ABSTRACT
The Advisory Committee on Immunization Practices of the US Centers for Disease Control (ACIP) has recently recommended the 13-valent protein-conjugate pneumococcal vaccine (PCV13) for routine use in adults age 18–65 who have immunocompromising conditions as well as in all adults over the age of 65. By comparison to 23-valent pneumococcal polysaccharide vaccine (PPSV23), antibody responses to PCV13 are similar or modestly better one month after vaccination. The implication that PCV13 will provide more persistent immunity has been disproven; 12 months later, recipients of PPSV23 or PCV13 have identical anti-pneumococcal activity. The theoretical concept that a protein-based vaccine will be followed by a booster effect when pure polysaccharide antigens are administered is based on remarkably little evidence. The strongest objection to the current recommendations is that, since PCVs stimulate mucosal antibodies, the widespread use of these PCVs has led to a near-disappearance of vaccine serotypes from the population. This phenomenon has been amply documented for PCV7, and PCV13 is well on its way to doing the same. Thus, as US physicians are convincing their adult patients to receive 2 “pneumonia shots” instead of one, the use of PCV13 in the USA is rapidly becoming irrelevant.
Introduction
The purpose of this communication is to consider the relevance of the recently published recommendations from the Advisory Committee on Immunization Practices of the US Centers for Disease Control (ACIP) regarding vaccination of adults with 13-valent protein conjugate pneumococcal vaccine (PCV13).Citation1 During those deliberations much emphasis was placed on: (a) the lack of data supporting benefit of the existing pneumococcal capsular polysaccharide vaccine (PPSV23); (b) in vitro data comparing immune responses to PPSV and PCV, (c) studies of PPSV23 and PCV in AIDS patients in Africa and, (d) at the time of the recommendation, the still-unpublished CAPiTA study. It is important, therefore, to: (a) review, briefly, data on the efficacy of PPSV; (b) consider studies that have compared immune responses to PPSV23 PCV in vitro and that might allow such comparison in vivo; (c) examine what new light the results of CAPiTA have shed on this subject; and, finally, (d) consider what benefit might be expected from implementing the ACIP recommendations.
Efficacy of PPSV
Controlled, prospective studies,Citation2-4 case-controlled studiesCitation5 and comparisons of serotypes that infect vaccinated vs. nonvaccinated individualsCitation6 have all shown that PPSV reduces the incidence of invasive pneumococcal disease (IPD; S. pneumoniae isolated from a normally sterile body site) or nonbacteremic pneumococcal pneumonia (NBPP; clinical diagnosis of pneumonia with pneumococcus isolated from sputum) by about 60 to 90%. Cochrane analyses, by Moberley et al.Citation7,8 have concluded that administration of PPSV reduces serotype specific NBPP, IPD and bacteremic pneumococcal pneumonia (BPP) by 73%, 82% and 87%, respectively. These analyses are regularly misquoted as not showing protection against NBPP, a problem that greatly distorts the results of cost-effectiveness projections. A meta-analysis by Huss et al Citation9 showed a reduction of only 36% in NBPP, with no significant effect on other outcomes. These investigators rejected many studies that other authorities consider valid, and their conclusions on the lack of efficacy of PPSV23 rely heavily on 2 papers,Citation10,11 both of which were very well-designed but used non-validated diagnostic methods to diagnose pneumococcal infection. Most importantly, they did not restrict their analysis to serotype-specific pneumococcal disease. The same criticism can be applied to the recently reported Spanish cohort study (CAPAMIS).Citation12 It is not reasonable to fault PPSV for failing to protect against serotypes that are not contained in the vaccine.
