abstract
Recombinant adeno-associated virus (rAAV) 2 vector gene therapy offers promise for the healing of Rheumatoid arthritis. To support the clinical development of the candidate gene therapeutic product in China, a comprehensive preclinical safety assessment of rAAV2 encoding human TNF receptor-immunoglobulin Fc fusion gene (rAAV2/human TNFR:Fc), were conducted in 3 species of experimental animals. No abnormal findings were observed in mice following single intravenous administration with test article. Compared with the control group, no differences in mean body weight, food consumption in rats and monkeys following the repeated intraarticular administration with rAAV2/human TNFR:Fc. There were also no significant adverse effects due to treatment noted by clinical chemistry, hematology and pathology assessments. After intraarticular administration with rAAV2/human TNFR:Fc, the vector DNA initially distributed to spleen, lymph nodes, and joint synovium. The vector DNA cleared rapidly as it could be detected mainly at the site of injection by 91 d post-administration (182 d for monkey). Taken together, localized delivery of rAAV2/human TNFR:Fc showed no significant toxicity in mice, rats, and monkeys, which support the planned clinical evaluation of this product.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease, which can cause chronic inflammation of the joints and other areas of the body. According to the World Health Organization estimated, the prevalence of RA in the general population ranges from 0.3% to 1%.Citation1 In China, there are about 5 millions RA patients and it is reported that RA has been one of the main reasons for physical disability.Citation2 Although the etiology of RA is not fully understood, it has been demonstrated that proinflammatory cytokines, particularly the tumor necrosis factor (TNF)-α, have an important role in RA pathogenesis.Citation3,4 Indeed, a series of TNF-α antagonists, such as Infliximab (chimeric mAb specific for TNF-α), Etanercept (soluble p75 TNF receptor- human IgG1Fc fusion proteins) have successfully been developed in the past decades, which have revolutionized the management of RA and other chronic inflammatory diseases.Citation5,6 However, some serious adverse effects are also reported after long-term systemic treatment with these TNF-α antagonists in some RA patients.Citation7,8 In addition, the high cost of TNF-α antagonists treatment also limits their widespread applications in the developing countries.Citation7,9
With the advance of biotechnology, delivery of genes encoding anti-arthritis proteins, rather than administration of the proteins themselves, promises to obviate these problems.Citation10,11 It is reported that the transfer to the body of cDNA (cDNA) encoding anti-arthritis gene products such as cytokine antagonists, growth factors, immunomodulators, enables the body to synthesize these products endogenously in a continuous, and biologically activities in many arthritis animal models.Citation11-17 Moreover, these endogenously proteins synthesized using gene transfer techniques have greater biologic activity than their recombinant counterparts.Citation14
There are several strategies for target gene transferring in arthritis, such as the local intraarticular delivery to individual diseased jointsCitation12,13 as well as the systemic delivery that the vector or a secreted transgene product is introduced at extraarticular locations (such as intramuscular or intravenous injection),Citation15,16 The most progress has been made with the intraarticular delivery due to expressing the gene product using this approach minimizes exposure of non-target sites, thereby reducing the unwanted side effects. Furthermore, this local treatment has fewer requirements and cost less.
Our previous study have demonstrated that the intraarticular gene delivery of recombinant adeno-associated virus 2 (rAAV2) encoding TNF receptor-immunoglobulin Fc (TNFR:Fc) fusion gene (rAAV2/TNFR:Fc) could ameliorate the arthritis symptoms and suppress the development of in rats with collagen-induced arthritis.Citation18 Since gene therapy products are intended for therapeutic use in patients, preclinical safety evaluation should be investigated according to the principles used for all new drugs.Citation19 Therefore, in order to provide preclinical data to support the safety and suitability of the candidate gene product to proceed into human clinical trial in China, in the present study a comprehensive preclinical safety assessment of rAAV2/human TNFR:Fc including single and repeat-dose toxicity studies were conducted in mice, rats and rhesus monkeys, respectively.
Materials and methods
Animals
Kunming mice, at 6 weeks old of age, were obtained from the animal center at the National Institutes of Food and Drug Control, (Beijing, China). Wistar rats, at 7 weeks old of age, were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd (Beijing, China). All mice and rats were maintained in a room equipped with an air-filtering system. Animals had ad libitum access to the certified rodent diet and sterilized municipal tap water was given ad libitum via water bottles. Rhesus monkeys, at 2 to 3 y old of age, were purchased from Beijing Institute of Xieerxin Biology Resource (Beijing, China) and maintained in stainless steel cages (L·W·H: 80·70·75 cm) under conventional conditions. Each monkey was individually housed, and provided with 300 g of standard monkey keeping diet, and fruits per day and sterilized municipal tap water (Beijing) was available ad libitum throughout the study.
Before the study was conducted, mice and rats were quarantined for 7 days, and monkeys were quarantined for 50 days, respectively. Animals were accepted for use on the study based on body weights and physical examination performed during the quarantine period. All animal experiment protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of National Center for Safety Evaluation of Drugs (NCSED), Beijing and all procedures conducted in this experiment were in accordance with Good Laboratory Practice (GLP)-compliant protocols.
