Abstract
This study evaluated the immunogenicity of the human rotavirus (RV) vaccine (RIX4414) when co-administered with routine childhood vaccines in Chinese infants (NCT01171963). Healthy infants aged 6–16 weeks received 2 doses of either RIX4414 or placebo according to a 0, 1-month schedule. Infants received routine diphtheria-tetanus-acellular pertussis (DTPa) and oral poliovirus (OPV) vaccines either separately from or concomitantly with RIX4414/placebo (separate and co-administration cohorts, respectively). Anti-RV IgA seroconversion rates (one month post-dose-2) and seropositivity rates (at one year of age) were measured using ELISA. Immune responses against the DTPa and OPV antigens were measured one month post-DTPa dose-3 in the co-administration cohort. Solicited local and general symptoms were recorded for 8-days post-vaccination (total cohort). The according-to-protocol immunogenicity population included 511 infants in the separate cohort and 275 in the co-administration cohort. One month post-RIX4414 dose-2, anti-RV IgA seroconversion rates were 74.7% (95% confidence interval [CI]: 68.9–79.9) and 64.2% (95% CI: 55.4–72.3) in the separate and co-administration cohorts; seropositivity rates at one year of age were 71.5% (95% CI: 65.5–77.1) and 50.0% (95% CI: 40.9–59.1), respectively. One month post-DTPa dose-3, all infants in the co-administration cohort were seroprotected against diphtheria and tetanus, and seropositive for pertussis toxoid, pertactin and filamentous haemaglutinin. Two months post-OPV dose-3, seroprotection rates against anti-poliovirus types 1, 2 and 3 were >99% in the co-administration cohort. Reactogenicity profiles were similar in both cohorts. RIX4414 was immunogenic and well-tolerated in Chinese infants and did not appear to interfere with the immunogenicity and reactogenicity of co-administered routine childhood vaccines.
Introduction
Rotavirus (RV) is an important cause of severe gastroenteritis (GE) in children younger than 5 years of age throughout the world.Citation1,2 Estimates in 2008 from the World Health Organization (WHO) indicated that each year approximately 453,000 child deaths worldwide could be associated with RVGE.Citation3 In China, approximately 13,400 children less than 5 years of age died from RV in 2002, of which 70% of cases occurred in rural areas.Citation4 Between 2003 and 2007, 48% of children hospitalized in China with diarrhea had RV. The highest burden of disease was observed in children younger than 2 years of age.Citation5
Vaccination as a primary prophylactic measure, may substantially reduce the disease burden associated with RVGE.Citation3 Two, live-attenuated, oral RV vaccines are currently available in many countries: a monovalent human RV vaccine (RIX4414; Rotarix™, GSK, Belgium) and a pentavalent human-bovine RV vaccine (Rotateq→, Merck & Co., USA).Citation6-8 In China, the Lanzhou lamb RV vaccine (LLR; Lanzhou Institute of Biomedical Products, China) is the only currently approved RV vaccine; it has an immunization coverage of 25%.Citation9
RIX4414 is recommended as a 2-dose schedule in infants, which are often co-administered with other routine childhood vaccines. Although data are available on the immunogenicity of RIX4414 when co-administered with recommended routine childhood vaccines in other countries,Citation10-12 this study was undertaken to obtain additional data in Chinese infants. The primary results of the trial that evaluated the efficacy and safety of RIX4414 against severe RVGE in Chinese infants during the first 2 years of life have been published elsewhere.Citation13 In this paper, we present the immunogenicity and reactogenicity of RIX4414 with co-administered childhood vaccines.
Results
Study participants and demographics
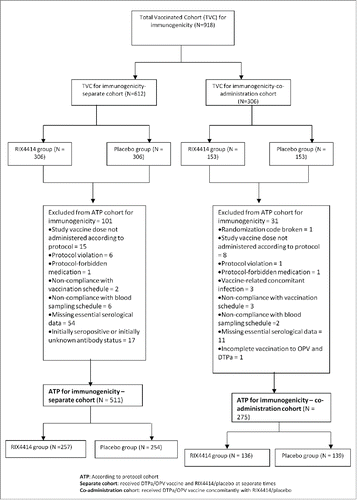
A total of 918 infants were included in the Total Vaccinated Cohort (TVC) for immunogenicity, of whom 612 and 306 were included in the separate administration and co-administration cohorts, respectively (). The According-to-Protocol (ATP) immunogenicity analysis included 511 subjects in the separate administration cohort (RIX4414 = 257; placebo = 254) and 275 in the co-administration cohort (RIX4414 = 136; placebo = 139). The demographic characteristics of the RIX4414 (N=391) or placebo (N=393) groups and both sub-cohorts are presented in . All infants in this study were Chinese.
