Abstract
As the subject of active research and development (R&D) in recent decades, monoclonal antibodies have emerged among the major classes of therapeutic agents for treatment of many human diseases, especially cancers, infections, and immunological disorders. This article surveys the landscape of R&D projects of therapeutic monoclonal antibodies (mAbs), which are mostly used for disease immunotherapy, from a number of perspectives, including therapeutic indications, development phases, participants, and citation of related patents. The results of this research can be used as a reference resource for pharmaceutical researchers, investors, and policymakers in the field of therapeutic mAbs
Abbreviations
| mAbs | = | monoclonal antibodies |
| R&D | = | research and development |
| US. FDA | = | US. Food and Drug Administration |
| HER2 | = | human epidermal growth factor receptor 2 |
| VEGF | = | vascular endothelial growth factor |
| CTLA-4 | = | cytotoxic T-lymphocyte-associated protein 4 |
| PD-1 | = | programmed cell death protein 1 |
| PD-L1 | = | programmed death-ligand 1 |
| Fc | = | crystallizable fragment |
| NK | = | natural killer |
| TA | = | tumor antigen |
| ADCC | = | antibody-dependent cellular cytotoxicity |
| VEGFR | = | vascular endothelial growth factor receptors |
| PDGFR | = | platelet-derived growth factor receptors |
| HHS | = | Health and Human Services |
| PDL | = | Protein Design Labs, Inc. |
| EPO | = | European Patent Office |
| USPTO | = | US. Patent and Trademark Office |
Introduction
Monoclonal antibodies (mAbs) are produced by a single clone of B cells and specifically target a particular antigen. With antibodies having been used as a treatment for disease in the 1890s, the hybridoma technique introduced by Kohler and Milstein in 1975 now makes it possible to obtain pure mAbs in large amounts,Citation1 enhancing their potential for clinical use. Given their advantages of specificity, efficacy, and safety, there is now widespread acceptance of mAbs as innovative therapeutic agents. Orthoclone OKT3 (muromonab-CD3) was the first therapeutic mAb approved by the US. Food and Drug Administration (US. FDA) in 1985, which represents a novel category of immunotherapy with a murine monoclonal antibody against CD3 antigen of human T cells that functions as an immunosuppressant in the treatment of acute transplant rejection and has the potential to induce immune tolerance and inhibit autoimmune inflammatory responses.Citation2,3 After that, the development of mAb as a major class of biopharmaceutical products has flourished,Citation4 with more than 40 mAb products currently approved in the US or Europe for treating cancers, autoimmune diseases, infectious diseases, and cardiovascular diseases.Citation5 To date, mAbs becomes the extremely valuable and successful modality of cancer therapeutics. For example, therapeutic mAbs that can modify tumor cell signaling cascade or tumor-stroma interaction such as trastuzumab (anti-human epidermal growth factor receptor 2 (HER2) mAb), rituximab (anti-CD20 mAb) and bevacizumab (anti-vascular endothelial growth factor (VEGF) mAb) are top-selling anti-cancer drugs.Citation6 More recently, a class of mAb-based immunotherapy named immune-checkpoint inhibitors, such as ipilimumab and tremelimumab (Abs that target cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)), pembrolizumab and nivolumab (Abs that target programmed cell death protein 1 (PD-1)), MPDL-3280A (an Ab target programmed death-ligand 1 (PD-L1)) which represent a breakthrough in the cancer treatment was approved by FDA.Citation7,8 As the highest-growing segment of the global biopharmaceutical market, mAbs generated revenues of US$51.1 billion in 2013 and are predicted to reach sales of US$70 billion by 2015.Citation9 Given the promising future of mAb therapeutics, most pharmaceutical and biotechnology companies are very interested in pursuing R&D in this area.
MAbs are products of immune cells and are a major effector molecule of immune response. The crystallizable fragment (Fc) region of mAbs binds to its receptor expressed by various immune cells, including natural killer (NK) cells, monocytes, macrophages, and granulocytes, which can result in a functional consequence in immune system.Citation10 Moreover, although not being considered as “professional” immunotherapeutics, many mAbs still have the capacity to up- or down-regulate the activation of immune cells, and their immunoregulatory activities are reportedly contribute to their therapeutic effect.Citation6,11-13 This can be exemplified by the mAbs used in the treatment of cancer. The mechanisms of mAbs-mediated anti-cancer effects can be summarized based on their targets. The anti-cancer effect of checkpoint inhibitors, such as ipilimumab, tremelimumab, pembrolizumab, nivolumab, and MPDL-3280A, can trigger anti-tumor immune responses directly by eliminating negative regulatory signals.Citation7,8 However, tumor antigen (TA)-targeting mAb-based treatment, one of the most successful strategies in cancer therapy, can also interact with immune cells through Fc-dependent mechanisms.Citation14 Such TA-specific mAb can also activate adaptive TA-specific immune responses through induction of antibody-dependent cellular cytotoxicity (ADCC), and promote antibody-targeted cross-presentation of tumor Ag, or trigger idiotypic network.Citation15 MAbs targeting tumor stroma which support tumor growth, such as those against VEGF, VEGFR, PDGFR, and C-kit, can also have a major impact on the tumor immunology.Citation16 Thus, it is not surprising that almost all therapeutic mAbs, even against diverse diseases including cancers,Citation10 immunological disorders,Citation17 infections,Citation18 neurological disorders,Citation19 and cardiovascular and cerebrovascular diseases,Citation20 can modulate immune responses directly or indirectly. Therefore, the therapeutic mAbs can be roughly divided into 2 categories based on their direct or indirect regulatory effect of immune cells.