Test articles
Test article rAAV2/human TNFR:Fc were developed by AGTC Gene Technology Company Ltd. China as described previously.Citation18 The vector titers of test article were expressed as vector genomes/ml (vg/mL). All test article was held at 2–8°C before use. PBS was used as negative control.
Design of toxicology study
The study design was outlined in . For the single-dose toxicity study, 15 male and 15 female mice were given a single intravenous injection of rAAV2/human TNFR:Fc at dose of 7.5 × 1012 vg/kg. The mice of control group were administrated equal volume of PBS. All animals were examined for mortality and clinical signs every day. Their body weight was measured on days 0, 1, 8, 14 and 21. All of the animals were sacrificed on day 22 and received a complete necropsy.
Table 1. The study design for preclinical safety evaluation of rAAV2/human TNFR:Fc.
A repeated dose toxicity study was performed in rats (). Animals were randomized into 4 groups and each group was composed of 15 male and 15 female rats. The ankle joints (left or right in turn for different injection) of rats were administrated intraarticularly with 50 μL of high (3 × 1012 vg/mL), middle (2 × 1012 vg/mL) and low (1 × 1012 vg/mL) dosages of rAAV2/human TNFR:Fc every day for continuous 8 d (a total of 8 injections), and then followed by 91 d of treatment-free period. The control group was treated with sterile PBS at an equivalent dose volume. Ten rats (female and male in half per time) of each group were sacrificed on study days 8, 22 and 99, respectively. The animals were given a full necropsy and histopathological examination. Before each necropsy, rats were anesthetized by sodium pentobarbital for the collection of blood samples. The blood samples were used for hematological and clinical chemistry analysis as well as CD4+ and CD8+ cell counting as described previously.Citation20 Furthermore, to evaluate the immune response of rAAV2/human TNFR:Fc in rat model, other 6 rats (female and male in half) were treated with PBS, high and low dosage of rAAV2/human TNFR:Fc as the above. The blood samples were collected on different time point for detection of antibodies against TNFR and AAV2 ().
A repeated dose toxicity study was performed in rhesus monkeys. Total 24 rhesus monkeys were randomized into 3 groups (female and male in half). The ankle joints (left or right in turn for different injections) of rhesus monkeys were injected intraarticularly with high and low dosages (1 × 1012 vg/mL and 3 × 1012 vg/mL) of rAAV2/human TNFR:Fc at a dose volume of 1 mL every day for continuous 8 d (a total of 8 injections), and then followed by 182 d of treatment-free period. Monkeys were sacrificed on days 8, 22, 99 and 190, respectively. The parameters for this study were basically the same as the rat study (). Additional parameters in this study were electrocardiogram (ECG) and IgG and IgM for the clinical chemistry analysis. Furthermore, part tissues of rats and monkeys from high dose groups were harvested at each necropsy for the biodistribution studies. A total of 16 tissues were tested: heart, liver, spleen, lung, kidneys, brain, intestines, mesenteric lymph nodes, muscle, testes, epididymis, prostate, uterus, ovaries, breast, and joint synovium. These tissues were stored at −80°C until use.
Detection of antibodies against TNFR and AAV2
The antibodies against TNFR:Fc in the sera were detected by ELISA. Microtiter plates were coated with 100 μl of human TNFR (2.5 μg/ml) overnight at 4°C, washed with phosphate buffered saline (PBS) containing 0.05% Tween 20, and then blocked with blocking buffer (1% bovine serum albumin in PBS) for 1 h at 37°C. Serial dilution of serum samples and a positive control (pooled normal human serum) were added to the plate. Following incubation for 1 h at 37°C, goat anti-rat IgG conjugated with horseradish peroxidase were added to the plates. After another incubation at the same condition, signals were measured by using 3,3′,5,5′-tetramethylbenzidine (TMB) substrate. Results were expressed as the highest dilution yielding the absorbance at 450 nm 2.1 times above the control values.
Furthermore, neutralizing antibodies to AAV2 were determined using luciferase assay as described previously.Citation21 In brief, AAV2 LUC was incubated with 3 dilutions (1:50, 1:200 and 1:800) of serum samples at room temperature for 1 h. BHK cells were infected with serum-treated AAV2 LUC at a multiplicity of infection of 105 genome copies per cell. At 48 h postinfection, luciferase activity was measured in the cell lysate using a luciferase assay kit (Promega, USA) according to the manufacture' s instructions. The positive result was defined as the less than 50% inhibition of AAV2 LUC was observed when compared to the negative control.
Similarly, serum samples were collected from the monkeys of each group at different time point (), the antibodies against TNFR and AAV2 in the sera of monkeys were detected as described above.