Table 1. Demographic characteristics (ATP immunogenicity cohort).
Immunogenicity
One month after the second RIX4414 dose, 71.1% (95% confidence interval [CI]: 66.3–75.5) of all RIX4414 recipients seroconverted for anti-RV Immunoglobulin A (IgA) compared with 5.6% (95% CI: 3.5–8.4) of placebo recipients. At one year of age, 64.3% (95% CI: 59.2–69.2) and 38.2% (95% CI: 33.3–43.2) of RIX4414 and placebo recipients, respectively, were seropositive for anti-RV IgA antibodies. One month after the second RIX4414 dose, the anti-RV IgA antibody geometric mean concentration (GMC) in seropositive subjects was 213.2 (95% CI: 180.3–252.0) in all RIX4414 recipients and 196.4 (95% CI: 108.9–354.3) in placebo recipients. At one year of age, the anti-RV IgA antibody GMCs in seropositive subjects were 128.4 (95% CI: 110.5–149.2) and 140.3 (95% CI: 118.4–166.4) in all RIX4414 and placebo recipients, respectively.
One month after the second RIX4414 dose, anti-RV IgA seroconversion rates were 74.7% (95% CI: 68.9–79.9) and 64.2% (95% CI: 55.4–72.3) in the separate and co-administration cohorts, respectively, (). At one year of age, 71.5% (95% CI: 65.5–77.1) of RIX4414 recipients in the separate cohort and 50.0% (95% CI: 40.9–59.1) in the co-administration cohort remained seropositive for anti-RV IgA antibodies ().
Figure 2. Anti-RV IgA seroconversion (one month post-dose-2)/seropositivity rates (at one year of age) and GMCs in seropositive subjects (separate and co-administration cohort [vaccine group]) (ATP- cohort): (a) Seroconversion / Seropositivity rates; (b) GMCs.
![Figure 2. Anti-RV IgA seroconversion (one month post-dose-2)/seropositivity rates (at one year of age) and GMCs in seropositive subjects (separate and co-administration cohort [vaccine group]) (ATP- cohort): (a) Seroconversion / Seropositivity rates; (b) GMCs.](/cms/asset/2e7e388f-67bb-42f7-ab63-e25266030e20/khvi_a_1085143_f0002_b.gif)
One month after the second dose of RIX4414, 3.5% and 9.4% of placebo recipients in the separate and co-administration cohorts respectively, were seropositive for anti-RV IgA due to natural infection. At one year of age, the anti-RV IgA seropositivity rates had also increased in the placebo recipients (separate cohort = 46.8%; co-administration cohort = 21.8%).
Anti-RV IgA antibody GMCs rose sharply from pre-vaccination until one month post-dose 2 and decreased at one year of age in subjects who received RIX4414 in both the separate and co-administration cohorts ().
One month after the third dose of combined diphtheria-tetanus-acellular pertussis (DTPa, Infanrix™, GSK, Belgium), infants who received RIX4414/placebo concomitantly with DTPa and the oral poliovirus vaccine (OPV, Institute of Medical Biology, Chinese Academy of Medical Sciences, China), had 100% seroprotection rates against diphtheria and tetanus. Seropositivity rates against pertussis toxoid (PT), filamentous haemagglutinin (FHA) and pertactin (PRN) were 100% in both the RIX4414 and placebo groups. The overall vaccine response rates for anti-PT and anti-FHA were 100% in both the RIX4414 and placebo groups, and were 98.4% (95% CI: 94.5–99.8) and 99.3% (95% CI: 96.1–100), respectively against PRN. Two months after the third dose of OPV, seroprotection rates for anti-poliovirus types 1, 2 and 3 were over 99% in both the RIX4414 and placebo groups. The corresponding antibody GMCs against the co-administered vaccine antigens are presented in .
Table 2. Immune responses toward co-administered vaccines one month after the third vaccine dose (co-administration cohort).
Reactogenicity
All symptoms during the 8-day post-vaccination follow-up period
In the TVC excluding subjects in the co-administration cohort, the incidence of all symptoms (solicited [general only] and unsolicited) was 44.2% (95% CI: 41.7–46.8) and 47.3% (95% CI: 44.8–49.8) in the RIX4414 and placebo groups, respectively. Grade 3 symptoms were similar in the RIX4414 (11.2% [95% CI: 9.7–12.9]) and placebo groups (10.1% [95% CI: 8.6–11.7]) ().
Table 3. Solicited symptoms (any symptom and general symptoms).