As commonly understood, research and development (R&D) activity is a well-organized process of creation, production, diffusion, and application of knowledge.Citation21 Currently available innovative products are the outcome of earlier R&D while current R&D hopes to generate future new commercial products. A comprehensive analysis of R&D activities, in terms of both technology and the commercial environment, is therefore of great interest to all stakeholders. Recently, several studies have analyzed therapeutic mAb R&D from the viewpoints of pure science,Citation22,23 milestone cases,Citation24 market and policy,Citation25 and patented technologies.Citation26 However, few studies to date have analyzed this R&D with reference to pipeline projects, which not only contain technological and commercial information but can be conducted on a large scale and, more importantly, can capture technology flow and evolution through related patent citations. The present study therefore aims to overview the landscape of therapeutic mAb R&D by looking at some of these pipeline projects from a number of perspectives, including therapeutic indications, development phases, participants, and citations of related patents, with potentially valuable insights for pharmaceutical researchers, investors, and policymakers in this field.
Therapeutic Applications of MAbs
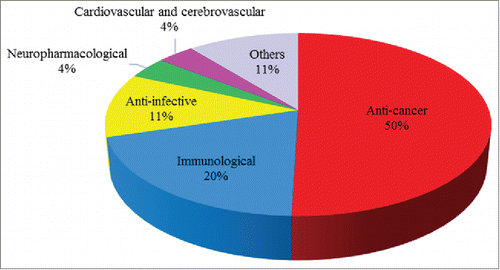
In recent years, mAbs have been widely studied in the treatment of various diseases, including cancers, autoimmune diseases, poisoning, cardiovascular diseases, and infections.Citation27,28 To better depict the status and trends of therapeutic mAbs research, we excluded the diagnostic mAbs and organized remaining mAbs into 6 therapeutic categories (). Cancer is clearly the most important and dominating area of therapeutic application, accounting for 50% of all mAb-related R&D programs. Immunological indication programs (20%) are the second largest category, while anti-infective programs account for 11%. These three are recognized as the most common therapeutic areas,Citation29,30 followed by relatively new therapeutic applications of mAbs on neuropharmacological indications and cardiovascular and cerebrovascular indications. The remaining programs are classified as “others,” including eye diseases, respiratory diseases, metabolic diseases, etc
Figure 1. Therapeutic categories of mAbs. Category of “others” includes eye diseases, respiratory diseases, metabolic diseases, etc.
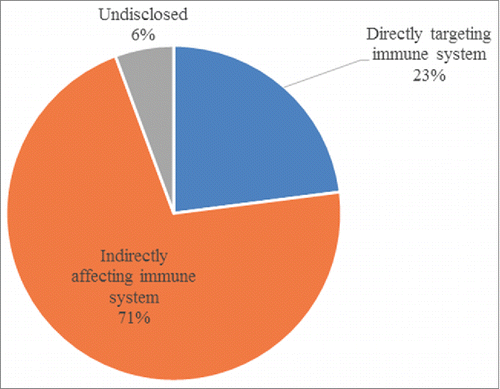
Taking anti-cancer effect of mAbs as example, immunotherapeutic patterns based on the mechanism of action were further investigated. Accordingly, by screening the R&D projects of anti-cancer mAbs, 1324 projects with application in cancer treatment were extracted from the total 2441 projects relevant to mAbs and divided into 3 classes: directly targeting immune system (such as checkpoint inhibitors, et al.), indirectly modulating immune system (TA-specific mAbs, mAbs targeting tumor vasculature and stroma, et al.), and a class of mAbs with undisclosed information on determining their effect mechanisms. The proportion of therapeutic patterns can be shown in . About 23% anti-cancer mAbs directly target immune system. In addition, half of the mAbs directly acting on immune system are against hematological cancers, and the most of such mAbs simultaneously target TAs. The therapeutic mAbs dual-targeting specific TAs or tumor stroma and immune system, account for more than 70% of anti-cancer projects
.Growth Trends in mAb-Related R&D Activities
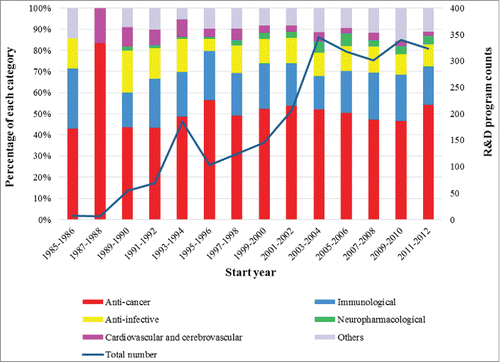
Following early enthusiasm in the 1970s and 1980s, therapeutic mAbs have become established as one of the most important and fastest-growing classes of therapeutic agents.Citation31 For present purposes, the start date of each mAb-related R&D program was retrieved from the summary information in the IMS R&D Focus database. To indicate R&D trends in the field, programs started over time are depicted in . The columns show percentages for the various therapeutic categories in 2-year cohorts; the line graph captures growth trends in mAb therapeutics research