Biodistribution study
To assess the biodistribution of vectors in animal (included rat and rhesus monkeys) following the repeated treatment with high dose rAAV2/human TNFR:Fc (3 × 1012 vg/mL), the vector DNAs were detected using quantitative PCR (QPCR) for specific AAV2 sequences as previously described.Citation22 Briefly, DNA was extracted from each frozen tissue type (approximately 25 mg) using DNA Purification Kit (TIANGEN, Beijing) according to the manufacturer's instructions. The concentration of eluted DNA was determined by UV spectrophotometry, and adjusted to a final concentration suitable for QPCR. Primers (PF: 5′-TCCTTCCTGCTCCCAATGG-3′ and PR: 5′-ACGGTGGGCATGTGTGAGT-3′) and probe (5′-FAM-AGCACTGGCGACGAGCCCAAA-TAMRA-3′) were designed using Primer Express software (Applied Biosystems, USA). The QPCR consisted of an initial heating step at 94°C for 3 min, and then 40 cycles of incubation at 94°C for 30s and at 60°C for 1 min. A standards curve of plasmid DNA (101 to 106 copies), no template controls, and background DNA controls were all run in duplicate reactions. Furthermore, to monitor QPCR inhibition, tissue from a naïve animal was separately spiked with plasmid DNA (102 to 104 copies) were also analyzed and no inhibition was found. The results were expressed as copy numbers per microgram of tissue and the limit of detection was 10 copies of target sequence.
Statistical analyses
Group means and standard deviations were calculated for body weight, food consumption, body temperature, clinical chemistry, hematology, organ weights, percentages of CD4+ and CD8+ cells. These data were analyzed using the statistics software Toxstat 2006, NCSED. Bartlett test was performed to test for variance homogeneity. When the result showed no significance (P ≥ 0.05) one-way analysis of variance (ANOVA) was used. When ANOVA showed significance (P < 0.05), Dunnett's test was done for multiple comparisons (all dose groups versus control group). The abnormal pathological changes were analyzed by Fisher's exact test using SPSS 19.0 software. P<0.05 was considered as indicative of significant difference.
Results
Single-dose toxicity study in mice
Compared to the control group, noabnormalities were observed in the rAAV2/human TNFR:Fc group in the single-dose toxicity experiment. These results suggested that the maximum tolerated dose of rAAV2/human TNFR:Fc, when injected intravenously, is greater than 7.5 × 1012 vg/kg.
Repeat-dose toxicity study in rats
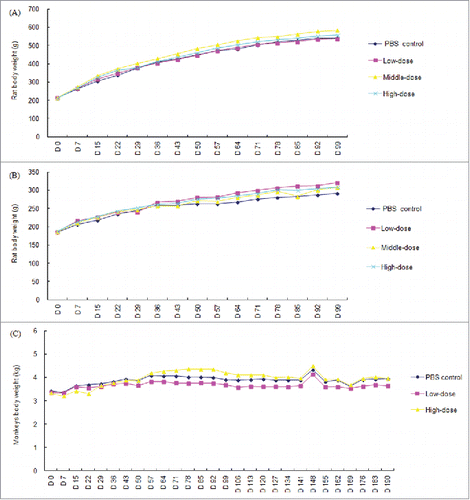
Intraarticular injection of rAAV2/human TNFR:Fc at high, middle and low dosages was well tolerated in rats. During this study, no early deaths and no obviously signs of systemic toxicity or abnormalities was noted, and there was no test article-related effects on food consumption. Animal body weights of all groups were increased and there was no significant difference between different groups at any time point (). Minimal red swell of injection site were noted in both control and treated rats with increased incidence and/or duration following several administration in treated animals. These irritation reactions were considered to be associated with intraarticular injection. Compared with the control group, body temperature increased significantly at one gender animal or one time point in the test article-treated groups, such as high, middle and low dosages of rAAV2/human TNFR:Fc male groups at 24 h after the last injection (on day 8), and female middle- and low-dose groups on day 22 (all P < 0.05) (Supplementary Table 1).
Figure 1. Group mean body weights of rats and rhesus monkeys following the repeated administration with rAAV2/human TNFR:Fc. (A) Mean body weights of male rat; (B) Mean body weights of female rat; (C) Mean body weights of rhesus monkeys (n = 18 on study day 2, 13 on day 3–42 and 5 on day 43–84 in each gender per group for rats; n = 8 on day 2–42, and 4 on day 43–70 in each group for cynomolgus monkeys except for the animal number of saline group were halved as described in Materials and Methods, *P<0.05 vs. the saline control group).
The hematological findings were: (1) on days 8, increased CD4+ and CD8+ cell levels in the middle dosage of rAAV2/human TNFR:Fc male group. (2) Increased mean corpuscular volume (MCV) and mean cell hemoglobin concentration (MCHC) in the high-dose male group on study day 22. (3) On recovery period (on study day 99), decreased white blood cell (WBC) level in the female high- and middle-dose groups and in the female high dose groups (Supplementary Table 2). For hematological analysis, it was only at the middle dosage of rAAV2/human TNFR:Fc male group that the alanine aminotransferase (ALT) level decreased on study day 22 in comparison with the control group (Supplementary Table 3).