In the co-administration cohort, all symptoms (solicited [local and general] and unsolicited) were reported for 57.5% (95% CI: 49.3–65.5) of RIX4414 and 52.9% (95% CI: 44.7–61.1) of placebo recipients. Grade 3 symptoms were reported for fewer than 6.0% of infants in both the RIX4414 and placebo groups ().
Solicited general AEs (8-day post-vaccination follow-up period)
Of RIX4414 recipients, 44.2% (95% CI: 41.7–46.8) in the TVC excluding subjects in the co-administration cohort and 57.5% (95% CI: 44.7–61.1) belonging to the co-administration cohort recorded any symptoms.
In the TVC excluding subjects in the co-administration cohort, irritability/fussiness was the most common solicited general symptom in both the RIX4414 and placebo groups, followed by cough/runny nose. Diarrhea was the most common grade 3 solicited general symptom observed in both the RIX4414 and placebo groups (). In the co-administration cohort, irritability/fussiness (all and grade 3) was the most commonly reported solicited general symptom in both the RIX4414 and placebo groups, followed by drowsiness and gastrointestinal symptoms ().
Solicited local AEs (8-day post-vaccination follow-up period)
In infants who received concomitant DTPa, redness was the most common solicited local symptom, which occurred in 13.3% (95% CI: 8.3–19.8) and 8.6% (95% CI: 4.7–14.3) of RIX4414 and placebo recipients, respectively. Injection site pain (RIX4414 = 9.3% [95% CI: 5.2–15.2]; placebo = 6.0% [95% CI: 2.8–11.0]) and swelling (RIX4414 = 8.7% [95% CI: 4.7–14.4]; placebo = 4.0% [95% CI: 1.5–8.4]) were reported at similar frequencies in both the RIX4414 and placebo groups. Grade 3 pain was reported in 2 RIX4414 recipients and grade 3 redness in one placebo recipient.
Discussion
This study evaluated the efficacy, immunogenicity and safety of RIX4414 in Chinese infants.
Primary efficacy, and safety results from the trial have been previously published,Citation13 and this paper presents the results for the secondary objective, the immune response of routine childhood vaccines (DTPa and OPV) when administered either separately from or concomitantly with RIX4414/placebo.
In our study, one month after the second dose of RIX4414, the anti-RV IgA seroconversion rate was 64.2% (95% CI: 55.4–72.3) in the co-administration cohort and 74.7% (95% CI: 68.9–79.9) in the separate cohort; the corresponding anti-RV IgA antibody GMCs in seropositive subjects was 275.8 (95% CI: 193.1–393.8) and 189.9 (95% CI: 158.3–227.9), respectively. The anti-RV IgA seroconversion rate in the co-administration cohort are consistent with studies conducted in Bangladesh (56.5% [95% CI: 44.0–68.4]) and South Africa (57.1% [95% CI: 44.7–68.9]) in which RIX4414 was co-administered with OPV.Citation14,15 Although the anti-RV IgA seroconversion rate in the co-administration cohort seems to be lower than that in the separate cohort, the 95% CIs overlapped. Interestingly, in the study from Bangladesh, a similar trend was observed, where the anti-RV IgA seroconversion rate was 66.7% (95% CI: 54.0–77.8) when RIX4414 and OPV were administered separately.Citation14 This difference in anti-RV IgA seroconversion rates between the groups receiving RIX4414 concomitantly or separately with routine OPV could be attributed to interference between RIX4414 and OPV when the 2 vaccines are co-administered. Research has shown that since OPV and RV vaccines contain live, attenuated vaccine virus strains which replicate in the gut, the potential for mutual interference exists following the first dose of RV vaccine.Citation16 A previous study in South Africa showed that OPV co-administration interfered with the immune response observed after the first dose of RIX4414, but was overcome after the second dose of RIX4414; there was no interference with the immune response to OPV.Citation16,17 In 2 studies from Latin America, one with and the other without co-administered OPV, similar efficacy results against severe RVGE were observed [without OPV: 84.8% (95% CI: 71.1–92.7); with OPV: 84.3% (95% CI: 59.0–94.9)].Citation6,18
Anti-RV IgA seroconversion rates are also affected by the socio-economic status of a country. The anti-RV IgA seroconversion rate in all RIX4414 recipients one month after the second dose (71.1% [95% CI: 66.3–75.5]) was lower than that which has been observed in Europe (86.5% [95% CI: 83.9–88.8]), Japan (85.3% [95% CI: 68.9–95.0]), Korea (88.1% [95% CI: 84.0–91.4]), Singapore (91.0% [95% CI: 85.2–95.1]) and Hong Kong (97.5% [95% CI: 86.8–99.9]); all countries whose populations belong to a higher socio-economic status compared our study population.Citation11,19,20-22 However, in South Africa and India, which have comparable socio-economic conditions to China, anti-RV IgA seroconversion rates comparable with the current study have been reported.Citation23,24
