- De Serres G, Pilishvili T, Link-Gelles R, Reingold A, Gershman K, Petit S, Farley MM, Harrison LH, Lynfield R, Bennett NM, et al. Use of surveillance data to estimate the effectiveness of the 7-valent conjugate pneumococcal vaccine in children less than 5 years of age over a 9 year period. Vaccine 2012; 30:4067-72; PMID:22525797; http://dx.doi.org/10.1016/j.vaccine.2012.04.017
- Vesikari T, Wysocki J, Chevallier B, Karvonen A, Czajka H, Arsène JP, Lommel P, Dieussaert I, Schuerman L. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr Infect Dis J 2009; 28: S66-76; PMID:19325449; http://dx.doi.org/10.1097/INF.0b013e318199f8ef
- Institute of Environmental Science and Research Ltd (ESR). Invasive pneumococcal disease in New Zealand, 2013. https://surv.esr.cri.nz/PDF_surveillance/IPD/2013/2013AnnualIPDRpt.pdf Accessed 17 May 2015
- Davis SM, Deloria-Knoll M, Kassa HT, O'Brien KL. Impact of pneumococcal conjugate vaccines on nasopharyngeal carriage and invasive disease among unvaccinated people: review of evidence on indirect effects. Vaccine 2013; 32:133-45; PMID:23684824; http://dx.doi.org/10.1016/j.vaccine.2013.05.005
- Jardine A, Menzies RI, McIntyre PB. Reduction in hospitalizations for pneumonia associated with the introduction of a pneumococcal conjugate vaccination schedule without a booster dose in Australia. Pediatr Infect Dis J 2010; 29:607-12; PMID:20589980; http://dx.doi.org/10.1097/INF.0b013e3181d7d09c
- Cohen R, Levy C, Bingen E, Koskas M, Nave I, Varon E. Impact of 13-valent pneumococcal conjugate vaccine on pneumococcal nasopharyngeal carriage in children with acute otitis media. Pediatr Infect Dis J 2012; 31:297-301; PMID:22330166; http://dx.doi.org/10.1097/INF.0b013e318247ef84
- Becker-Dreps S, Amaya E, Liu L, Moreno G, Rocha J, Briceño R, Alemán J, Hudgens MG, Woods CW, Weber DJ. Changes in childhood pneumonia and infant mortality rates following introduction of the 13-valent pneumococcal conjugate vaccine in Nicaragua. Pediatr Infect Dis J 2014; 33:637-42; PMID:24445827; http://dx.doi.org/10.1097/INF.0000000000000269
- Guevara M, Ezpeleta C, Gil-Setas A, Torroba L, Beristain X, Aguinaga A, García-Irure JJ, Navascués A, García-Cenoz M, Castilla J, et al. Reduced incidence of invasive pneumococcal disease after introduction of the 13-valent conjugate vaccine in Navarre, Spain,2001–2013. Vaccine 2014; 32:2553-62; PMID:24674661; http://dx.doi.org/10.1016/j.vaccine.2014.03.054
- Harboe ZB, Dalby T, Weinberger DM, Benfield T, Mølbak K, Slotved HC, Suppli CH, Konradsen HB, Valentiner-Branth P. Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clin Infect Dis 2014; 59:1066-73; PMID:25034421; http://dx.doi.org/10.1093/cid/ciu524
- Lepoutre A, Varon E, Georges S, Dorléans F, Janoir C, Gutmann L, Lévy-Bruhl D, Microbiologists of Epibac, ORP Networks. Impact of pneumococcal conjugate vaccines on invasive pneumococcal disease in France, 2001-2012. Vaccine 2015; 33:359-66; PMID:25448105; http://dx.doi.org/10.1016/j.vaccine.2014.11.011
- Lim GH, Wormsbecker AE, McGeer A, Pillai DR, Gubbay JB, Rudnick W, Low DE, Green K, Crowcroft NS, Deeks SL. Have changing pneumococcal vaccination programmes impacted disease in Ontario? Vaccine 2013; 31:2680-5; PMID:23597716; http://dx.doi.org/10.1016/j.vaccine.2013.04.007
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis 2015; 15:301-9; PMID:25656600; http://dx.doi.org/10.1016/S1473-3099(14)71081-3
- Regev-Yochay G, Paran Y, Bishara J, Oren I, Chowers M, Tziba Y, Istomin V, Weinberger M, Miron D, Temper V, et al. Early impact of PCV7/PCV13 sequential introduction to the national pediatric immunization plan, on adult invasive pneumococcal disease: a nationwide surveillance study. Vaccine 2015; 33:1135-42; PMID:25613717; http://dx.doi.org/10.1016/j.vaccine.2015.01.030
- Simonsen L, Taylor RJ, Schuck-Paim C, Lustig R, Haber M, Klugman KP. Effect of 13-valent pneumococcal conjugate vaccine on admissions to hospital 2 years after its introduction in the USA: a time series analysis. Lancet Respir Med 2014; 2:387-94; PMID:24815804; http://dx.doi.org/10.1016/S2213-2600(14)70032-3
- Ho PL, Chiu SS, Cheung CH, Lee R, Tsai TF, Lau YL. Invasive pneumococcal disease burden in Hong Kong children. Pediatr Infect Dis J 2006 May; 25:454-5; PMID:16645513; http://dx.doi.org/10.1097/01.inf.0000215004.85582.30
- Rubin JL, McGarry LJ, Strutton DR, Klugman KP, Pelton SI, Gilmore KE, Weinstein MC. Public health and economic impact of the 13-valent pneumococcal conjugate vaccine (PCV13) in the United States. Vaccine 2010; 28:7634-43; PMID:20883739; http://dx.doi.org/10.1016/j.vaccine.2010.09.049
- The Center's for Disease Control and Prevention Active Bacterial Core Surveillance (ABCs) program 2009. Unpublished report.
