Abstract
Pneumococcal conjugate vaccines (PCVs) have been successful in preventing invasive pneumococcal disease but effectiveness has been challenged by replacement of vaccine serotypes with non-vaccine serotypes. Vaccines targeting common pneumococcal protein(s) found in most/all pneumococci may overcome this limitation. This phase II study assessed safety and immunogenicity of a new protein-based pneumococcal vaccine containing polysaccharide conjugates of 10 pneumococcal serotypes combined with pneumolysin toxoid(dPly) and pneumococcal histidine triad protein D(PhtD) (PHiD-CV/dPly/PhtD-30) in African children. 120 Gambian children (2–4 years, not previously vaccinated against Streptococcus pneumoniae) randomized (1:1) received a single dose of PHiD-CV/dPly/PhtD-30 or PCV13. Adverse events occurring over 4 d post-vaccination were reported, and blood samples obtained pre- and 1-month post-vaccination. Serious adverse events were reported for 6 months post-vaccination. Solicited local and systemic adverse events were reported at similar frequency in each group. One child (PHiD-CV/dPly/PhtD-30 group) reported a grade 3 local reaction to vaccination. Haematological and biochemical parameters seemed similar pre- and 1-month post-vaccination in each group. High pre-vaccination Ply and PhtD antibody concentrations were observed in each group, but only increased in PHiD-CV/dPly/PhtD-30 vaccinees one month post-vaccination. One month post-vaccination, for each vaccine serotype ≥96.2% of PHiD-CV/dPly/PhtD-30 vaccinees had serotype-specific polysaccharide antibody concentrations ≥0.20µg/mL except serotypes 6B (80.8%) and 23F (65.4%), and ≥94.1% had OPA titres of ≥8 except serotypes 1 (51.9%), 5 (38.5%) and 6B (78.0%), within ranges seen in PCV13-vaccinated children. A single dose of PHiD-CV/dPly/PhtD-30 vaccine, administered to Gambian children aged 2–4 y not previously vaccinated with a pneumococcal vaccine, was well-tolerated and immunogenic.
Abbreviations
| AE | = | adverse event |
| ALT | = | alanine transaminase |
| ATP | = | according-to-protocol |
| CI | = | confidence interval |
| dPly | = | pneumolysin toxoid |
| ELISA | = | enzyme-linked immunosorbent assay |
| GMC | = | geometric mean antibody concentration |
| GMT | = | geometric mean titres |
| IPD | = | Invasive pneumococcal disease |
| LU | = | Luminex units |
| NTHi | = | non-typeable Haemophilus influenzae |
| OPA | = | opsonophagocytic activity |
| PCV | = | pneumococcal conjugate vaccine |
| PhtD | = | histidine-triad protein D |
| Ply | = | pneumolysin |
| PS | = | capsular polysaccharide |
| SAE | = | serious adverse event |
| TVC | = | total vaccinated cohort |
Introduction
Pneumococcal pneumonia and invasive pneumococcal disease (IPD), caused by Streptococcus pneumoniae, are responsible for about 500,000 deaths each year among children <5 years, with the majority of these deaths occurring in developing countries.Citation1 S. pneumoniae has over 90 serotypes and normally inhabits the nasopharynx from where it can spread to cause otitis media, sinusitis or pneumonia, or invade the circulation to cause bacteraemia, septicaemia or meningitis.
Pneumococcal conjugate vaccines (PCVs) containing polysaccharides (PS) coupled to a non-pneumococcal protein have reduced the burden of pneumococcal diseases due to vaccine serotypes worldwide. Citation2-5 In the US, PCV7 covered >80% of the serotypes responsible for IPD prior to vaccination and dramatically decreased vaccine type IPD. Coverage in Africa was less, about 60%.Citation6,7 The restricted coverage provided by PCV7 led to the development of PCVs with additional serotypes, in particular serotypes 1 and 5, common in Africa, as recognized by WHO in the target profile for global PCVs targeted for the advance market commitment. However, global control of pneumococcal disease may be difficult to achieve due to serotype replacement, technical limitations in the number of PS that can be included and the high cost of PCVs.Citation6-8 A potential solution to overcome the PCVs' limitations is the development of vaccines containing pneumococcal protein(s) well conserved across all pneumococci.