.reveals 2 obvious periods of growth in R&D programs between 1985 and 2012. The first period of rapid increase happened between 1987 and 1994. There are 2 possible explanations for this significant growth. The first FDA-approved mAb-based immunotherapeutics in 1986 inspired a large amount of pharmaceutical companies to invest effort in mAb therapeutics R&D. Additionally, phage display technology—the simplest robust technology for raising mAbs against a given therapeutic target protein—was developed during that same period,Citation32 which may also have renewed pharmaceutical companies' interest in the development of human mAbs. However, patent disputes impeded the broad use of these methods until the end of 1990s, and this may have contributed to the second period of increase in 1999–2004. In addition, ribosome display,Citation33 and yeast display,Citation34 technologies for generation of human antibodies were developed in 1997; these had the potential to generate larger libraries by eliminating the need to transform cells to generate such libraries, which also significantly impacted the R&D performance of mAb therapeutics.
As shown in , the percentages of anti-cancer, immunological, and anti-infective programs in each 2 years are relatively large and stable, indicating the mainstream concerns in therapeutic mAbs research. R&D relating to cardiovascular and cerebrovascular indications as well as neuropharmacological indications first appeared in the late 1980s and early 1990s, during the first period of rapid increase. The “others” category has also expanded since then, as mAb R&D therapeutics modulating immune response became more diverse (see Supplementary 1). These trends indicate how the advance of mAb technology has opened the door to a wide range of possible clinical applications.
Distribution of mAbs Projects by Pipeline Phase
The development of new drugs is known to be a long, complex, and expensive activity, requiring a discovery stage and several clinical phases before commercial release. shows the latest phases of mAb-related R&D programs by percentage. It is worth noting that the designations of the phases of the projects are based not fully on definitions by regulatory authorities but on company definitions endorsed by IMS Health. Thus, designations of the phases of the projects may not be accurate definitely, especially given that there can be a financial or other incentive on the part of the developer to lean toward naming a more advanced stage of development (e.g., phase II rather than phase I) for the benefit of the company. According to IMS Health, discovery phase qualifies all discovery agreements and research programs, where no lead compounds have yet been identified; preclinical phase covers early basic research, as well as in vitro and in vivo studies. Obviously, such allocations are based on the judgment not rigorous definition of the developing group, and that there also can be an incentive - financial or otherwise - for a company to call a given MAb ‘preclinical’ rather than ‘discovery’, hence potentially skewing the reported numbers of such MAbs. As far as sample mAbs projects are concerned, most (more than one-third) are in preclinical phase, followed by discontinued and discovery phase programs. It must be noted that the share of discontinued programs may be underestimated because many companies, in the real world, do not report a discontinuation, but instead simply stop discussing these projects. Moreover, phase I and phase II account for approximately 10% while only less than 3% are at phase III which is the most pivotal phase of R&D before the drug reaches the market. Clearly, although clinical applications of mAbs have been in development for several decades, considerable difficulties and risks remain in this area of research
Figure 4. Share of mAbs-related R&D programs at different pipeline phases. The designations of the phases of the projects are based not fully on definitions by regulatory authorities but on company definitions endorsed by IMS Health.