Our interpretation is that, using a variety of methodologic approaches, a large body of scientific literature has shown that PPSV effectively reduces the risk of IPD, BPP and NBPP, although this effect is less apparent in those most in need of protection, namely, immune compromised, frail and very elderly persons.Citation13-15
Direct comparisons of PPSV and PCV
No clinical study has directly compared PPSV with PCV. Comparisons have been based on ‘surrogate’ studies in which antibody to capsular polysaccharide and/or opsonic activity of serum for S. pneumoniae are measured after vaccination with PPSV or PCV. In a careful review of the studies published through 2011 (most of these were small-scaled),Citation13 we concluded that PCV was at least as immunogenic as PPSV but that there was no consistent advantage of either vaccine in immunocompetent or immunocompromised hosts. Since that time, additional small-scaled studies have yielded similar findings.Citation16
In 2013, however, Jackson et al.Citation17 published results of a study of >800 subjects, 60–64 y old, which showed that, one month after vaccination, opsonophagocytic activity for most pneumococcal serotypes was greater in recipients of PCV13 than in those who were given PPSV23. Although differences were statistically significant, it is unclear whether they would translate into clinical significance. Importantly, the assumption that higher levels would yield longer-lasting protection was clearly refuted; one year after vaccination, opsonic activity was essentially identical in recipients of PCV13 or PPSV23. In other words, except for a statistically significant greater opsonophagocytic activity one month (but not one year) after vaccination, the data do not favor PCV13 over PPSV23. In frail, elderly patients, Ridda et al.Citation15 reached the same conclusion, showing that one month post vaccination with PCV7 or PPSV23 antibody levels were higher for some antigens after PCV7, but that, 6 months later, antibody in the 2 groups was essentially identical.
Evidence for immunologic priming by PCV
When a polysaccharide is covalently conjugated to a carrier protein, the resulting antigen is thought to be recognized as T cell–dependent, stimulating a good serum antibody response, mucosal immunity, and immunologic memory.Citation18 An attractive hypothesis has been that initial vaccination with a protein conjugate polysaccharide would generate a primary response that will be followed by a booster response upon re-exposure to the same polysaccharide, whether conjugated or not. Abzug et al. reported a ‘booster’ phenomenon in HIV-infected children on antiretroviral therapy,Citation19 although Bhorat et al.,Citation20 working within a shorter time frame, did not find evidence for such a phenomenon.
Existing data in adults do not provide strong support for the priming/booster hypothesis. We Citation21 reported that conjugate vaccine followed by PPSV23 booster led to better antibody levels at one month. However, by 6 months later, antibody in booster recipients had fallen to baseline levels. Two recent large-scaled studies have examined the immunologic priming hypothesis, using sequential administration of PCV and PPSV. Jackson et alCitation22 showed that, among adults 60–64 y old, who had received PCV13 or PPSV23 >3 y prior, responses to PPSV23 after PCV elicited higher antibody responses when compared to initial PPSV23, while PPSV23 following PPSV23 elicited lower or non-inferior responses. In contrast, in a similar study population, Greenberg et al.Citation23 found that initial PCV13 enhanced the antibody response to PPSV23 given one year later compared to initial PPSV 23; but the antibody response after PCV13-PPSV 23 or PCV13-PCV13 sequence was not enhanced when compared to PCV13 alone, suggesting either that there is no booster effect or that a 1-year interval for PPSV23 following PCV13 may not be sufficient to elicit booster responses. This latter study does not necessarily support the current recommendation that there should be a one-year interval between PCV13 and PPSV23. Importantly, neither of these studies measured the putative booster response more than one month following the second injection.
Among immunosuppressed adults, immunologic priming has also been difficult to demonstrate regardless of the population studied,Citation20 and no further response to PPSV was seen when PPSV23 was given after multiple doses of PCV in allogeneic stem cell transplant recipients.Citation24 In a study that showed an advantage of the sequential PCV-PPSV23 over PPSV23 strategy 8 weeks after the polysaccharide vaccine, the benefit of the booster was no longer present after 6 months.Citation25 The ACIP has changed its recommendations for sequential administration of PCV followed by PPSV23, now recommending a one-year interval between the first and second vaccine.Citation26 As the reader can infer, this recommendation is not strongly supported by currently available data.