- Population estimates from Malaysia Census and Statistics Department. www.statistics.gov.my/portal/download_Population/files/census2010/Taburan_Penduduk_dan_Ciri-ciri_Asas_Demografi.pdf Accessed 23 June 2013
- Population estimates from Hong Kong Census and Statistics Department. http://www.censtatd.gov.hk/hong_kong_statistics/statistical_tables/index.jsp?charsetID=1&subjectID=1&tableID=152 Accessed 23 June 2013
- WHO. Malaysia: WHO and UNICEF estimates of immunization coverage: 2013 revision. http://www.who.int/immunization/monitoring_surveillance/data/mys.pdf Accessed 17 May 2015
- Weinstein MC, O'Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, Luce BR; ISPOR Task Force on Good Research Practices–Modeling Studies. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices–Modeling Studies. Value Health 2003; 6:9-17; PMID:12535234; http://dx.doi.org/10.1046/j.1524-4733.2003.00234.x
- Hsieh YC, Lin PY, Chiu CH, Huang YC, Chang KY, Liao CH, Chiu NC, Chuang YC, Chen PY, Chang SC, et al. National survey of invasive pneumococcal diseases in Taiwan under partial PCV7 vaccination in 2007: emergence of serotype 19A with high invasive potential. Vaccine 2009; 27:5513-8; PMID:19615960; http://dx.doi.org/10.1016/j.vaccine.2009.06.091
- Maimaiti N, Aljunid S, Ahmed Z, Nur AM, Isa ZM, Sulung S. Clinical burden of pneumococcal disease in Malaysia APCP 2012 (Abstract). Kuching, Malaysia, 2012.
- Wu DB, Chang CJ, Huang YC, Wen YW, Wu CL, Fann CS. Cost-effectiveness analysis of pneumococcal conjugate vaccine in Taiwan: a transmission dynamic modeling approach. Value Health 2012; 15: S15-9; PMID:22265061; http://dx.doi.org/10.1016/j.jval.2012.03.1391
- Sung RYT, Yu CW, Chan JTS. How common is acute otitis media in Hong Kong? HK Pract 2003; 20:114-9
- Lin TY, Shah NK, Brooks D, Garcia CS. Summary of invasive pneumococcal disease burden among children in the Asia-Pacific region. Vaccine 2010; 28:7589-605; PMID:20674872; http://dx.doi.org/10.1016/j.vaccine.2010.07.053
- Low S, Chan FL, Cutter J, Ma S, Goh KT, Chew SK. A national study of the epidemiology of pneumococcal disease among hospitalised patients in Singapore: 1995 to 2004. Singapore Med J 2007; 48:824-9; PMID:17728963
- Sirinavin S, Vorachit M, Thakkinstian A, Hongsanguensri S, Wittayawongsruji P. Pediatric invasive pneumococcal disease in a teaching hospital in Bangkok. Int J Infect Dis 2003; 7:183-9; PMID:14563221; http://dx.doi.org/10.1016/S1201-9712(03)90050-6
- Wardlaw TM, Johansson EW, Hodge M, UNICEF/WHO. Pneumonia: the forgotten killer of children. 2006.
- Ahmed Z, Aljunid S, Maimaiti N, Nur AM, Isa ZM, Sulung S. Economic burden of pneumococcal disease in Malaysia. APCP 2012 (abstract). Kuching, Malaysia, 2012.
- Total expenditure in Malaysia. Available at http://www.who.int/countries/mys/en/ Accessed 14 Oct 2012.
- Total expenditure in the US. Available at http://www.who.int/countries/usa/en/ Accessed 14 Oct 2012.
- Nakamura MM, Tasslimi A, Lieu TA, Levine OS, Knoll MD, Russell LB, Sinha A. Cost effectiveness of child pneumococcal conjugate vaccination in middle-income countries. Int Health 2011; 3:270-81; PMID:24038500; http://dx.doi.org/10.1016/j.inhe.2011.08.004
- Malaysian Investment Development Authority. Available at www.mida.gov.my/env3/index.php?page=wage-rates. Accessed 10 May 2013.