In the second necropsy, significant increase in liver weight of low-, middle- and high dose male group, kidneys weight of middle-dose male group as well as in spleen weight of high-dose male group was observed compared to the control group (all P< 0.05), but there was no corresponding histopathological finding. Furthermore, it was noted that brain weights decreased in the low-, middle- and high dose male group (all P < 0.05). In contrast, these changes had been alleviated, and no significant difference was found in the article test-treated groups at the recovery periods (Supplementary Table 4). In addition, no treatment-related histopathological findings were observed during the whole study periods.
Repeat-dose toxicity study in rhesus monkeys
Intraarticular injection with high- and low-dose of rAAV2/human TNFR:Fc were also well tolerated in rhesus monkeys and no obvious signs of systemic toxicity were observed clinically during the whole study period. Any obvious irritation at injection sites were not found after administration. There were no test article-related effects on food consumption, body weight () and body temperature (supplementary Table 5). It was shown that no abnormal were observed at ECG in the rAAV2/human TNFR:Fc treated groups compared with the control group (data not shown).
There was an increase in numbers of red blood cell (RBC) while eosinophiles (EOS) was decreased significantly in the low-dose group on 3 months after the last injections (on study day 99) (all P < 0.05). A significant increase in MCHC was found in the high-dose group on day 134 when compared with the control group, respectively (P < 0.05) (supplementary Table 6). For the clinical chemistry analysis, alanine aminotransferase (ALT) level in the high-dose group was increased significantly on study day 22 when compared with the control group (P < 0.05) (supplementary Table 7). Furthermore, obvious increases were shown in albumin (ALB) concentration of rhesus monkeys from the low-dose group on study day 64 and in glucose (GLU) level in the high-dose group on study day 99, respectively (all P < 0.05).
In necropsy, slight abnormalities on weight of several organs such as liver, thymus were found occasionally in the test article-treated groups (Supplementary Table 8), but no corresponding histopathological change were observed. Furthermore, these changes had also been improved greatly at the end of recovery period and had no difference in comparison with the control group.
Antibodies to TNFR and AAV2
Antibodies to TNFR were detected in high and low dose of rAAV2/human TNFR:Fc groups. The results showed that antibodies could be detected in 2 out of 6 rats in the high dose of group at 2 weeks after the last injection (on day 22). An obvious dose-response was observed over time, and almost all rats in the high dose of group showed the obvious antibody response since the study day 57 onward ().
Table 2. Antibodies to TNFR in rats and monkeys after administration with rAAV2/human TNFR:Fc.
In order to determine neutralizing antibodies to AAV2, 3 different dilutions of serum samples at different time points were analyzed. It was showed that low level of antibodies (titers 1:50 and 1:200) can be detected in the high- and low-dose rAAV2/human TNFR:Fc groups, and then the antibody level against AAV2 continued to decrease post injection monitored (Table 3). On end of the recovery period (day 99), only one animal from high- and low-dose was positive when sera were diluted to be 1:50, respectively. Antibody titers of none of animals at any time points exceeded 1:800.
Different to the findings in rats, no obvious anti-TNFR antibody response were found in rhesus monkeys after the repeated administration with rAAV2/human TNFR:Fc (). Furthermore, no positive antibody response to AAV2 was found in monkey treated with test articles even when sera were diluted to be 1:50 ().
Table 3. Antibodies to AAV2 in rats and monkeys after administration with rAAV2/human TNFR:Fc.
Biodistribution analysis following the repeated administration with rAAV2/human TNFR:Fc
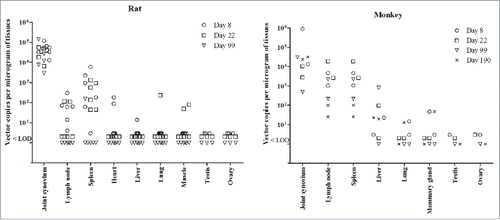
Similar biodistribution trends of adeno-associated vector DNA were identified in rats and monkeys following the repeated high dose rAAV2/human TNFR:Fc (). With some variation from animal to animal, the adeno-associated vector DNA remained largely at the site of injection (in the joint synovium). Furthermore, certain levels of the vector DNA were also detected in spleen and lymph nodes, but vector copy number of lymph nodes in monkeys seem be higher than that of rats at study days 8 and 22. Vector copy numbers had no difference between female and male animals. It was also noted that vector DNA was not found in gonad specimens (such as testis and ovary) in either animal species (). Only sporadically quantifiably positive signals were observed in other tissues. At the end of recovery period, the rAAV DNA was almost not detected in tissues other than at the injection site in rats and monkeys.
Figure 2. Biodistribution of AAV2 DNA in different tissues of rats and rhesus monkeys following the repeated administration with rAAV2/human TNFR:Fc. Part tissues of rats (n = 6) and monkeys (n = 2) from high dose groups were harvested for biodistribution analysis at each necropsy. Each symbol represents the value for an individual animal. LOD represents lower limit of detection.