The CAPITA study
The CAPiTA trial Citation27 was a very well-designed, randomized controlled trial in which nearly 85,000 Dutch adults received either PCV13 or a placebo with careful observation for the ensuing 4 y to determine the efficacy of the vaccine in preventing pneumococcal pneumonia or invasive pneumococcal disease. The results showed 46 percent efficacy of PCV13 against vaccine-type pneumococcal pneumonia and 75 percent efficacy against vaccine-type invasive pneumococcal disease. Efficacy persisted for the duration of the trial. This study was designed to exclude immunocompromised individuals. It is important to note that, in a relatively small number of subjects who developed an immune-compromising condition or who were given immunosuppressive drugs during the period of study, PCV13 exhibited no protective effect (14 cases of pneumococcal disease in vaccine-recipients vs. 11 cases in placebo-recipients; Table S4,Citation27 P= .69). Thus, (a) the CAPiTA study did not compare PCV13 to PPSV23 and PCV13; (b) the study excluded immune compromised subjects; and (c) when persons already enrolled in the study developed an immunocompromising condition, PCV13 was specifically shown to be of no benefit.
Relevance of PCV13 in the adult population
PCV stimulates systemic and mucosal immunity, providing generalized protection against pneumococcal infection and also preventing nasal colonization with vaccine types. Widespread use of PCV7 has virtually eliminated pneumococcal infection due to these 7 vaccine types among vaccinated toddlers and young children.Citation28,29 Importantly, because of its effect on colonization of infants and toddler, an indirect effect has been the more gradual near-elimination of infection in adults due to vaccine strains (Fig. 1).Citation28 This indirect effect has also extended to some groups of immunocompromised individuals such as those with AIDS,Citation30 albeit to a lesser degree, and the incidence continues to remain several times fold that of the non-HIV population Since the direct and indirect effect of conjugate vaccine appears to be less effective in patients with AIDS, it is clear that alternative approaches are needed.
Figure 1. Decline in vaccine-type specific cases of invasive pneumococcal disease in children <5 years of age (upper panel) and adults >65 years of age (lower panel). PCV7 = seven valent protein-conjugate pneumococcal vaccine. Reprinted with permission from Ref. 28: Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010; 201(1):32-41; PMID:19947881; http://dx.doi.org/10.1086/648593. © 2010 Oxford University Press. Reproduced by permission of Oxford University Press. Permission to reuse must be obtained from the rightsholder.
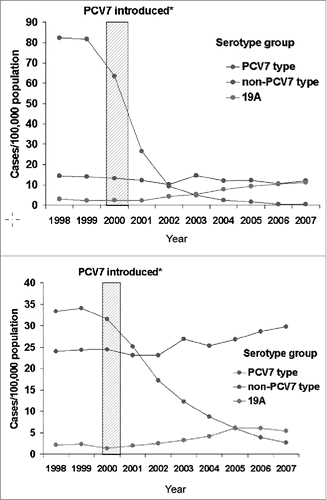
There was never any reason to doubt that PCV13 would have the same kind of indirect effect as that seen with PCV7. Dagan et al documented decreased colonization by vaccine types among children who have received PCV13 Citation31 just as had previously been done following vaccination with PCV7.Citation32,33 Moore et al. Citation34 showed that, between 2010 and 2013, there was a 58–72% decline in IPD due to serotypes specifically contained in PCV13 but not in PCV7, and workers in France have shown similar results. Finally, the CAPiTA study, itself, observed a 38% decline in pneumonia in adults due to strains contained in PCV13 comparing the early years to the last years of the study. Detailed studies in the US Citation28 show that the indirect effect on pneumococcal disease in adults lags the effect on colonization of children by several years.