At one year of age, anti-RV seropositivity rates in RIX4414 recipients were higher in the separate cohort (71.5% [95% CI: 65.5–77.1]) as compared with the co-administration cohort (50.0% [95% CI: 40.9–59.1]). This difference could be due to OPV co-administration, but the GMC values calculated on seropositive subjects in the 2 sub-cohorts were in the same range (separate cohort=141.3 [95% CI: 118.0–169.1]; co-administration cohort=97.8 [95% CI: 75.0–127.6]). Furthermore, studies conducted in Latin America showed similar efficacy results against severe RVGE when RIX4414 was administered with and without OPV.Citation6,18
The seroconversion rate one month after the second dose of RIX4414 (71.1% [95% CI: 66.3–75.5]) and the seropositivity rate at one year of age (64.3% [95% CI: 59.2–69.2]) in the pooled RIX4414 group are comparable with the observed estimate of vaccine efficacy against severe RVGE at one year of age (75%),Citation13 implying that the anti-RV IgA seroconversion and seropositivity rates could be reasonable estimates for vaccine efficacy. A recent paper has also suggested that anti-RV IgA seropositivity may serve as a useful correlate of RIX4414 efficacy against RVGE.Citation25 Further studies to support this may be needed. On the other hand, the observed seropositivity rate at one year of age in placebo recipients in the separate cohort (46.8% [95% CI: 40.5–53.2]) indicates a high rate of natural RV infection in this population during the first year of life, and supports the need for the early RV prevention.
As observed in studies conducted in the United States, Europe, Singapore and Latin America, we demonstrated no interference between RIX4414 and DTPa when routinely administered, with respect to the seropositivity/seroprotection rates and GMCs against the DTPa antigens.Citation10,11,21,26 The OPV seroprotection rates (>99%) observed in this study were higher than those seen in Bangladesh (69.6%–98.5%),Citation14 but similar to those recorded in South Africa (>98%) and Latin America (>98%).Citation17,26 Despite suggested OPV interference, RIX4414 was immunogenic when co-administered with OPV and did not interfere with the OPV seroprotection rates, demonstrating that the RIX4414 vaccine had no impact on the immune response of the routinely co-administered vaccines.
Overall, the reactogenicity profiles were similar in the RIX4414 and placebo groups in both sub-cohorts. In addition, co-administration with routine childhood vaccines was not associated with an increase in solicited general symptoms. As has been previously observed, irritability was the most common solicited general symptom reported in both sub-cohorts.Citation14,21,22,27,28
The results of this study should be interpreted bearing in mind that immunogenicity and reactogenicity were secondary objectives and the study was not powered to draw definitive conclusions. It is also important to consider that infants were mostly enrolled from rural areas in the Guangxi Province, the southern part of China, and that this study population may not be fully representative of the infant population in China as a whole.
In conclusion, our study shows that 2 oral doses of the liquid formulation of RIX4414 are immunogenic and well-tolerated in Chinese infants and do not interfere with the immunogenicity and reactogenicity of co-administered DTPa and OPV vaccines. These data support the concomitant administration of RIX4414 with routine childhood vaccines in China.
Materials and methods
Study design and participants
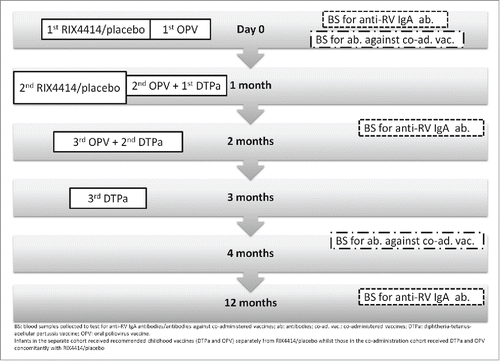
This phase III, double-blind, randomized, placebo-controlled study (NCT01171963) was conducted at 4 centers in China between August 2010 and May 2012. Healthy infants aged 6–16 weeks were randomized (1:1) to receive 2 doses of either RIX4414 or placebo according to a 0, 1 month schedule. A subset of infants from the primary efficacy cohortCitation13 was grouped into 2 immunogenicity sub-cohorts. Infants in the separate cohort received the recommended childhood vaccines (DTPa and OPV) separately from RIX4414/placebo while those in the co-administration cohort received DTPa and OPV concomitantly with RIX4414/placebo (). The pooled cohort for immunogenicity comprised the 2 combined sub-cohorts. Exclusion criteria were described in the primary report.Citation13
The study was carried out in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The Institutional Ethics Committee of each participating center reviewed and approved all study-related documents. Parents/guardians provided written informed consent before any study procedures were performed.