Discussion
Although many preclinical studies of local and systemic gene therapy for RA have been reported,Citation12,13,16,17 most are focused on the efficacies of gene products and few preclinical safety study has been published. It is generally accepted that efficacies as well as safety are the core of gene products. Therefore, in order to identify potential toxicity from rAAV2/human TNFR:Fc, a comprehensive preclinical safety assessment were investigated in rodent and primate animal model.
The selection of animal species was critical for toxicity study. Ideally, the animal species should be able to expressed gene product in response to the relevant regarding biological,and also sensitive to the viral vector.Citation19 Although the pharmacodynamic studies of rAAV2/human TNFR:Fc were not performed in the monkey RA model, it is reported that non-human primates are the only relevant animal species with regard to both the expressed protein and sensitivity to the viral vector.Citation23,24 Non-human primates are often used as the preferred animal species in the preclinical safety study of gene products. Furthermore, it is required that preclinical safety study for human gene therapy products should include both specific and non-specific evaluations.Citation19 Therefore, to maximize the exposure of potential non-specific and specific toxic responses that might predict human toxicity of rAAV2/human TNFR:Fc, both rodent species (Kunming mouse and Wistar rat) were used in the single- and repeat-dose toxicity studies. The intended human dose of rAAV2/human TNFR:Fc developed in this study is 1 × 1012 vg/mL according to our previous studiesCitation18 and unpublished data. The dose levels used in the single- and repeat-dose toxicity studies were approximately 3–7 folds higher than the proposed clinical dose. Despite the relatively high dose administered and the repeated inoculations (8 consecutive doses in the repeated toxicity study), there were no obviously signs of systemic toxicity or abnormalities noted in all animal species. The repeated intrarticular injection of rAAV2/human TNFR:Fc in rats and monkeys resulted in only a few sporadic statistically significant changes in body temperature, organ weight, clinical chemistry and hematology parameters. Such findings were deemed to be incidental due to its observation only in one gender or one time point, and no dose-dependent change was revealed. More importantly, these observed changes could not be correlated with any other indication of toxicity or histopathology on microscopic examination of the tissues, further supporting that these findings were be not attributed to the administration of rAAV2/human TNFR:Fc. On the other hand, previous studies also reported that rAAV gene transfer to specific tissue liver was strongly influenced by sex. The male rodents have been shown to express proteins at a higher level than female rodents receiving identical vector dosesCitation25,26 The different efficient transduction may be explained for the abnormalities that the decrease of ALT level was only found at male middle dose group.
Previous studies have demonstrated that the TNFR is a 55- to 80-kDa glycoprotein that binds TNF-α.Citation27,28 Heterogeneity in TNFR from different species is also found, which amino acid sequence of human TNFR have 64% similarity with rat TNFR, and 6 amino acids less than the latter.Citation29 Therefore, it is not surprising that antibody response to human TNFR were found in rats treated with rAAV2/human TNFR:Fc due to species heterogeneity. This antibody might neutralize the therapeutic agent, and affect the efficacy.Citation30 To expose the potential toxicity by heterogenous immune response, another repeat-dose toxicity assessments in rats were also administrated in parallelwith rAAV2/rat TNFR:Fc. No difference besides heterogenous immune response was observed in rats treated with rAAV2/human TNFR:Fc and rAAV2/rat TNFR:Fc (data not shown). The results suggested that immune response by heterogenous target gene product might not induce obvious toxicological change, further supporting the use of rodent species in the preclinical safety study of human gene therapy product. Consistent with data from previous study,Citation30 no antibody to TNFR was detected in non-human primate RA model because there is a high level of homology between human and monkey.
Recombinant AAV2 vectors do not contain viral genes, and has been engineered for use as a vector in human gene therapy, which is widely considered as one of the most safety of currently used viral vectors.Citation31-33 In agreement with the previous studies,Citation31,34 low level of humoral immune response to AAV2 were detected in rats following the repeated administration, and then diminished over time. In contrast, neutralizing antibody response to AAV2 was not exhibited in monkeys. Although the exact cause is not clear, the description may be associated with distinct immune response rAAV2 in different species.
Considering the vector DNA is usually at very low levels in most tissues following administration with adeno-associated vectors,Citation22,35 only tissues of rats and monkeys from high-dose groups were analyzed for the biodistribution analysis at each necropsy in this study. The results showed that the adeno-associated vector DNA largely remained at the site of injection (joint synovium) in both rodent and non-human primate species following the repeated administration of rAAV2/human TNFR:Fc. No differences were noted in these biodistribution and clearance patterns between genders. In agreement with the previous study,Citation36 low level vector DNA biodistributed to spleen, lymph nodes and liver. These results also reflected the relatively rapid clear of vector in the lymphoid tissues as fewer animals remained positive at study endpoints despite of the repeated delivery of up to 10Citation12 copies in animals. It is reported that adeno-associated viral vectors can persist for long time at the site of inoculation, and in lymphatic tissues as well as liver,Citation37 although the mechanism of vector persistence remains unknown. It is speculated that these exogenous DNA molecules are captured by antigen-presenting cells and trafficked to the lymphoid organs and liver.Citation35 The biodistribution studies suggested that potential target organs for toxicity might be spleen, lymph nodes and/or liver, as well as the sites of injection. However, no corresponding histological abnormalities were found in the tissues positive for vector. Collectively, these data suggest that the vector DNA would be maintained in joint synovium for a long period of time without inducing any clinical sysmptoms. Furthermore, it was also noted that adeno-associated vector DNA was never found in gonad specimens in this study, indicating that the administration with rAAV2/human TNFR:Fc had a low risk for adeno-associated vector to biodistribute to reproductive organs.