An investigational vaccine containing 2 proteins - pneumococcal histidine triad protein D (PhtD) and pneumolysin toxoid (dPly standing for “detoxified pneumolysin”) is being developed. PhtD, one of the proteins expressed on the surface of the pneumococcus, is thought to be involved in invasionCitation9 and in inhibition of complement deposition through binding to factor H.Citation10,11 PhtD is involved in zinc homeostasis and is crucial for host colonization and invasion.Citation12 Pneumolysin (Ply) is an exotoxin released during bacterial autolysis.Citation13 Ply is a multifunctional haemolytic cytolysin that plays a role in the early pathogenesis of IPD by facilitating intrapulmonary bacterial growth and invasion of the blood.Citation13 Antibodies to these proteins could promote neutralization of important toxic or enzymatic functions of pneumococci and inhibit adherence of the bacteria to epithelial cells.Citation14,15 In animal studies, immunization with dPly and/or PhtD protected against nasopharyngeal colonization, septicaemia, lethal challenge and pneumonia due to various serotypes.Citation10,14-17
dPly and PhtD, administered alone or in combination with a 10-valent PCV (PCV10), were well tolerated and immunogenic in healthy young adults,Citation18,19 children and infants in Europe.Citation20-22 The safety and immunogenicity of this pneumococcal protein-based vaccine could, however, be different in African settings where there is a high prevalence of nasopharyngeal carriage of S. pneumoniae and a high incidence of pneumococcal disease. Therefore, a cautious approach was adopted to evaluate the safety profile of this vaccine in African children. We describe here the results of a pilot safety assessment of an investigational vaccine containing 30 µg of each dPly and PhtD combined with a 10-valent pneumococcal conjugate vaccine (PHiD-CV/dPly/PhtD-30) in Gambian children aged 2–4 y prior to the conduct of a larger trial in infants. (www.clinicalTrials.gov NCT01262872). However, this study was not powered to detect differences between study groups in immune responses to the vaccines.
Results
Study participants
One hundred and twenty children aged 2–4 y were enrolled and randomized, all of whom received one dose of either PHiD-CV/dPly/PhtD-30 or PCV13. All completed the last study visit. Seventeen children (8 receiving PHiD-CV/dPly/PhtD-30; 9 receiving PCV13) were excluded from the ATP safety and immunogenicity cohort as they received a concomitant vaccine (OPV) given during a mass campaign against polio after receiving the study vaccine (). The demographic characteristics of the 2 groups were comparable. The mean (SD) age of PHiD-CV/dPly/PhtD-30 children was 2.8 (0.40) years and that of the PCV13-vaccinated children was 2.9 (0.36) years. There were 41 (68.3%) girls in the PHiD-CV/dPly/PhtD-30 group and 26 (43.3 %) in the PCV13 group. All the children were of African ancestry.
Figure 1. Trial Consort. N: number of enrolled children; ATP: according-to-protocol; PHiD-CV/dPly/PhtD-30: Children receiving a single dose of an investigational vaccine containing polysaccharide conjugates of PHiD-CV combined with 30 µg each of dPly and PhtD pneumococcal proteins; PCV13: Children receiving a single dose of Prevnar13.
Safety and reactogenicity
Grade 3 vaccine-related swelling was reported at the injection site in one child receiving PHiD-CV/dPly/PhtD-30. There were no episodes of general swelling of the vaccinated limb in either study groups during the 4-day post-vaccination period. The overall incidence of solicited general AEs was in similar ranges in both groups. No grade 3 general solicited AEs were reported. Fever, the most frequently reported solicited general AE, was reported in 4 (6.7%) children receiving PHiD-CV/dPly/PhtD-30 and in 2 (3.3%) children receiving PCV13; for one subject in each group, fever was considered to be causally related to vaccination by the investigator. Loss of appetite was reported for one child receiving PHiD-CV/dPly/PhtD-30. No other solicited local or general AEs in either group were reported. At least one unsolicited AE was reported for 21.7% (95% CI 12.1%–34.2%) of children receiving PHiD-CV/dPly/PhtD-30 and for 11.7% (95% CI 4.8%–22.6%) receiving PCV13. The most frequently reported unsolicited AE in the PHiD-CV/dPly/PhtD-30 group was respiratory tract infection (8.3%) while tinea capitis was the most frequently reported AE in the PCV13 group (3.3%). Prophylactic use of an antipyretic was not reported in either group and for only one child in the PCV13 group antipyretic use was reported during the 4-days post-vaccination period. No SAEs were reported throughout the extended safety period of 6 months.