Cost-analysis projections
Results of cost analysis are largely determined by the predictions and estimates on which they are based. Smith et al. Citation30 reported that PCV13 might be more cost-effective than PPSV23 in adults >50 y of age. This study utilized a Delphi expert analysis which assumed that PPSV23 had no impact on preventing nonbacteremic pneumococcal pneumonia based on a misreading of Moberley et al. Citation7 and excessive emphasis on Huss et al.Citation9 as alluded to above. Even more important, although the authors were cognizant of the indirect effect of PCV13, (“…these conclusions are sensitive to assumptions regarding PCV13 effectiveness … PPSV23 effectiveness against IPD and NPP, and herd immunity…”), they underestimated its extent. In other words, estimates of cost-effectiveness of PCV13 have been based on underestimates both of the efficacy of PPSV23 and the indirect effect of PCV13. Their figures have also failed to consider the actual impact of pneumococcal disease. These authors estimated that 30% of all hospitalized pneumonia was due to pneumococcus, whereas recent studies Citation35,36 suggest that no more than 10–12% of community acquired pneumonia is due to S. pneumoniae. In a UK study, Jiang et al. Citation37 predicted greater benefit/cost ratio for PPSV23 when compared to PCV13.
It should fnally be pointed out that sequential administration of 2 pneumococcal vaccines greatly increases the complexity of vaccine delivery, both for practicing physicians and for institutional providers. A concern is that patients may receive only one pneumococcal vaccine – namely, PCV13 and not return for the second one.
Conclusion
In summary, the ACIP recommendations for widespread use of PCV 13 in immunocompromised adults and in adults >65 y of age are based on very few supporting data. It is not at all clear that vaccinating at-risk individuals induces any lasting benefit compared to PPSV23. A booster effect from administering PPSV23 after PCV13 has not been consistently demonstrated The pneumococcal serotypes contained in this vaccine are rapidly disappearing from the population which renders these recommendations largely irrelevant. Cost-benefit analyses have erroneously assumed that PPSV23 does not reduce the risk of NBPP and have overestimated, perhaps by threefold, the number of cases of pneumococcal pneumonia each year in the US. It is, therefore, unfortunate that, with all the other medical and social needs for dollars, a major vaccination campaign for adult vaccination with PCV13 is being undertaken in the US at the present time.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged >/=65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014;63:822-5; PMID:25233284
- Felton LD, Ekwurtzel GM, Simmons JS, Dublin LI. Studies on immunizing substances in pneumococci. Public Health Rep 1938;53:1855; PMID:19315683; http://dx.doi.org/10.2307/4582685
- Kaufman P. Studies on old age pneumonia : prophylactic effect of pneumococcus polysaccharide against pneumonia. Archives of internal medicine 1941;67:304-19; http://dx.doi.org/10.1001/archinte.1941.00200020066004
- MacLeod CM, Hodges RG, Heidelberger M, Bernhard WG. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J Exp Med 1945;82:445-65; http://dx.doi.org/10.1084/jem.82.6.445
- Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, Adair RK, Clemens JD. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med 1991;325:1453-60; PMID:1944423; http://dx.doi.org/10.1056/NEJM199111213252101
- Bolan G, Broome CV, Facklam RR, Plikaytis BD, Fraser DW, Schlech WF, 3rd. Pneumococcal vaccine efficacy in selected populations in the United States. Ann Intern Med 1986;104:1-6; PMID:3940476; http://dx.doi.org/10.7326/0003-4819-104-1-1
- Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2008:CD000422; PMID:18253977
- Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2013; 1:CD000422; PMID:23440780
- Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ 2009;180:48-58; PMID:19124790; http://dx.doi.org/10.1503/cmaj.080734
- Koivula I, Sten M, Leinonen M, Makela PH. Clinical efficacy of pneumococcal vaccine in the elderly: a randomized, single-blind population-based trial. Am J Med 1997;103:281-90; PMID:9382120; http://dx.doi.org/10.1016/S0002-9343(97)00149-6
- Ortqvist A, Hedlund J, Burman LA, et al. Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Swedish Pneumococcal Vaccination Study Group. Lancet 1998;351:399-403; PMID:9482293; http://dx.doi.org/10.1016/S0140-6736(97)07358-3
- Ochoa-Gondar O, Vila-Corcoles A, Rodriguez-Blanco T, Gomez-Bertomeu F, Figuerola-Massana E, Raga-Luria X, Hospital-Guardiola I. Effectiveness of the 23-Valent Pneumococcal Polysaccharide Vaccine Against Community-Acquired Pneumonia in the General Population Aged >=60 Years: 3 Years of Follow-up in the CAPAMIS Study. Clin Infect Dis 2014;58:909-17; PMID:24532544; http://dx.doi.org/10.1093/cid/ciu002
- Musher DM, Sampath R, Rodriguez-Barradas MC. The potential role for protein-conjugate pneumococcal vaccine in adults: what is the supporting evidence? Clin Infect Dis 2011;52:633-40; PMID:21292668; http://dx.doi.org/10.1093/cid/ciq207
- van Werkhoven CH, Huijts SM, Bolkenbaas M, Grobbee DE, Bonten MJ. The impact of age on the efficacy of 13-valent pneumococcal conjugate vaccine in elderly. Clin Infect Dis 2015; PMID:26265498
- Ridda I, Macintyre CR, Lindley R, Gao Z, Sullivan JS, Yuan FF, McIntyre PB. Immunological responses to pneumococcal vaccine in frail older people. Vaccine 2009;27:1628-36; PMID:19100304; http://dx.doi.org/10.1016/j.vaccine.2008.11.098
- Karlsson J, Hogevik H, Anderssson K, Roshani L, Andreasson B, Wenneras C. Pneumococcal vaccine responses in elderly patients with multiple myeloma, Waldenstrom's macroglobulinemia, and monoclonal gammopathy of undetermined significance Trials Vaccinol 2013;2:31-8; http://dx.doi.org/10.1016/j.trivac.2013.09.001
- Jackson LA, Gurtman A, van Cleeff M, Jansen KU, Jayawardene D, Devlin C, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults. Vaccine 2013;31:3577-84; PMID:23688526; http://dx.doi.org/10.1016/j.vaccine.2013.04.085
- Dinleyici EC, Yargic ZA. Current knowledge regarding the investigational 13-valent pneumococcal conjugate vaccine. Expert Rev Vaccines 2009;8:977-86; PMID:19627181; http://dx.doi.org/10.1586/erv.09.68
- Abzug MJ, Song LY, Levin MJ, Nachman SA, Borkowsky W, Pelton SI; International Maternal Pediatric Adolescent AIDS Clinical Trials Group P1024 and P1061s Protocol Teams. Antibody persistence and immunologic memory after sequential pneumococcal conjugate and polysaccharide vaccination in HIV-infected children on highly active antiretroviral therapy. Vaccine 2013;31:4782-90; PMID:23954381; http://dx.doi.org/10.1016/j.vaccine.2013.08.002
- Bhorat AE, Madhi SA, Laudat F, Sundaraiyer V, Gurtman A, Jansen KU, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Immunogenicity and safety of the 13-valent pneumococcal conjugate vaccine in HIV-infected individuals naive to pneumococcal vaccination. AIDS 2015;29:1345-54; PMID:25888646; http://dx.doi.org/10.1097/QAD.0000000000000689
- Musher DM, Rueda AM, Nahm MH, Graviss EA, Rodriguez-Barradas MC. Initial and subsequent response to pneumococcal polysaccharide and protein-conjugate vaccines administered sequentially to adults who have recovered from pneumococcal pneumonia. J Infect Dis 2008;198:1019-27; PMID:18710324; http://dx.doi.org/10.1086/591629
- Jackson LA, Gurtman A, van Cleeff M, Frenck RW, Treanor J, Jansen KU, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine 2013;31:3594-602; PMID:23688525; http://dx.doi.org/10.1016/j.vaccine.2013.04.084