It is generally accepted that QPCR can be used to quantity the integrated vector DNA in different tissues when compared with immunohistochemical assay. Therefore, QPCR is widely employed for biodistribution analysis in preclinical safety evaluation of gene therapy products.Citation35,38,39 Howerver, the real-time PCR is so exquisitely sensitive that the smallest contamination in the course of collection of the tissues during necropsy would be tested although rigorous measures are performed to prevent cross-contamination in this study. It is likely that the sporadically positive cases where a weak signal (close to the limit of detection) was seen in one animal at single time point, could be the result of inadvertent contamination. This is a limitation of using the sensitive real-time PCR for biodistribution analysis.
In conclusion, there were no significant toxicity effects due to treatment in the preclinical safety assessment of rAAV2/human TNFR:Fc described herein. Long term distribution of vector appeared to be localized within the site of injection. These results support the planned clinical evaluation of this product.
Disclosure of potential conflicts of interest
Aizhi Zhao and Xiaobing Wu were employees of AGTC Gene Technology Company Ltd as the work was done. The other authors declare no conflicts of interest.
Supplemental_Tables.zip
Download Zip (68.3 KB)Acknowledgments
We would like to thank Ming Li, Li Sun, Xin Li, Yufa Miao, Fang Liu, Yanwei Yang, and Di Zhang for excellent technical assistant in animal and pathological studies. We also thank Dr Hairuo Wen for reviewing and revising this manuscript.
Funding
This work was partly supported by National Major Scientific and Technological Special Project for “Significant New Drugs Development” (No. 2012ZX09302001).
References
- Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ 2003; 81:646-56; PMID:14710506
- Zeng QY, Chen R, Darmawan J, Xiao ZY, Chen SB, Wigley R, Le Chen S, Zhang NZ. Rheumatic diseases in China. Arthritis Res Ther 2008; 10:R17; PMID:18237382; http://dx.doi.org/10.1186/ar2368
- Feldmann M, Maini RN. The role of cytokines in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 1999; 38 Suppl 2:3-7; PMID:10646481
- Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest 2008; 118:3537-45; PMID:18982160; http://dx.doi.org/10.1172/JCI36389
- Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, Genovese MC, Wasko MC, Moreland LW, Weaver AL, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med 2000; 343:1586-93; PMID:11096165; http://dx.doi.org/10.1056/NEJM200011303432201
- Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, Smolen JS, Weisman M, Emery P, Feldmann M, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study group. N Engl J Med 2000; 343:1594-602; PMID:11096166; http://dx.doi.org/10.1056/NEJM200011303432202
- Gomez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum 2003; 48:2122-7; PMID:12905464; http://dx.doi.org/10.1002/art.11137
- Askling J, Fored CM, Brandt L, Baecklund E, Bertilsson L, Coster L, Geborek P, Jacobsson LT, Lindblad S, Lysholm J, et al. Risk and case characteristics of tuberculosis in rheumatoid arthritis associated with tumor necrosis factor antagonists in Sweden. Arthritis Rheum 2005; 52:1986-92; PMID:15986370; http://dx.doi.org/10.1002/art.21137
- Woolacott N, Bravo Vergel Y, Hawkins N, Kainth A, Khadjesari Z, Misso K, Light K, Asseburg C, Palmer S, Claxton K, et al. Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation. Health Technol Assess 2006; 10:iii-iv, xiii-xvi, 1-239; PMID:17083854
- Evans CH, Ghivizzani SC, Kang R, Muzzonigro T, Wasko MC, Herndon JH, Robbins PD. Gene therapy for rheumatic diseases. Arthritis Rheum 1999; 42:1-16; PMID:9920008; http://dx.doi.org/10.1002/1529-0131(199901)42:1%3c1::AID-ANR1%3e3.0.CO;2-4
- Evans CH, Robbins PD, Ghivizzani SC, Wasko MC, Tomaino MM, Kang R, Muzzonigro TA, Vogt M, Elder EM, Whiteside TL, et al. Gene transfer to human joints: progress toward a gene therapy of arthritis. Proc Natl Acad Sci U S A 2005; 102:8698-703; PMID:15939878; http://dx.doi.org/10.1073/pnas.0502854102