The pre-vaccination ALT level of one child receiving PHiD-CV/dPly/PhtD-30 was of a grade 3 reaction, but this fell to a normal level one month post-vaccination. The result of biochemical parameters were not known before vaccination. There were no other clinically significant abnormalities in the haematological or biochemical measurements.
Immune response to Ply and PhtD
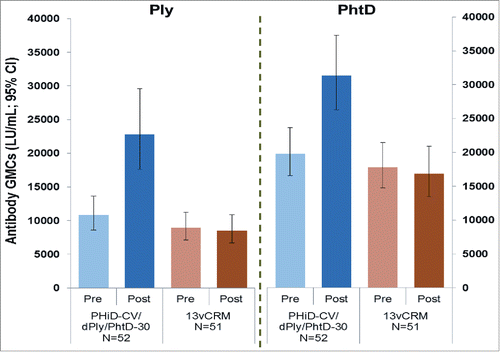
Pre-vaccination, Ply and PhtD GMCs were in similar ranges in each group. All children receiving PHiD-CV/dPly/PhtD-30 and ≥98% of children receiving PCV13 had Ply antibody titres ≥599 LU/ml. Similarly all the children in each group had PhtD antibody titres ≥399 LU/ml. One month after vaccination, Ply and PhtD GMCs tended to be higher in the PHiD-CV/dPly/PhtD-30 group compared to pre-vaccination levels. No increase was observed in the PCV13 group ().
Figure 2. Ply and PhtD antibody geometric mean concentrations pre- and 1-month post vaccination. N: number of enrolled children; Pre: before vaccination; Post: one month after vaccination; GMC: geometric mean antibody concentrations; PHiD-CV/dPly/PhtD-30: Children receiving a single dose of an investigational vaccine containing polysaccharide conjugates of PHiD-CV combined with 30 µg each of dPly and PhtD pneumococcal proteins; PCV13: Children receiving a single dose of Prevenar 13. (The error bars represent standard deviation.
Immune response to pneumococcal serotype-specific PS conjugates
For each vaccine serotype there were increases in PS GMCs and GMTs from pre- to post-vaccination in each group (). For each of the 10 common vaccine serotypes, the percentage of children with an antibody concentration of ≥0.2 µg/ml pre-vaccination ranged from 9.6% to 63.5% in the PHiD-CV/dPly/PhtD-30 group and from 9.8% to 62.7% in the PCV13 group reflecting an existing baseline sero-positivity rate; one month post-vaccination, ≥96.2% of the PHiD-CV/dPly/PhtD-30-vaccinated children had serotype-specific PS GMCs ≥0.2 µg/ml except for antibodies to serotypes 6B (80.8%) and 23F (65.4%), figures seem to be comparable to those found in PCV13-vaccinated children (≥96.1% had PS GMCs of ≥0.2 µg/ml) except for antibodies to serotypes 6B (90.2%) and 23F (90.2%). Cross-reactivity is said to occur when a vaccine containing a serotype (e.g. 6B) but no other serotypes in the same serogroup (serogroup 6) is administered to an individual and induces antibody production against other serotype(s) (e.g. 6A) in the same serogroup. Pre-vaccination, 30.8% and 17.6% of PHiD-CV/dPly/PhtD-30-vaccinated and PCV13-vaccinated children respectively had antibody concentrations of ≥0.2 µg/ml against serotype 6A while 61.5% and 49.0% of PHiD-CV/dPly/PhtD-30-vaccinated and PCV13-vaccinated children respectively had GMCs of ≥0.2 µg/ml against serotype 19A. Post-vaccination, for cross-reactive serotypes 6A and 19A, the percentages of children with PS GMCs ≥0.2 µg/ml in the PHiD-CV/dPly/PhtD-30-vaccinated group were 42.3% and 92.3% respectively compared to values of 90.2% and 100% respectively in the PCV13 vaccinated group. For each of the 10 common vaccine serotypes, over 94.1% of PHiD-CV/dPly/PhtD-30-vaccinated children had GMTs of ≥8 except for serotypes 1 (51.9%), 5 (38.5%) and 6B (78.0%). For each of the 10 common vaccine serotypes, over 96.1% of the PCV13-vaccinated children had GMTs of ≥8 except for serotypes 1 (90.0%) and 5 (89.8%).