- Wages and Labour Earnings 2011. http://www.censtatd.gov.hk/m/o210.jsp. Accessed 13 May 2013.
- Department of Statistics Malaysia, official portal 2012. www.statistics.gov.my Accessed 13 May 2013.
- Census and Statistics Department. www.censtatd.gov.hk/hkstat/sub/sp270.jsp Accessed 13 May 2013.
- Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, Elvin L, Ensor KM, Hackell J, Siber G, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J 2000; 19:187-95; PMID:10749457; http://dx.doi.org/10.1097/00006454-200003000-00003
- Jauneikaite E, Jefferies JM, Hibberd ML, Clarke SC. Prevalence of Streptococcus pneumoniae serotypes causing invasive and non-invasive disease in South East Asia: a review. Vaccine 2012; 30:3503-14; PMID:22475858; http://dx.doi.org/10.1016/j.vaccine.2012.03.066
- Ho PL, Chiu SS, Ang I, Lau YL. Serotypes and antimicrobial susceptibilities of invasive Streptococcus pneumoniae before and after introduction of 7-valent pneumococcal conjugate vaccine, Hong Kong, 1995-2009. Vaccine 2011; 29:3270-5; PMID:21352937; http://dx.doi.org/10.1016/j.vaccine.2011.02.025
- Hansen J, Black S, Shinefield H, Cherian T, Benson J, Fireman B, Lewis E, Ray P, Lee J. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using World Health Organization standardized interpretation of chest radiographs. Pediatr Infect Dis J 2006; 25:779-81; PMID:16940833; http://dx.doi.org/10.1097/01.inf.0000232706.35674.2f
- Fireman B, Black SB, Shinefield HR, Lee J, Lewis E, Ray P. Impact of the pneumococcal conjugate vaccine on otitis media. Pediatr Infect Dis J 2003; 22:10-6; PMID:12544402; http://dx.doi.org/10.1097/00006454-200301000-00006
- Lieu TA, Ray GT, Black SB, Butler JC, Klein JO, Breiman RF, Miller MA, Shinefield HR. Projected cost-effectiveness of pneumococcal conjugate vaccination of healthy infants and young children. JAMA 2000; 283:1460-8; PMID:10732936; http://dx.doi.org/10.1001/jama.283.11.1460
- Ray GT, Whitney CG, Fireman BH, Ciuryla V, Black SB. Cost-effectiveness of pneumococcal conjugate vaccine: evidence from the first 5 years of use in the United States incorporating herd effects. Pediatr Infect Dis J 2006; 25:494-501; PMID:16732146; http://dx.doi.org/10.1097/01.inf.0000222403.42974.8b
- Zhou F, Kyaw MH, Shefer A, Winston CA, Nuorti JP. Health care utilization for pneumonia in young children after routine pneumococcal conjugate vaccine use in the United States. Arch Pediatr Adolesc Med 2007; 161:1162-8; PMID:18056561; http://dx.doi.org/10.1001/archpedi.161.12.1162
- Zhou F, Shefer A, Kong Y, Nuorti JP. Trends in acute otitis media-related health care utilization by privately insured young children in the United States, 1997-2004. Pediatrics 2008; 121:253-60; PMID:18245415; http://dx.doi.org/10.1542/peds.2007-0619
- Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet 2007; 369:1179-86; PMID:17416262; http://dx.doi.org/10.1016/S0140-6736(07)60564-9
- Prymula R, Peeters P, Chrobok V, Kriz P, Novakova E, Kaliskova E, Kohl I, Lommel P, Poolman J, Prieels JP, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet 2006; 367:740-8; PMID:16517274; http://dx.doi.org/10.1016/S0140-6736(06)68304-9
- Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine 2004; 22:4203-14; PMID:15474710; http://dx.doi.org/10.1016/j.vaccine.2004.05.003
- Shepard CW, Ortega-Sanchez IR, Scott II RD, Rosenstein NE, ABCs Team. Cost-effectiveness of conjugate meningococcal vaccination strategies in the United States. Pediatrics 2005; 115:1220-32; PMID:15867028; http://dx.doi.org/10.1542/peds.2004-2514
- Erickson LJ, De Wals P, McMahon J, Heim S. Complications of meningococcal disease in college students. Clin Infect Dis 2001; 33:737-9; PMID:11477523; http://dx.doi.org/10.1086/322587
- Oostenbrook R, Moll H, Essink-Bot ML. The EQ-5D and the Health Utilities Index for permanent sequelae after meningitis: a head-to-head comparison. J Clin Epidemiol 2002; 55:791-99; PMID:12384194; http://dx.doi.org/10.1016/S0895-4356(02)00448-1
- World Health Organization Cost-effectiveness Threshold Values. Available from: www.who.int/choice/en/. Accessed 10 May 2013.
- Malaysian and Hong Kong GDP per capita 2011. Available from: http://en.wikipedia.org/wiki/List_of_countries_by_GDP_(nominal)_per_capita. Accessed 10 May 2013.