- Bandara G, Mueller GM, Galea-Lauri J, Tindal MH, Georgescu HI, Suchanek MK, Hung GL, Glorioso JC, Robbins PD, Evans CH. Intraarticular expression of biologically active interleukin 1-receptor-antagonist protein by ex vivo gene transfer. Proc Natl Acad Sci U S A 1993; 90:10764-8; PMID:8248169; http://dx.doi.org/10.1073/pnas.90.22.10764
- Yao Q, Seol DW, Mi Z, Robbins PD. Intra-articular injection of recombinant TRAIL induces synovial apoptosis and reduces inflammation in a rabbit knee model of arthritis. Arthritis Res Ther 2006; 8:R16; PMID:16507116; http://dx.doi.org/10.1186/ar1867
- Gouze JN, Gouze E, Palmer GD, Liew VS, Pascher A, Betz OB, Thornhill TS, Evans CH, Grodzinsky AJ, Ghivizzani SC. A comparative study of the inhibitory effects of interleukin-1 receptor antagonist following administration as a recombinant protein or by gene transfer. Arthritis Res Ther 2003; 5:R301-9; PMID:12932294; http://dx.doi.org/10.1186/ar795
- Imagawa T, Watanabe S, Katakura S, Boivin GP, Hirsch R. Gene transfer of a fibronectin peptide inhibits leukocyte recruitment and suppresses inflammation in mouse collagen-induced arthritis. Arthritis Rheum 2002; 46:1102-8; PMID:11953990; http://dx.doi.org/10.1002/art.10188
- Apparailly F, Millet V, Noel D, Jacquet C, Sany J, Jorgensen C. Tetracycline-inducible interleukin-10 gene transfer mediated by an adeno-associated virus: application to experimental arthritis. Hum Gene Ther 2002; 13:1179-88; PMID:12133271; http://dx.doi.org/10.1089/104303402320138961
- Chan JM, Villarreal G, Jin WW, Stepan T, Burstein H, Wahl SM. Intraarticular gene transfer of TNFR:Fc suppresses experimental arthritis with reduced systemic distribution of the gene product. Mol Ther 2002; 6:727-36; PMID:12498769; http://dx.doi.org/10.1006/mthe.2002.0808
- Zhou X, Gao K, Shen L, Zhao A, Wu X, Wang C, Wang J, Li B. Modulation of immune and inflammatory responses on experimental arthritis following intraarticular gene transfer of tumor necrosis factor receptor-immunoglobulin Fc. Rheumatol Int 2012; 32:2605-14; PMID:21833532; http://dx.doi.org/10.1007/s00296-011-1974-z
- Verdier F, Descotes J. Preclinical safety evaluation of human gene therapy products. Toxicol Sci 1999; 47:9-15; PMID:10048148; http://dx.doi.org/10.1093/toxsci/47.1.9
- Huo Y, Li B, Zhang Y, Wang S, Bao M, Gao X, Li D, Wang L, Yu Y, Wang J. Pre-clinical safety evaluation of heat shock protein 65-MUC1 peptide fusion protein. Regul Toxicol Pharmacol 2007; 49:63-74; PMID:17600604; http://dx.doi.org/10.1016/j.yrtph.2007.05.005
- Peng J, Tan S, Dong X, Wu Z, Yuan H, Chen F, Wu X. Study on transduction of mammal ian cells with recombinant adeno-associated virus 2 in vitro. Chinese J Virol 2004; 20:128-32
- Wang X, Miao Y, Zhou X, Shen L, Li B. Biodistribution of gag gene transferred by adenovirus vector in mice. Chin J Biologicals 2008; 21:938-40
- San Sebastian W, Kells AP, Bringas J, Samaranch L, Hadaczek P, Ciesielska A, Macayan M, Pivirotto PJ, Forsayeth J, Osborne S, et al. Safety and tolerability of Mri-guided infusion of AAV2-hAADC into the midbrain of non-human primate. Mol Ther Methods Clin Dev 2014; 3: 14049; PMID:25541617; http://dx.doi.org/10.1038/mtm.2014.49
- Conrad CK, Allen SS, Afione SA, Reynolds TC, Beck SE, Fee-Maki M, Barrazza-Ortiz X, Adams R, Askin FB, Carter BJ, et al. Safety of single-dose administration of an adeno-associated virus (AAV)-CFTR vector in the primate lung. Gene Ther 1996; 3:658-68; PMID:8854091
- Guenzel AJ, Hillestad ML, Matern D, Barry MA. Effects of adeno-associated virus serotype and tissue-specific expression on circulating biomarkers of propionic acidemia. Hum Gene Ther 2014; 25:837-43; PMID:25046265; http://dx.doi.org/10.1089/hum.2014.012
- Davidoff AM, Ng CY, Zhou J, Spence Y, Nathwani AC. Sex significantly influences transduction of murine liver by recombinant adeno-associated viral vectors through an androgen-dependent pathway. Blood 2003; 102:480-8; PMID:12637328; http://dx.doi.org/10.1182/blood-2002-09-2889