Table 1. Antibody concentrations (22F-inhibition ELISA) for pneumococcal serotype-specific responses, pre- and one month post-vaccination (ATP immunogenicity cohort).
For cross-reactive serotypes 6A and 19A, the percentages of children who had GMTs ≥8 were 78.7% and 98% respectively in the PHiD-CV/dPly/PhtD-30-vaccinated (cross-reactive antibodies) compared with 100% and 100% respectively in the PCV13-vaccinated group ().
Table 2. Serotype-specific opsonophagocytic titres one month post-vaccination (ATP immunogenicity cohort).
Immune response to Haemophilus influenzae protein D
The percentage of children who had pre-vaccination antibody titres to NTHi protein D above the cut-off value was 15.4% in the PHiD-CV/dPly/PhtD-30 group vs. 13.7% in the PCV13 group. One month post-vaccination, the percentage was 61.5% for the PHiD-CV/dPly/PhtD-30-vaccinated children while no increase in GMC or seropositivity rate was observed in the PCV13 group (13.7% in PCV13) ().
Table 3. Immune responses to dPly, PhtD and PD pre- and one month post-vaccination (ATP immunogenicity cohort).
Discussion
Pneumococcal proteins are promising antigens for vaccine development as they induce functional antibodies Citation23 and protect animals against pneumococcal infection.Citation14,15 We have evaluated the reactogenicity, safety and immunogenicity of PHiD-CV/dPly/PhtD-30 when given as a single dose to Gambian children aged 2–4 y. This was the first time that a protein-based pneumococcal vaccine has been administered to African children.
Vaccine tolerability seemed to be comparable between the PHiD-CV/dPly/PhtD-30 and PCV13 groups. There were no safety concerns raised throughout the study period; only one grade 3 local reaction (swelling) was reported in the PHiD-CV/dPly/PhtD-30 group. There were no clinically significant haematological or biochemical toxicities observed post-vaccination. One child receiving PHiD-CV/dPly/PhtD-30 had an elevated ALT level pre-vaccination but this was normal one month post-vaccination. The results of the biochemistry tests were not known at the time of vaccination. The elevated ALT pre-vaccination could have been due to a subclinical liver infection such as hepatitis A. No SAEs were reported throughout the study period including the safety follow-up period of 6 months.
All children had measurable Ply and PhtD antibody titres pre-vaccination. The background antibody titres may be due to the high nasopharyngeal carriage of S. pneumoniae reported in this population.Citation24-26 Regardless of this high background, both dPly and PhtD were still highly immunogenic in African children as in European adultsCitation19 and children.Citation20 The post-vaccination GMCs against pneumococcal proteins that are associated with protection against pneumococcal diseases in humans have not been determined so far. The licensure pathway for pneumococcal protein-based vaccines has not been defined yet. Therefore, in addition to immunogenicity results, further assessment of the efficacy of this pneumococcal protein vaccine against nasopharyngeal carriage is currently ongoing.
In our study population, despite the existing pre-vaccination antibody levels, there was a substantial increase from pre-vaccination to post-vaccination levels for anti-Ply and anti-PhtD which is within a 2-fold increase. These existing pre-vaccination levels of anti-Ply and anti-PhtD may be because the participants were already exposed to S. pneumoniae. The immune response in infants, the desired target population who have not had such exposure may be different; following 2 or 3 primary doses the responses may be higher and long lasting compared to one dose. Furthermore, the functionality of these antibodies was not quantified. However, the proteins have been shown to protect against pneumococcal infection in preclinical studies. In the infant cohort of this study we assess the immune response and effect of nasopharyngeal carriage of S. pneumoniae.