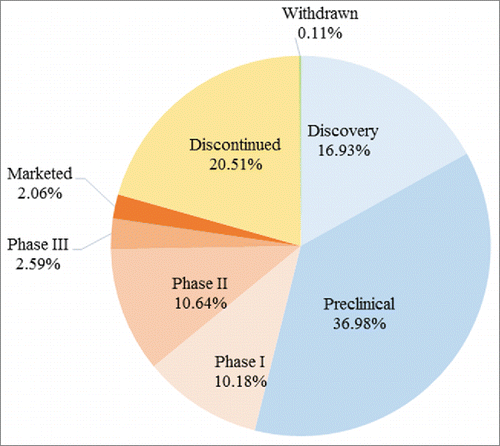
To clarify the research status of the different therapeutic categories, we calculated the percentage of programs at different phases in each category (). The biggest share in all categories represents the early stages of drug discovery; the discovery and preclinical stages, during which promising candidates are identified, account for more than 40%. The small percentage reaching phase III and marketed phases, as well as the large percentage of discontinued programs, indicate the difficulty of successfully bringing a therapeutic mAb agent to market. For anti-infective and neuropharmacological indications in particular, only a small fraction (or minority) of R&D programs reach the clinical and marketed phases, with a relatively large percentage ending in the early stage of discovery or discontinued phase. This clearly indicates that mAb R&D related to anti-infective and neuropharmacological indications is high-risk
Table 1. Comparison of the share of mAb-related R&D programs at different pipeline phases across therapeutic areas
Companies Involved in Therapeutic mAbs Research
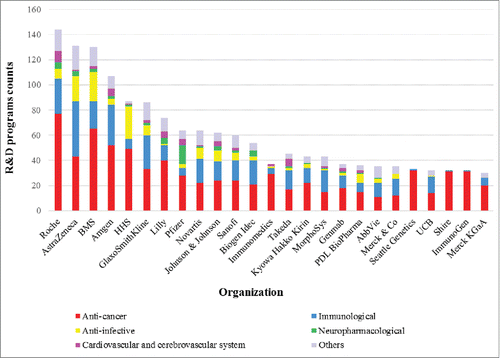
Given these promising development trends, it is not surprising that mAb therapeutics has attracted a great deal of attention from the pharmaceutical industry, and market incentives and further technological advances have encouraged some companies to build their own or partnered pipelines. At the end of 2013, a total of 816 companies were involved in mAb therapeutics R&D, most of them small, young biopharmaceutical companies. Many of these have focused on basic technological platforms and preclinical studies, enabling them to achieve competitive status in the biopharmaceutical market.Citation25 However, their lack of funds or market experience means that the likely destiny of these small companies is to be acquired or incorporated into large pharmaceutical enterprises,Citation35 for example, GlycArt and ARIUS Research were acquired by Roche; Johnson & Johnson completed the acquisition of Centocor and Crucell; and Lilly acquired ImClone Systems and Applied Molecular Evolution as its wholly-owned subsidiaries. In addition, big pharmaceutical firms seem much more willing to cooperate with biotechnology firms, as in Roche's cooperation with Genmab and AstraZeneca's collaboration with Amgen.
It is clear from that larger pharmaceutical and biotech companies account for the majority of mAb-related R&D programs, as they can sustain the huge manpower, materials, and financial resources needed to implement R&D programs and guide new mAbs through development, production, and licensing.Citation36 Only one public sector agency, the US Department of Health and Human Services (HHS), makes the top 25. As a government agency, HHS has significant financial backing, affording it an important position in mAb R&D through its involvement in multiple programs. Given its goal of protecting the health of all Americans and providing essential human services, HHS's mAb R&D activities focus primarily on anti-cancer and anti-infective indications. Other public sector agencies, which include academic or nonprofit research institutions, participate in fewer R&D programs, and most of them choose to partner with pharmaceutical companies in the co-development of therapeutic mAbs. Other than Seattle Genetics, Shire, and ImmunoGen, which focus mainly on cancer treatments, pharmaceutical companies with the largest number of programs tend to cover multiple therapeutic areas and develop immunotherapeutic mAbs of both directly and indirectly targeting immune system. This further confirms the trend to diversification in mAb R&D followed by most of the companies involved
.Patent Citation Network of mAbs
Over decades of development, mAb technology has evolved and flourished. Tracing the development trajectory of mAb technology is essential to understanding this evolving process. As an important element of R&D outcomes, patents can reflect value and shed light on possibilities for development.Citation37 Patent citations are effective tools in providing useful insights into technology transfer processes and flows.Citation38-41 In the present study, a large number of sampled pipeline projects were found to contain relevant patent data, which was extracted and processed to construct the patent citation network in
Figure 6. Patent citation network of mAbs. Nodes represent patents; edges represent the citation relationship between patents. Different colors indicate patents associated with different therapeutic areas. Colors corresponding to the specific therapeutic areas mentioned above, with the exception of purple nodes, which represent patents in multiple therapeutic areas.