Vaccines
A single dose of liquid RIX4414 vaccine (Rotarix™, GSK, Belgium) contained at least 106.0 median cell culture infectious doses (CCID50) of live, attenuated human RV strain. The placebo (GSK, Belgium) contained the same constituents as RIX4414 vaccine without any viral content. The DTPa vaccine (Infanrix™, GSK, Belgium) contained diphtheria toxoid (≥30 IU), tetanus toxoid (≥40 IU), PT (25 µg), FHA (25 µg) and PRN (8 µg); OPV vaccine (Institute of Medical Biology, Chinese Academy of Medical Sciences) contained total polio-virus not less than 6.15 lg CCID50, ≥6.0 Ig CCID50 of poliovirus type 1, ≥5.0 Ig CCID50 of poliovirus type 2, and ≥5.5 Ig CCID50 of poliovirus type 3 per dose.
RIX4414, OPV and placebo were all administered orally, while the DTPa vaccine was administered intramuscularly into the left anterolateral thigh.
Immunogenicity
The blood sampling schedule is shown in . Anti-RV IgA antibody concentrations were measured using an in-house enzyme-linked immunosorbent assay (ELISA; assay cut-off = 20 U/ml). Seroconversion was defined as the appearance of anti-RV IgA antibodies (≥20 U/ml) in the sera of infants who were seronegative before vaccination.
An ELISA technique developed by The National Institutes for Food and Drug Control, China was used to antibodies against diphtheria, tetanus, PT, FHA and PRN.Citation29-31 Anti-polio antibodies were assessed using a microneutralization test adapted from the WHO guidelines (assay cut-off = 8 ED50).Citation32 Seroprotection was defined as an antibody concentration of ≥0.1 IU/ml for diphtheria and tetanus and 1:8 dilution for poliovirus types 1, 2, and 3. Seropositivity was defined as ≥5 EL.U/ml for each of the pertussis antigens. Vaccine response was evaluated for anti-PT and anti-FHA (defined as ≥20 EL.U/ml), and anti-PRN (calculated as ≥4-fold increase in antibody concentration from pre- to post-vaccination) during the efficacy follow-up period (2 weeks post-dose 2 of RIX4414 up to study end).
Reactogenicity
Solicited general symptoms (fever, fussiness/irritability, loss of appetite, cough/runny nose, diarrhea and vomiting) were recorded in diary cards by parents/guardians during the 8-day post-vaccination follow-up period. For infants in the co-administration cohort, local symptoms (pain, swelling and redness) were also recorded.
The intensity of each solicited symptom was assessed on a 4-point scale (ranging from grade 0: normal to grade 3: severe), where grade 3 was defined as follows: infant cried when limb was moved/spontaneously painful (pain); >30 mm injection site surface diameter (redness and swelling); prevented normal daily activities (cough/runny nose, irritability/fussiness, drowsiness, gastrointestinal symptoms); ≥6 looser than normal stools per day (diarrhea); ≥3 episodes of vomiting per day (vomiting), axillary temperature >39°C (fever); did not eat at all (loss of appetite).
Unsolicited symptoms were measured during the 31-day post-RIX4414/placebo vaccination follow-up period and serious adverse events were recorded throughout the study period as previously reported.Citation13
Statistical analysis
All statistical analyses were performed using SAS Drug and Development web portal version 3.5.
The TVC included all infants who received at least one dose of RIX4414 or placebo. Anti-RV IgA antibody analysis was performed on the ATP cohort for immunogenicity, which included infants who: complied with vaccination and blood sampling schedules; were seronegative for serum anti-RV IgA antibodies before vaccination; had available immunogenicity data at pre- and post-sampling time points. Antibody analysis of the antigens in the co-administered vaccines was performed on subjects in the co-administration cohort, who complied with DTPa and OPV vaccination and blood sampling schedules; completed all vaccinations; had available immunogenicity data at the post-sampling time point.
Serum anti-RV IgA antibody seroconversion rates (one month post-dose 2)/seropositivity rates (at one year of age)/ GMCs (at both time points) of RIX4414 versus placebo were tabulated with 95% CI. GMCs were calculated by taking the anti-log of the mean of the log antibody concentration transformations.
For subjects in the co-administration cohort, the antibody seroprotection/seropositivity rates for anti-diphtheria, anti-tetanus, anti-PT, anti-FHA anti-PRN (one month post-dose 3 of DTPa), anti-poliovirus types 1, 2 and 3 (2 months post-dose-3 of OPV) were calculated with 95% CI.