Increases in serotype-specific PS GMCs and GMTs were observed in children receiving PHiD-CV/dPly/PhtD-30. It is difficult to compare the immunogenicity of the investigational vaccine with PHiD-CV as it was not used in this study. Also there is insufficient data from studies of PHiD-CV with similar settings, vaccine schedules and age group. However, the percentages of children who attained the cut-off threshold of 0.2 µg/mL in our study seemed to be similar to those seen in a study where African infants were given PHiD-CV as a 3-primary dose schedule using the 6–10–14 week schedule,Citation27 for all the common vaccine serotypes except 23F. The immune response to serotype 6B in the current study seemed to be lower compared to other serotypes as seen in most PCV studies.Citation28-31 Despite generally low GMCs to serotypes 6B and 23F, the efficacy of PHiD-CV against IPD, acute otitis media and pneumonia caused by these serotypes has been demonstrated following infant vaccination. Citation29,32,Citation33 Also, no enhancement of the immune responses measured by OPA GMTs was observed in PHiD-CV/dPly/PhtD-30 vaccinees for serotype 3, suggesting that no detectable OPA response was induced by the pneumococcal proteins against this serotype. It should be noted that the immune response to pneumococcal proteins may be acting on the bacteria through other mechanisms of action than opsonic antibodies (e.g. anti-adhesion, neutralizing of toxin activity).To the best of our knowledge, the protective effect of PCVs following 1-dose catch-up vaccination in 2–4-years-old has not been assessed. The high proportion of children with high GMCs prior to vaccination may be due to high nasopharyngeal carriage and environmental exposure to PCV7 serotypesCitation24 which has been associated with a reduced immune response to PCVs in early infancy.Citation34
In our study, the seropositivity rate of antibodies to protein D one month post-vaccination increased to 61.5% for the PHiD-CV/dPly/PhtD-30-vaccinated children. This response is lower compared to the values obtained in European toddlers.Citation20 This may be explained by the fact that these European toddlers received 2 doses of the vaccine while children in our study received only one dose. In another study, European children 2–5 y of age also received a single dose catch-up vaccination with PHiD-CV. The seropositivity rate for antibodies to protein D one month post-vaccination was 76.3%,Citation35 which is in similar range to the seropositivity rate found in our study.
The human microbiome is known to vary depending on the diet/nutrition, geographic location and living conditions of the population and affects immune responses to vaccines.Citation36 The microbiome of Gambian children may be considered a confounder to their responses to this investigational vaccine. Therefore, generalization of the results should be done with caution.
One limitation of this study is that it was not designed to assess the impact of pneumococcal proteins on the PS-conjugate vaccineas the controls in this study received PCV13. To do this PHiD-CV should have been used as the comparator vaccine but PCV13 was chosen for this study primarily addressing safety in African children aged 2 to 4 y as PCV13 was about to be introduced into the Gambian National Immunization Program. However, a study evaluating the safety and immunogenicity of this investigational product in European toddlers using PHiD-CV as a comparator vaccine showed that the pneumococcal proteins do not negatively impact antibody responses to vaccine serotypes. Citation20 Another limitation includes the small sample size in this study. However, the primary objective was to assess the safety of the vaccine; immunogenicity analyses were mainly descriptive. A larger sample size will be required to make conclusions on the immune responses induced by these proteins and their potential impact on pneumococcal carriage and disease. In this study we used a threshold value of 0.2µg/ml, corresponding to WHO working group's 0.35µg/ml antibody concentration of IgG anticapsular PS, in order to characterize the vaccine response. This threshold applied for immune responses measured one month after completion of a primary series in infancy has been recommended by WHO as non-inferiority threshold (0.35) for licensure on new PCV against IPD. The clinical relevance of this threshold after one dose in an older age group like in this study is unknown. Lastly, there was an imbalance in the proportion of girls in the PHiD-CV/dPly/PhtD-30 group compared to the control group. The reason for this imbalance is that the randomization system does not account for gender when assigning groups. Thus, the results are by chance and differences were not expected because of this.