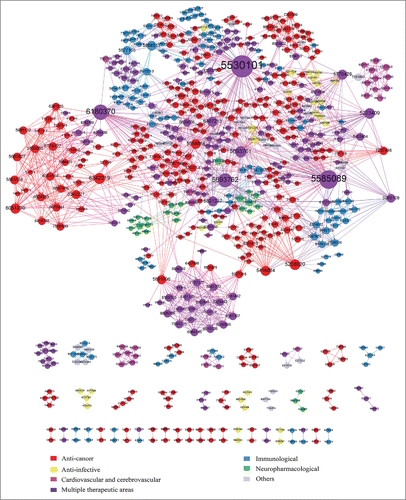
In total, 669 patents were used to construct this network, with 1848 internal citation relations (). The direction of arrows represents the direction of technology flow captured by the citations. Patent citations are usually divided into backward (citations made by a patent to previously issued patents) and forward (citations received by a patent from subsequently issued patents). In the citation network, in-degree of a node refers to the number of its backward citations, and out-degree refers to the number of forward citations, reflecting the position of the patents in this citation network. Patents with a high in-degree are “summarizers” that have gleaned knowledge from many previous patents, often protecting new products or compositions, such as US 8557780 (2013), US 8895266 (2014), and US 8883980 (2014). Most of the important summarizers have been granted in recent years. In contrast, patents with a high out-degree are cited mostly by subsequent patents, indicating that these patents refer to basic and essential technologies of great influence and play a significant role in the further development of mAb therapeutics. The five patents with highest out-degree in this network are US 5530101 (1996), US 5585089 (1996), US 6180370 (2001), US 5693762 (1997), and US 5693761 (1997). Interestingly, all of these are held by Protein Design Labs, Inc.. (PDL). Moreover, they belong to the same patent family, protecting novel methods for producing humanized antibodies, specifically reactive with strong affinity to a predetermined antigen, and overcoming the limitations of mAb technology in developing from chimeric mAbs to humanized mAbs.Citation42 Their wide forward citations enrich the entire mAbs patent citation network, which reflects the technically fundamental and dominant position of humanized mAbs in mAb therapeutics R&D.
It is worth noting the technology flow across different communities, consisting of a group of nodes that are relatively densely connected to each other but sparsely connected to other dense groups in the network.Citation43 It is obvious from that the biggest block includes several communities (e.g.,, the lower central purple one and the red community on the left) while many small communities are isolated in the lower part of the network. Interestingly, these dense communities are basically composed of nodes with same colors—that is, patents in the same therapeutic areas. All communities above middle sized (e.g., anti-cancer, cardiovascular and cerebrovascular) are connected to the biggest block through technology flow across therapeutic areas. In other words, for mAb, internal technology flow within specific therapeutic areas is very frequent. Where technologies can develop to an extent flow across areas, such flows may be an important attribute in forming large-size technology communities. By comparison with a previous study,Citation40 the network in this study is completely different, with a patent citation network on anti-Alzheimer's drugs showing the high dispersion and great difficulty of technology flow across therapeutic mechanisms. This may be seen to imply that mAbs as a powerful tool has strong diffusion capability and can be widely applied in different areas.
Discussion
After three decades of research and development, mAbs have dramatically transformed from scientific tools to powerful human therapeutics to become the fastest growing area in the biopharmaceutical sector.Citation44,45 As a fundamental contribution, this study describes the overall landscape and developing trends in mAb therapeutic development by reference to multiple parameters of mAb-related R&D programs.
The rapid increase in the scale of research confirms the great promise of therapeutic mAbs. More than 2000 R&D programs have been conducted since 1985, with accelerated increases after 1987 and 1999. In both of these periods of rapid increase, the mAb industry has been inspired by new technology's emergence or new product approvals, either of which may account for these spikes in growth. Typically, technological advances in a field will promote the industry's prosperity, and it is reasonable to believe that the rapid increase in mAb therapeutic development owes to advances in basic research technology. It seems likely that the emergence of further new techniques will again stimulate a research upsurge in mAb therapeutics, requiring companies to pay close attention to emergent technologies.
With the improvement of mAb-related technologies and the expansion of emerging market demand, the therapeutic applications of mAbs have gradually broadened. Initially used in the diagnosis, monitoring, and prognosis of diseases, research has led to the use of mAbs as therapeutic agents in a range of diseases, including cancers, autoimmune diseases, and infections. Herceptin, approved by the FDA in 1998, was the first mAb to be used for treating women with metastatic breast cancers overexpressing HER2, to excellent therapeutic effect.Citation46 Given the outstanding therapeutic advantages of mAbs—high targeting specificity, high affinity binding to targets, and limited side effects—research now encompasses a wide range of diseases beyond the 3 main therapeutic areas,Citation47 including disorders of the nervous system, respiratory system, and cardiovascular system, as well as eye disease, diabetes, and skin diseases. Diversification has become a seemingly inevitable trend; analyzing the latest phase profile of each therapeutic category, it is surprising to find that some new therapeutic areas show more programs in clinical phases than those in traditional therapeutic fields, indicating their obvious promise in new drug research and development. Nevertheless, the original 3 therapeutic areas still account for most of the market share, indicating a high level of ongoing interest in these areas. More noteworthy is that mAb R&D is still high-risk, especially considering discontinued programs in the real world but underreported in IMS R&D Focus database.