The safety analysis was performed on the TVC. The percentage of all infants with solicited general symptoms reported during the 8-day post-vaccination follow-up period was tabulated with 95% CI. For infants in the co-administration cohort solicited local symptoms were also tabulated.
Abbreviations
| ATP | = | According-to-Protocol |
| CCID50 | = | Median Cell Culture Infectious Dose |
| CI | = | Confidence Interval |
| DTPa | = | combined diphtheria tetanus and acellular pertussis vaccine |
| ED50 | = | Estimated dose 50% |
| EL.U/ml | = | ELISA units per milliliter |
| ELISA | = | Enzyme-Linked Immunosorbent Assay |
| FHA | = | Filamentous Haemagglutinin |
| GE | = | Gastroenteritis |
| GMC | = | Geometric mean concentration |
| IgA | = | Immunoglobulin A |
| IU/ml | = | International units per milliliter |
| IPV | = | inactivated poliovirus vaccine |
| LLR | = | Lanzhou Lamb Rotavirus |
| OPV | = | oral poliovirus vaccine |
| PRN | = | pertactin |
| PT | = | Pertussis toxoid |
| RV | = | Rotavirus |
| SAS | = | Statistical Analysis System |
| SD | = | Standard Deviation |
| TVC | = | Total Vaccinated Cohort |
| WHO | = | World Health Organization |
Trademarks
Rotarix and Infanrix are registered trademarks of the GSK group of companies. Rotateq is a registered trademark of Merck & Co., USA. Lanzhou lamb rotavirus vaccine is a registered trademark of Lanzhou Institute of Biomedical Products, China.
Disclosure of potential conflicts of interest
Authors RCL, TH, YL, LW, JT, BF, GS, YN, ZM declare to have received institutional funding/grants from the GSK group of companies for the conduct of Rota trials. Authors IL, HT, NK, HHH are employees of the GSK group of companies. HHH also holds stock options/restricted shares from the sponsoring company. XL and NR were employees of the GSK group of companies at the time of this study. XL is currently employee of Merck China, working in the department of Clinical Research and is responsible for Rotateq clinical study.
Acknowledgments
The authors thank all the investigators, study nurses and staff members who were involved in this clinical trial and the participating parents and children. The authors acknowledge the following members of the GSK group of companies (current and ex-employees): Ben Wang, Ying Lu, Alvin Zhang, Dan Lin, John Hou and Eva Liu for their monitoring support; Clay Churchill and Jenny Jiang (for providing inputs on the protocol design), Johny Jiang (for providing inputs on the study report), Ramya Basavaraju (for global study management), Mudit Mehrotra (for study data management), Julia Donnelly (freelance for language editing), Ashmita Ravishankar and Mark Franco (for manuscript writing) and Manjula K (for publication coordination and editorial support).
Funding
Funding
This study was sponsored and funded by GlaxoSmithKline Biologicals SA. GlaxoSmithKline Biologicals SA was involved in all stages of the study conduct and analysis; and also took charge of all costs associated with developing and publishing this manuscript.
References
- Kawai K, O'Brien MA, Goveia MG, Mast TC, El Khoury AC. Burden of rotavirus gastroenteritis and distribution of rotavirus strains in Asia: a systematic review. Vaccine 2012; 30:1244-54; PMID:22212128; http://dx.doi.org/10.1016/j.vaccine.2011.12.092
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:136-41; PMID:22030330; http://dx.doi.org/10.1016/S1473-3099(11)70253-5
- Rotavirus vaccines. WHO position paper - January 2013. Wkly Epidemiol Rec 2013; 88:49-64; PMID:23424730
- Yee EL, Fang ZY, Liu N, Hadler SC, Liang X, Wang H, Zhu X, Jiang B, Parashar U, Widdowson MA, et al. Importance and challenges of accurately counting rotavirus deaths in China, 2002. Vaccine 2009; 27 Suppl 5:F46-9; PMID:19931719; http://dx.doi.org/10.1016/j.vaccine.2009.08.065
- Duan ZJ, Liu N, Yang SH, Zhang J, Sun LW, Tang JY, Jin Y, Du ZQ, Xu J, Wu QB, et al. Hospital-based surveillance of rotavirus diarrhea in the People's Republic of China, August 2003-July 2007. J Infect Dis 2009; 200 Suppl 1:S167-73; PMID:19817597; http://dx.doi.org/10.1086/605039
- Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11-22; PMID:16394298; http://dx.doi.org/10.1056/NEJMoa052434
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354:23-33; PMID:16394299; http://dx.doi.org/10.1056/NEJMoa052664
- Soares-Weiser K, Maclehose H, Bergman H, Ben-Aharon I, Nagpal S, Goldberg E, Pitan F, Cunliffe N. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Sys Rev 2012; 11:CD008521
- He Q, Wang M, Xu J, Zhang C, Wang H, Zhu W, Fu C. Rotavirus vaccination coverage among children aged 2-59 months: a report from Guangzhou, China. PloS One 2013; 8:e68169; PMID:23840828; http://dx.doi.org/10.1371/journal.pone.0068169
- Dennehy PH, Bertrand HR, Silas PE, Damaso S, Friedland LR, Abu-Elyazeed R. Coadministration of RIX4414 oral human rotavirus vaccine does not impact the immune response to antigens contained in routine infant vaccines in the United States. Pediatrics 2008; 122:e1062-6; PMID:18977955; http://dx.doi.org/10.1542/peds.2008-1059
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Thollot F, Garcia-Corbeira P, Damaso S, Han HH, Bouckenooghee A. Immunogenicity and safety of the human rotavirus vaccine Rotarix co-administered with routine infant vaccines following the vaccination schedules in Europe. Vaccine 2010; 28:5272-9; PMID:20538094; http://dx.doi.org/10.1016/j.vaccine.2010.05.057
- Steele AD, De Vos B, Tumbo J, Reynders J, Scholtz F, Bos P, de Beer MC, Van der Merwe CF, Delem A. Co-administration study in South African infants of a live-attenuated oral human rotavirus vaccine (RIX4414) and poliovirus vaccines. Vaccine 2010; 28:6542-8; PMID:18786585; http://dx.doi.org/10.1016/j.vaccine.2008.08.034
- Li RC, Huang T, Li Y, Luo D, Tao J, Fu B, Si G, Nong Y, Mo Z, Liao X, et al. Human rotavirus vaccine (RIX4414) efficacy in the first two years of life: A randomized, placebo-controlled trial in China. Hum Vaccin Immunother 2014; 10:1-8; PMID:24832715; http://dx.doi.org/10.4161/hv.28050
- Zaman K, Sack DA, Yunus M, Arifeen SE, Podder G, Azim T, Luby S, Breiman RF, Neuzil K, Datta SK, et al. Successful co-administration of a human rotavirus and oral poliovirus vaccines in Bangladeshi infants in a 2-dose schedule at 12 and 16 weeks of age. Vaccine 2009; 27:1333-9; PMID:19162114; http://dx.doi.org/10.1016/j.vaccine.2008.12.059
- Madhi SA, Kirsten M, Louw C, Bos P, Aspinall S, Bouckenooghe A, Neuzil KM, Steele AD. Efficacy and immunogenicity of two or three dose rotavirus-vaccine regimen in South African children over two consecutive rotavirus-seasons: a randomized, double-blind, placebo-controlled trial. Vaccine 2012; 30 Suppl 1:A44-51; PMID:22520136; http://dx.doi.org/10.1016/j.vaccine.2011.08.080
- Patel M, Steele AD, Parashar UD. Influence of oral polio vaccines on performance of the monovalent and pentavalent rotavirus vaccines. Vaccine 2012; 30 Suppl 1:A30-5; PMID:22520134; http://dx.doi.org/10.1016/j.vaccine.2011.11.093
- Steele AD, Reynders J, Scholtz F, Bos P, de Beer MC, Tumbo J, Van der Merwe CF, Delem A, De Vos B. Comparison of 2 different regimens for reactogenicity, safety, and immunogenicity of the live attenuated oral rotavirus vaccine RIX4414 coadministered with oral polio vaccine in South African infants. J Infect Dis 2010; 202 Suppl:S93-100; PMID:20684724; http://dx.doi.org/10.1086/653550
- Tregnaghi MW, Abate HJ, Valencia A, Lopez P, Da Silveira TR, Rivera, L, Medina DMR, Saez-Llorens X, Ayala SEG, De Leon T, et al. Human rotavirus vaccine is highly efficacious when co-administered with routine expanded program of immunization vaccines including oral poliovirus vaccine in Latin America. Pediatr Infect Dis J 2011; 30(6): p.e103-8; PMID:21378594
- Kawamura N, Tokoeda Y, Oshima M, Okahata H, Tsutsumi H, Van Doorn LJ, Muto H, Smolenov I, Suryakiran PV, Han HH. Efficacy, safety and immunogenicity of RIX4414 in Japanese infants during the first two years of life. Vaccine 2011; 29:6335-41; PMID:21640780; http://dx.doi.org/10.1016/j.vaccine.2011.05.017