Conclusion
This study has shown that PHiD-CV/dPly/PhtD-30 was well tolerated and immunogenic when administered as a single dose to 2–4-year-old Gambian children who had not been previously vaccinated against S. pneumoniae. The investigators have proceeded to a larger dose-and schedule-finding trial in Gambian infants, assessing impact on nasopharyngeal carriage.
Methods
Study design
This phase II, randomized, observer-blind, controlled study was conducted in the Fajikunda district of the Western Region of The Gambia between February 2011 and September 2011.
Recruitment and randomization
Mothers who brought their infants for routine vaccination at the Fajikunda Health Centre were asked if they had children aged 2–4 y. According to routine Expanded Programme on Immunization (EPI) vaccines in The Gambia, 2–4 year-old children were expected to have completed their immunization comprising one dose each of Bacille-Calmette-Guerin, oral polio vaccine (OPV) and hepatitis B vaccines given at birth, 3 doses each of OPV and pentavalent vaccine containing diphtheria, pertussis, tetanus, hepatitis B and Haemophilus influenzae type b antigens administered at 2, 3 and 4 months of age and measles and yellow fever vaccines given at 9 months of age. PCV had not been introduced in The Gambia when these children were infants. Children were eligible for inclusion if they were healthy, had completed the primary series of EPI vaccines and had not received any vaccination against S. pneumoniae. Exclusion criteria included having received or planning to use other investigational products during the study period, having received immunoglobulins or other blood products, chronic use of immunosuppressive therapy, having a confirmed or suspected immunodeficient state, having an allergic disease likely to be worsened by any component of the vaccines or being severely malnourished (defined as weight for age Z-score less than -3). A computer-generated, block-randomization program was used to assign randomly eligible participants in a 1:1 ratio to receive either a single dose of PHiD-CV/dPly/PhtD-30 or PCV13 (Prevenar13™; Pfizer, USA). Children were assigned sequentially to the next available study participant number. During the study, the parents of the vaccinees and study staff responsible for endpoint evaluations were blinded to which vaccine had been administered to an individual child.
Vaccines
PHiD-CV/dPly/PhtD-30 contained 30 µg of each, dPly and PhtD combined with the 10 polysaccharide conjugates of the pneumococcal H. influenzae protein D conjugate vaccine (PHiD-CV; Synflorix™, GSK Vaccines), consisting of 1 μg of PS for serotypes 1, 5, 6B, 7F, 9V, 14 and 23F and 3 μg for serotype 4 conjugated to protein D, 3 μg of PS for serotype 18C and 19F conjugated to tetanus toxoid and diphtheria toxoid, respectively. The proteins (dPly and PhtD) were adsorbed on Aluminum Phosphate (500 µg Aluminum content per dose of 0.5 mL). An investigational vaccine containing 30 µg each of dPly and PhtD was chosen as it has been shown to elicit higher immune responses and no safety concerns in European young adults Citation18 compared to the lower dose (10 µg). Safety of the higher dose was to be confirmed in African children before initiating Cohort 2 study in infants. The safety and reactogenicity results of the 2-dose primary vaccination part of the study in European toddlersCitation20 were available before the initiation of the Cohort 1 of this study (February 2011) and did not raise any safety concerns in those vaccinated with PHiD-CV/dPly/PhtD-30. Antigens were adsorbed onto aluminum phosphate adjuvant. The comparator vaccine, PCV13, contains serotypes 1, 6A and 19A in addition to PHiD-CV serotypes, all individually conjugated to CRM197. Both vaccines were provided as a suspension in pre-filled syringes. Vaccines were administered intramuscularly into the deltoid region of the non-dominant arm.