The development of therapeutic mAbs is closely related to underlying technical innovations. To date, several key technologies such as CDR grafted technology, phage display, transgenic mice, and Fc sequences have been well developed. The relevant technology flow is relatively compact and centralized, and our patent citation network presents the dense and complex connections among different technologies associated with mAb applications. Patents with the largest out-degree (i.e., humanized mAbs) clearly dominate therapeutic mAb R&D. It has been noted that fully human mAbs have gradually become the hottest topic in the field since the 2000s.Citation29,30,48,49 This pattern has not as yet been explained by reference to the citation network. By comparison with humanized mAbs, fully human mAbs form less complicated patent citation relationships, probably because patents usually follow long after R&D activities.
In summary, the present study maps the landscape of therapeutic mAb R&D activities and the technology flow of mAb techniques. These technologies have made unceasing progress in recent decades, and a significant number of R&D programs are promoted by technological advances. These advances have also helped to diversify the therapeutic applications of mAbs, indicating the prospective research and development trend for mAbs-related clinical applications.
Methods
IMS R&D Focus is a comprehensive and well-structured database that monitors pharmaceutical R&D projects worldwide, from discovery phase to marketed phase. In total, the present study retrieved 2441 such projects from the database, searching the criteria “action: monoclonal antibody,” further collecting comprehensive and detailed data for each of these R&D projects such as indicationsa, latest phase, starting year, relevant patents, and companies. Additionally, descriptive statistics for the research sample were assembled in relation to therapeutic areas, years, R&D phases, and companies.
For the purpose of understanding technology flow and diffusion in this field, we explored the profile of patents relevant to mAbs by constructing the patent citation network. First, the original patent information for these R&D programs was extracted. Second, the same method was applied to the existing literature,Citation40 to retrieve stable citation data. That is to say, these original patents were transformed into corresponding United States patents using the patent family system of the European Patent Office (EPO), and citation data for these sampled United States patents were obtained from the US. Patent and Trademark Office (USPTO). Finally, the evolution of mAb technology was visualized by constructing the patent citation network, using the software Gephi.Citation50 In the network, each node represents a patent, and the edge between 2 nodes indicates the patent citation relationship. Different colors indicate patents associated with different therapeutic areas. The arrow denotes technology flow, with the head pointing from cited to citing patents. In the directed citation network, in-degree of a node refers to the number of its backward citations, and out-degree denotes the number of forward citations.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Note
aIndications of mAb-related R&D programs were classified for simplicity into six therapeutic categories (anti-cancer, immunological, anti-infective, neuropharmacological, cardiovascular and cerebrovascular, and others). Anti-cancer indications included mAbs for treating various types of cancers; anti-infective indications included mAbs against viral infection, bacterial infection, or fungal infection; immunological indications were assigned to mAbs as a treatment for transplant rejection, inflammation, systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, or several other autoimmune diseases.
Supplemental_Figure_1.zip
Download Zip (14.7 KB)Funding
We thank the University of Macau for financial support for this research by the project MYRG2015-00172-ICMS-QRCM.