- Gohda T, Niewczas MA, Ficociello LH, Walker WH, Skupien J, Rosetti F, Cullere X, Johnson AC, Crabtree G, Smiles AM, et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol 2012; 23:516-24; PMID:22266664; http://dx.doi.org/10.1681/ASN.2011060628
- Palanisamy R, Kumaresan V, Harikrishnan R, Arasu MV, Al-Dhabi NA, Arockiaraj J. Functional roles and gene regulation of tumor necrosis factor receptor 1 in freshwater striped murrel. Mol Immunol 2015; 66:240-52; PMID:25841174; http://dx.doi.org/10.1016/j.molimm.2015.03.015
- Himmler A, Maurer-Fogy I, Kronke M, Scheurich P, Pfizenmaier K, Lantz M, Olsson I, Hauptmann R, Stratowa C, Adolf GR. Molecular cloning and expression of human and rat tumor necrosis factor receptor chain (p60) and its soluble derivative, tumor necrosis factor-binding protein. DNA Cell Biol 1990; 9:705-15; PMID:1702293; http://dx.doi.org/10.1089/dna.1990.9.705
- Moreland LW, Baumgartner SW, Schiff MH, Tindall EA, Fleischmann RM, Weaver AL, Ettlinger RE, Cohen S, Koopman WJ, Mohler K, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med 1997; 337:141-7; PMID:9219699; http://dx.doi.org/10.1056/NEJM199707173370301
- McPhee SW, Janson CG, Li C, Samulski RJ, Camp AS, Francis J, Shera D, Lioutermann L, Feely M, Freese A, et al. Immune responses to AAV in a phase I study for Canavan disease. J Gene Med 2006; 8:577-88; PMID:16532510; http://dx.doi.org/10.1002/jgm.885
- Mease PJ, Hobbs K, Chalmers A, El-Gabalawy H, Bookman A, Keystone E, Furst DE, Anklesaria P, Heald AE. Local delivery of a recombinant adenoassociated vector containing a tumour necrosis factor alpha antagonist gene in inflammatory arthritis: a phase 1 dose-escalation safety and tolerability study. Ann Rheum Dis 2009; 68:1247-54; PMID:18678578; http://dx.doi.org/10.1136/ard.2008.089375
- Mease PJ, Wei N, Fudman EJ, Kivitz AJ, Schechtman J, Trapp RG, Hobbs KF, Greenwald M, Hou A, Bookbinder SA, et al. Safety, tolerability, and clinical outcomes after intraarticular injection of a recombinant adeno-associated vector containing a tumor necrosis factor antagonist gene: results of a phase 1/2 Study. J Rheumatol 2010; 37:692-703; PMID:20032102; http://dx.doi.org/10.3899/jrheum.090817
- Chirmule N, Xiao W, Truneh A, Schnell MA, Hughes JV, Zoltick P, Wilson JM. Humoral immunity to adeno-associated virus type 2 vectors following administration to murine and nonhuman primate muscle. J Virol 2000; 74:2420-5; PMID:10666273; http://dx.doi.org/10.1128/JVI.74.5.2420-2425.2000
- Sheets RL, Stein J, Bailer RT, Koup RA, Andrews C, Nason M, He B, Koo E, Trotter H, Duffy C, et al. Biodistribution and toxicological safety of adenovirus type 5 and type 35 vectored vaccines against human immunodeficiency virus-1 (HIV-1), Ebola, or Marburg are similar despite differing adenovirus serotype vector, manufacturer's construct, or gene inserts. J Immunotoxicol 2008; 5:315-35; PMID:18830892; http://dx.doi.org/10.1080/15376510802312464
- Mori S, Takeuchi T, Enomoto Y, Kondo K, Sato K, Ono F, Iwata N, Sata T, Kanda T. Biodistribution of a low dose of intravenously administered AAV-2, 10, and 11 vectors to cynomolgus monkeys. Jpn J Infect Dis 2006; 59:285-93; PMID:17060693
- Tatsis N, Fitzgerald JC, Reyes-Sandoval A, Harris-McCoy KC, Hensley SE, Zhou D, Lin SW, Bian A, Xiang ZQ, Iparraguirre A, et al. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood 2007; 110:1916-23; PMID:17510320; http://dx.doi.org/10.1182/blood-2007-02-062117
- Puntel M, A KMG, Farrokhi C, Vanderveen N, Paran C, Appelhans A, Kroeger KM, Salem A, Lacayo L, Pechnick RN, et al. Safety profile, efficacy, and biodistribution of a bicistronic high-capacity adenovirus vector encoding a combined immunostimulation and cytotoxic gene therapy as a prelude to a phase I clinical trial for glioblastoma. Toxicol Appl Pharmacol 2013; 268:318-30; PMID:23403069; http://dx.doi.org/10.1016/j.taap.2013.02.001
- Chulay JD, Ye GJ, Thomas DL, Knop DR, Benson JM, Hutt JA, Wang G, Humphries M, Flotte TR. Preclinical evaluation of a recombinant adeno-associated virus vector expressing human alpha-1 antitrypsin made using a recombinant herpes simplex virus production method. Hum Gene Ther 2011; 22:155-65; PMID:20812844; http://dx.doi.org/10.1089/hum.2010.118