Assessment of safety and reactogenicity
Each child was observed in the clinic for 30 minutes post-vaccination and vital signs were recorded. For 3 d post-vaccination trained field staff visited each child at home daily and recorded local (pain, redness, swelling) and general (fever, drowsiness, irritability or loss of appetite) adverse events (AEs). The intensity of all solicited AEs was graded on a scale of 1–3. Pain at the injection site was considered grade 3 if the child cried when the limb was moved or if the limb was spontaneously painful. Redness and swelling at the injection site were considered grade 3 if the diameter was >30 mm. The presence of diffuse swelling or a noticeable increase of limb circumference following vaccination was also recorded. Irritability or drowsiness was considered grade 3 if a participant cried and would not be comforted or was drowsy to an extent that prevented normal activity, respectively. All solicited local symptoms were considered causally related to vaccination. Mothers/guardians were encouraged to bring their child to the health center at any time if the child was unwell. Unsolicited AEs were recorded for 31 d post-vaccination. Serious adverse events (SAEs), defined as any untoward medical occurrence that resulted in death, was life-threatening, required hospitalization or prolongation of existing hospitalization or resulted in disability/incapacity, were reported throughout the study period. SAEs were reported over a period of 6 months post-vaccination. Assessment of the causal relationship of solicited general AEs, unsolicited AEs and SAEs to vaccination was based on the clinical judgment of the investigators. Blood samples were obtained pre- and one month post-vaccination for serological, haematological (haemoglobin, white cell and platelet counts) and biochemical (serum alanine transaminase (ALT) and creatinine) measurements. Haematological and biochemical measurements were performed at the clinical laboratory of the Medical Research Council Unit, The Gambia as described previously.Citation37 Safety oversight for the study was provided by an Independent Data Monitoring Committee.
Immunological assays
Sera were stored at −20°C until analyzed. Serotype-specific antibodies against the vaccine serotypes were measured using GSK Biologicals' 22F-inhibition ELISA, as described previously.Citation38-40 GMCs of 0.2 µg/ml corresponded to the threshold value of 0.35 µg/ml in the WHO reference ELISA for comparison of new PCVs.Citation38,39 Opsonophagocyctic activity (OPA) against bacteria of the vaccine serotypes was measured by a pneumococcal killing assay with a cut-off titer of 8 as described previously.Citation41,42 Differentiated HL-60 cells were harvested at 37°C in a 5% CO2 atmosphere. Cells were resuspended in opsonophagocytosis buffer 10 minutes before use. For the functional assay, serially diluted samples were mixed with the appropriately diluted bacterial suspension and incubated before adding the complement source and differentiated HL-60 cells. Viable colony counts were performed after an 18 h incubation period. Test reagents, with the exception of antibodies to pneumococci, were included in the complement control wells. Sandoglobulin was used as reference preparation with known OPA titer for each tested serotype. Opsonophagocytic titers were calculated as the reciprocal of the serum dilution causing ≥50% killing compared to the growth in the complement control wells.Citation41 Antibodies against non-typeable Haemophilus influenzae (NTHi) protein D, Ply and PhtD were quantified using GSK's multiplex immunoassays with cut-offs of 112 LU/mL, 599 LU/mL and 391 LU/mL respectively. These cut-offs were based on the lower limit of quantification, the global variability of the assay at the highest dilution and the lower limit of linearity.Citation43
Statistical analyses
Safety analyses were performed on the total vaccinated cohort (TVC) which comprised all children who were vaccinated. Immunogenicity analyses were performed on the according-to-protocol (ATP) cohort which was defined as vaccinated children who met all eligibility criteria and complied with all study procedures and for whom immunogenicity data were available. Geometric mean antibody concentrations (GMC) and geometric mean OPA titers (GMT) with their 95% confidence intervals (CI) and percentages of children who attained the predefined thresholds were determined.
Ethical consideration
The trial protocol was approved by The Gambia Government/Medical Research Council Unit Joint Ethics Committee and by the Western Institutional Review Board, USA. Preliminary discussions about the nature of the study and its purpose were conducted with the village heads, religious and women leaders in Fajikunda. Individual, written consent was obtained from the parents/guardians of each child before enrolment. The study was conducted in accordance with the Good Clinical Practice Guidelines and the Declaration of Helsinki, and the protocol and associated documents were reviewed and approved by local ethics committees and was monitored by an independent contract research organization.