- Kim JS, Bae CW, Lee KY, Park MS, Choi YY, Kim KN, Kim JD, Park WS, Sin JB, Kim EA, et al. Immunogenicity, reactogenicity and safety of a human rotavirus vaccine (RIX4414) in Korean infants: a randomized, double-blind, placebo-controlled, phase IV study. Human vaccines & immunotherapeutics 2012; 8:806-12; PMID:22699440; http://dx.doi.org/10.4161/hv.19853
- Phua KB, Emmanuel SC, Goh P, Quak SH, Lee BW, Han HH, Ward RL, Bernstein DI, De Vos B, Bock HL. A rotavirus vaccine for infants: the Asian experience. Ann Acad Med Singapore 2006; 35:38-44; PMID:16470273
- Lau YL, Nelson EA, Poon KH, Chan PK, Chiu S, Sung R, Leung CW, Ng D, Ma YM, Chan D. Efficacy, safety and immunogenicity of a human rotavirus vaccine (RIX4414) in Hong Kong children up to three years of age: A randomized, controlled trial. Vaccine 2013; 31:2253-9; PMID:23499605; http://dx.doi.org/10.1016/j.vaccine.2013.03.001
- Steele AD, De Vos B, Tumbo J, Reynders J, Scholtz F, Bos P, de Beer MC, Van der Merwe CF, Delem A. Co-administration study in South African infants of a live-attenuated oral human rotavirus vaccine (RIX4414) and poliovirus vaccines. Vaccine 2010; 28:6542-8; PMID:18786585; http://dx.doi.org/10.1016/j.vaccine.2008.08.034
- Narang A, Bose A, Pandit AN, Dutta P, Kang G, Bhattacharya SK, Datta SK, Suryakiran PV, Delem A, Han HH, et al. Immunogenicity, reactogenicity and safety of human rotavirus vaccine (RIX4414) in Indian infants. Hum Vaccin 2009; 5:414-9; PMID:19276664; http://dx.doi.org/10.4161/hv.5.6.8176
- Cheuvart B, Neuzil KM, Steele AD, Cunliffe N, Madhi SA, Karkada N, Han HH, Vinals C. Association of serum anti-rotavirus immunoglobulin A antibody seropositivity and protection against severe rotavirus gastroenteritis: analysis of clinical trials of human rotavirus vaccine. Hum Vaccin Immunother 2014; 10:505-11; PMID:24240068; http://dx.doi.org/10.4161/hv.27097
- Salinas B, Perez Schael I, Linhares AC, Ruiz Palacios GM, Guerrero ML, Yarzabal JP, Cervantes Y, Clemens C, Damaso S, Hardt K, et al. Evaluation of safety, immunogenicity and efficacy of an attenuated rotavirus vaccine, RIX4414: A randomized, placebo-controlled trial in Latin American infants. Pediatr Infect Dis J 2005; 24:807-16; PMID:16148848; http://dx.doi.org/10.1097/01.inf.0000178294.13954.a1
- Vesikari T, Karvonen A, Bouckenooghe A, Suryakiran PV, Smolenov I, Han HH. Immunogenicity, reactogenicity and safety of the human rotavirus vaccine RIX4414 oral suspension (liquid formulation) in Finnish infants. Vaccine 2011; 29:2079-84; PMID:21238572; http://dx.doi.org/10.1016/j.vaccine.2011.01.004
- Cheuvart B, Friedland LR, Abu-Elyazeed R, Han HH, Guerra Y, Verstraeten T. The human rotavirus vaccine RIX4414 in infants: a review of safety and tolerability. Pediatr Infect Dis J 2009; 28:225-32; PMID:19209095; http://dx.doi.org/10.1097/INF.0b013e31819715fa
- Camargo ME, Silveira L, Furuta JA, Oliveira EP, Germek OA. Immunoenzymatic assay of anti-diphtheric toxin antibodies in human serum. J Clin Microbiol 1984; 20:772-4; PMID:6386879
- Melville-Smith ME, Seagroatt VA, Watkins JT. A comparison of enzyme-linked immunosorbent assay (ELISA) with the toxin neutralisation test in mice as a method for the estimation of tetanus antitoxin in human sera. J Biol Stand 1983; 11:137-44; PMID:6345548; http://dx.doi.org/10.1016/S0092-1157(83)80038-9
- Riffelmann M, Thiel K, Schmetz J, Wirsing von Koenig CH. Performance of Commercial Enzyme-Linked Immunosorbent Assays for Detection of Antibodies to Bordetella pertussis. J Clin Microbiol 2010; 48:4459-63; PMID:20943873; http://dx.doi.org/10.1128/JCM.01371-10
- World Health Organization Expanded Programme on Immunization. Guidelines for WHO/EPI collaborative studies on poliomyelitis: standard procedure for determining immunity to poliovirus using the microneutralization test. Geneva: World Health Organization 1993