References
- Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975; 256:495-7; PMID:1172191; http://dx.doi.org/10.1038/256495a0
- Smith SL. Ten years of Orthoclone OKT3 (muromonab-CD3): a review. J Transpl Coord 1996; 6:109-19; PMID:9188368; http://dx.doi.org/10.7182/prtr.1.6.3.8145l3u185493182
- Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol 2007; 7:622-32; PMID:17641665; http://dx.doi.org/10.1038/nri2134
- Leavy O. Therapeutic antibodies: past, present and future. Nat Rev Immunol 2010; 10:297; PMID:20422787; http://dx.doi.org/10.1038/nri2763
- Niwa R, Satoh M. The Current Status and Prospects of Antibody Engineering for Therapeutic Use: Focus on Glycoengineering Technology. J Pharm Sci US 2015; 104:930-41; PMID:25583555
- Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer 2012; 12:278-87; PMID:22437872; http://dx.doi.org/10.1038/nrc3236
- Naidoo J, Page DB, Wolchok JD. Immune modulation for cancer therapy. Brit J Cancer 2014; 111:2214-9; PMID:25211661; http://dx.doi.org/10.1038/bjc.2014.348
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015; 348:56-61; PMID:25838373; http://dx.doi.org/10.1126/science.aaa8172
- Kuystermans D, Al-Rubeai M. Biopharmaceutical Products from Animal Cell Culture. Animal Cell Culture, Mohamed Al-Rubeai, editor; Springer, Dordrecht, The Netherlands; 2015; pp:717-57
- Weiner GJ. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer 2015; 15:361-70; PMID:25998715; http://dx.doi.org/10.1038/nrc3930
- Rimawi MF, Schiff R, Osborne CK. Targeting HER2 for the treatment of breast cancer. Annu Rev Med 2015; 66:111-28; PMID:25587647; http://dx.doi.org/10.1146/annurev-med-042513-015127
- Lavaud P, Andre F. Strategies to overcome trastuzumab resistance in HER2-overexpressing breast cancers: focus on new data from clinical trials. BMC Med 2014; 12:132; PMID:25285786; http://dx.doi.org/10.1186/s12916-014-0132-3
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Bio 2001; 2:127-37; PMID:11252954; http://dx.doi.org/10.1038/35052073
- Michaud HA, Eliaou JF, Lafont V, Bonnefoy N, Gros L. Tumor antigen-targeting monoclonal antibody-based immunotherapy: Orchestrating combined strategies for the development of long-term antitumor immunity. OncoImmunology 2014; 3:e955684; PMID:25941618; http://dx.doi.org/10.4161/21624011.2014.955684
- Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet 2009; 373:1033-40; PMID:19304016; http://dx.doi.org/10.1016/S0140-6736(09)60251-8
- Terme M, Colussi O, Marcheteau E, Tanchot, E. Tartour C, Tartour E, Taieb J. Modulation of immunity by antiangiogenic molecules in cancer. Clin Dev Immunol 2012; 2012:492920; PMID:23320019; http://dx.doi.org/10.1155/2012/492920
- Townsend MJ, Monroe JG, Chan AC. B-cell targeted therapies in human autoimmune diseases: an updated perspective. Immunol Rev 2010; 237:264-83; PMID:20727041; http://dx.doi.org/10.1111/j.1600-065X.2010.00945.x
- Reichert JM, Dewitz MC. Anti-infective monoclonal antibodies: perils and promise of development. Nat Rev Drug Discov 2006; 5:191-5; PMID:16518372; http://dx.doi.org/10.1038/nrd1987
- Bosch X, Saiz A, Ramos-Casals M. Monoclonal antibody therapy-associated neurological disorders. Nat Rev Neurol 2011; 7:165-72; PMID:21263460; http://dx.doi.org/10.1038/nrneurol.2011.1
- Foltz IN, Karow M, Wasserman SM. Evolution and Emergence of Therapeutic Monoclonal Antibodies What Cardiologists Need to Know. Circulation 2013; 127:2222-30; PMID:23733968; http://dx.doi.org/10.1161/CIRCULATIONAHA.113.002033
- Wang EC, Huang W. Relative efficiency of R&D activities: A cross-country study accounting for environmental factors in the DEA approach. Res Policy 2007; 36:260-73; http://dx.doi.org/10.1016/j.respol.2006.11.004
- Li J, Zhu Z. Research and development of next generation of antibody-based therapeutics. Acta Pharmacol Sin 2010; 31:1198-207; PMID:20694021; http://dx.doi.org/10.1038/aps.2010.120
- Liu JKH. The history of monoclonal antibody development–Progress, remaining challenges and future innovations. Ann Med Surg 2014; 3:113-6; PMID:25568796; http://dx.doi.org/10.1016/j.amsu.2014.09.001
- Kudrin A, Knezevic I, Joung J, Kang HN. Case studies on clinical evaluation of biosimilar monoclonal antibody: Scientific considerations for regulatory approval. Biologicals 2015; 43:1-10; PMID:25467836; http://dx.doi.org/10.1016/j.biologicals.2014.11.002
- Pavlou AK, Belsey MJ. The therapeutic antibodies market to 2008. Eur J Pharm Biopharm 2005; 59:389-96; PMID:15760719; http://dx.doi.org/10.1016/j.ejpb.2004.11.007