- Greenberg RN, Gurtman A, Frenck RW, Strout C, Jansen KU, Trammel J, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults 60-64 years of age. Vaccine 2014;32:2364-74; PMID:24606865; http://dx.doi.org/10.1016/j.vaccine.2014.02.002
- Cordonnier C, Ljungman P, Juergens C, Maertens J, Selleslag D, Sundaraiyer V, Giardina PC, Clarke K, Gruber WC, Scott DA, et al. Immunogenicity, Safety, and Tolerability of 13-Valent Pneumococcal Conjugate Vaccine Followed by 23-Valent Pneumococcal Polysaccharide Vaccine in Recipients of Allogeneic Hematopoietic Stem Cell Transplant Aged >/=2 Years: An Open-Label Study. Clin Infect Dis 2015;61:313-23; PMID:25870329; http://dx.doi.org/10.1093/cid/civ287
- Lesprit P, Pedrono G, Molina JM, Goujard C, Girard PM, Sarrazin N, Katlama C, Yéni P, Morineau P, Delfraissy JF, et al. Immunological efficacy of a prime-boost pneumococcal vaccination in HIV-infected adults. Aids 2007;21:2425-34; PMID:18025879; http://dx.doi.org/10.1097/QAD.0b013e3282887e91
- Kobayashi H. Intervals between PCV13 and PPSV23 vaccines: evidence supporting currently recommended intervals and proposed changes. 2015. Available at http:///wwwcdcgov/vaccinesacip/meetings/downloads/slides-2015-06/pneumo-02-kobayashipdf
- Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AM, Sanders EA, Verheij TJ, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015;372:1114-25; PMID:25785969; http://dx.doi.org/10.1056/NEJMoa1408544
- Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010;201(1):32-41; PMID:19947881; http://dx.doi.org/10.1086/648593
- Feikin DR, Kagucia EW, Loo JD, Link-Gelles R, Puhan MA, Cherian T, Levine OS, Whitney CG, O'Brien KL, Moore MR; Serotype Replacement Study Group. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites. PLoS Med 2013; 10:e1001517; PMID:24086113; http://dx.doi.org/10.1371/journal.pmed.1001517
- Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA 2012;307:804-12
- Dagan R, Juergens C, Trammel J, Patterson S, Greenberg D, Givon-Lavi N, Porat N, Gurtman A, Gruber WC, Scott DA. Efficacy of 13-Valent Pneumococcal Conjugate Vaccine (PCV13) Versus That of 7-Valent PCV (PCV7) Against Nasopharyngeal Colonization of Antibiotic-Nonsusceptible Streptococcus pneumoniae. J Infect Dis 2015;211:1144-53; PMID:25355940; http://dx.doi.org/10.1093/infdis/jiu576
- Dagan R. Impact of pneumococcal conjugate vaccine on infections caused by antibiotic-resistant Streptococcus pneumoniae. Clin Microbiol Infect 2009; 15 Suppl 3:16-20; PMID:19366365; http://dx.doi.org/10.1111/j.1469-0691.2009.02726.x
- Pelton SI, Loughlin AM, Marchant CD. Seven valent pneumococcal conjugate vaccine immunization in two Boston communities: changes in serotypes and antimicrobial susceptibility among Streptococcus pneumoniae isolates. Pediatr Infect Dis J 2004;23:1015-22; PMID:15545856; http://dx.doi.org/10.1097/01.inf.0000143645.58215.f0
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis 2015;15:301-9; PMID:25656600; http://dx.doi.org/10.1016/S1473-3099(14)71081-3
- Musher DM, Roig IL, Cazares G, Stager CE, Logan N, Safar H. Can an etiologic agent be identified in adults who are hospitalized for community-acquired pneumonia: results of a one-year study. J Infect 2013;67:11-8; PMID:23523447; http://dx.doi.org/10.1016/j.jinf.2013.03.003
- Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, Reed C, Grijalva CG, Anderson EJ, Courtney DM, et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N Engl J Med 2015;373:415-27; PMID:26172429; http://dx.doi.org/10.1056/NEJMoa1500245
- Jiang Y, Gauthier A, Keeping S, Carroll S. Cost-effectiveness of vaccinating the elderly and at-risk adults with the 23-valent pneumococcal polysaccharide vaccine or 13-valent pneumococcal conjugate vaccine in the UK. Expert Rev Pharmacoecon Outcomes Res 2014;14:913-27; PMID:25189087; http://dx.doi.org/10.1586/14737167.2014.950232