- Petering J, McManamny P, Honeyman J. Antibody therapeutics–the evolving patent landscape. N Biotechnol 2011; 28:538-44; PMID:21515427; http://dx.doi.org/10.1016/j.nbt.2011.03.023
- Berger M, Shankar V, Vafai A. Therapeutic applications of monoclonal antibodies. Am J Med Sci 2002; 324:14-30; PMID:12120821; http://dx.doi.org/10.1097/00000441-200207000-00004
- ElBakri A, Nelson PN, Odeh ROA. The state of antibody therapy. Hum Immunol 2010; 71:1243-50; PMID:20849901; http://dx.doi.org/10.1016/j.humimm.2010.09.007
- Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC. Monoclonal antibody successes in the clinic. Nat Biotechnol 2005; 3:1073-8; PMID:16151394; http://dx.doi.org/10.1038/nbt0905-1073
- Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov 2010; 9:767-74; PMID:20811384; http://dx.doi.org/10.1038/nrd3229
- Stern M, Herrmann R. Overview of monoclonal antibodies in cancer therapy: present and promise. Crit Rev Oncol Hematol 2005; 54:11-29; PMID:15780905; http://dx.doi.org/10.1016/j.critrevonc.2004.10.011
- Winter G, Griffiths AD, Hawkins RE, Hoogenboom HR. Making antibodies by phage display technology. Annu Rev Immunol 1994; 12:433-55; PMID:8011287; http://dx.doi.org/10.1146/annurev.iy.12.040194.002245
- Hanes J, Plückthun A. In vitro selection and evolution of functional proteins by using ribosome display. Proc Natl Acad Sci USA 1997; 94:4937-42; PMID:9144168; http://dx.doi.org/10.1073/pnas.94.10.4937
- Boder ET, Wittrup KD. Yeast surface display for screening combinatorial polypeptide libraries. Nat Biotechnol 1997; 15:553-7; PMID:9181578; http://dx.doi.org/10.1038/nbt0697-553
- Audretsch D, Feldman M. Small-firm strategic research partnerships: The case of biotechnology. Technol Anal Strateg 2003; 15:273-88; http://dx.doi.org/10.1080/0953732032000051154
- Piggee C. Therapeutic antibodies coming through the pipeline. Anal Chem 2008; 80:2305-10; PMID:18456912; http://dx.doi.org/10.1021/ac086033v
- Breitzman AF, Mogee ME. The many applications of patent analysis. J Inf Sci 2002; 28:187-205; http://dx.doi.org/10.1177/016555150202800302
- Narin F. Patent bibliometrics. Scientometrics 1994; 30:147-55; http://dx.doi.org/10.1007/BF02017219
- Stolpe M. Determinants of knowledge diffusion as evidenced in patent data: the case of liquid crystal display technology. Research Policy 2002; 31:1181-98; http://dx.doi.org/10.1016/S0048-7333(01)00192-5
- Xu J, Kong X, Qiu L, Geng X, Hu Y, Wang Y. Research and development of anti-Alzheimer's drugs: an analysis based on technology flows measured by patent citations. Expert Opin Ther Pat 2014; 24:791-800; PMID:24798577; http://dx.doi.org/10.1517/13543776.2014.915943
- Kong X, Hu Y, Cai Z, Yang F, Zhang Q. Dendritic-cell-based technology landscape: Insights from patents and citation networks. Human Vacc Immunother 2015; 11:682-8; PMID:25714961; http://dx.doi.org/10.1080/21645515.2015.1008857
- Queen CL, Selick HE, inventors; Humanized immunoglobulins. Unite States patent US 5,530,101. 1996 June 25
- Lancichinetti A, Kivela M, Saramaki J, Fortunato S. Characterizing the community structure of complex networks. PLoS One 2010; 5:e11976; PMID:20711338; http://dx.doi.org/10.1371/journal.pone.0011976
- Buss NA, Henderson SJ, McFarlane M, Shenton JM, de Haan L. Monoclonal antibody therapeutics: history and future. Curr Opin Pharmacol 2012; 12:615-22; PMID:22920732; http://dx.doi.org/10.1016/j.coph.2012.08.001
- Kim SJ, Park Y, Hong HJ. Antibody engineering for the development of therapeutic antibodies. Mol Cells 2005; 20:17-29; PMID:16258237
- Shak S. Overview of the trastuzumab (Herceptin) anti-HER2 monoclonal antibody clinical program in HER2-overexpressing metastatic breast cancer. Herceptin Multinational Investigator Study Group. Semin Oncol 1999; 26:71-7; PMID:10482196
- Alkan SS. Monoclonal antibodies: the story of a discovery that revolutionized science and medicine. Nat Rev Immunol 2004; 4:153-6; PMID:15040588; http://dx.doi.org/10.1038/nri1265
- Weiner LM. Fully human therapeutic monoclonal antibodies. J Immunother 2006; 29:1-9; PMID:16365595; http://dx.doi.org/10.1097/01.cji.0000192105.24583.83
- Zhang MY, Lu JJ, Wang L, Gao ZC, Hu H, Ung COL, Wang YT. Development of monoclonal antibodies in China: overview and prospects. BioMed Res Int 2015; 2015:168935; PMID:25811022
- Bastian M, Heymann S, Jacomy M. Gephi: an open source software for exploring and manipulating networks. ICWSM 2009; 8:361-2