Disclosure of Potential Conflicts of Interest
MAn has received non-financial support from the GSK group of companies. MAl has received grants from the Bill & Melinda Gates Foundation. MT, VV, KD and DB are employees of the GSK group of companies. VV is co-inventor of patents related to study vaccines. VV and DB have stock options/restricted shares; KD has restricted shares from the GSK group of companies. LSHTM and GSK received support from PATH for the conduct of this trial. AO, MOO, EOO, PO and AW have no conflict of interests to declare.
Authors' Contributions
AO was involved in planning, data collection, site coordination of study, review of the reported study and drafting the manuscript. MOO was involved in planning, data collection, review, project oversight on site. EOO was involved in planning/design/review of the reported study, interpretation of the results. MAn was involved in planning/design/review of the reported study, analysis plan and interpretation of the results. PO was involved in center coordination and data collection. AW was involved in center coordination, data collection and quality check. BG was involved in the study design, interpretation of the results. MAl was involved in planning/design/review of the reported study and interpretation of the results, MT was involved in planning/design/review of the reported study and statistical analysis of the data, VV was involved in planning/design/review of the reported study, interpretation of the results, and project oversight, KD was involved in interpretation of the results, coordination and reporting of the study. DB was involved in planning/design/review of the reported study, analysis plan, interpretation of the results, safety (interaction/reporting to IDMC) and project oversight. All authors have provided critical input in the manuscript and have approved the final version for submission and agreed on journal selection.
Acknowledgments
We thank the parents and their children who participated in this study, the Gambian government, the EPI program of Gambia, and staff of Fajikunda Health Centre for their collaboration. We thank Joan Vive Tomas and Elizabeth Stanley-Batchilly for project management, Basiru Sanyang and the clinical trials assistants for the study site coordination and the Fajikunda field team for their field work. We appreciate support from the staff of the MRC clinical laboratories. We also thank Uduak Okomo for safety monitoring, Yolanda Lewis for study coordination, Oforiwaa Gyasi-Baiden and John Attafuah for study monitoring, Liliana Manciu for drafting the protocol and study report, and Bart van Heertum for manuscript coordination.
Funding
The study was funded by Medical Research Council (MRC) UK, PATH, Seattle, USA and GlaxoSmithKline Biologicals SA. Synflorix is a trademark of the GSK group of companies.
References
- O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009; 374:893-902; PMID:19748398; http://dx.doi.org/10.1016/S0140-6736(09)61204-6
- Cutts FT, Zaman SM, Enwere G, Jaffar S, Levine OS, Okoko JB, Oluwalana C, Vaughan A, Obaro SK, Leach A, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet 2005; 365:1139-46; PMID:15794968; http://dx.doi.org/10.1016/S0140-6736(05)71876-6
- Whitney CG, Pilishvili T, Farley MM, Schaffner W, Craig AS, Lynfield R, Nyquist AC, Gershman KA, Vazquez M, Bennett NM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet 2006; 368:1495-502; PMID:17071283; http://dx.doi.org/10.1016/S0140-6736(06)69637-2
- Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease–United States, 1998-2003. MMWR Morb Mortal Wkly Rep 2005; 54:893-7; PMID:16163262
- Black SSH, Baxter R, Austrian R, Bracken L, Hansen J, Lewis E, Fireman B. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal conjugate vaccine in Northern California Kaiser Permanente. Pediat Infect Dis J 2004; 23:485-9; PMID:15194827; http://dx.doi.org/10.1097/01.inf.0000129685.04847.94
- Aguiar SI, Serrano I, Pinto FR, Melo-Cristino J, Ramirez M. Changes in Streptococcus pneumoniae serotypes causing invasive disease with non-universal vaccination coverage of the seven-valent conjugate vaccine. Clin Microbiol Infect 2008; 14:835-43; PMID:18844684; http://dx.doi.org/10.1111/j.1469-0691.2008.02031.x
- Cheung YB ZS, Nsekpong ED, Van Beneden CA, Adegbola RA, et al. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian children who participated in a 9-valent pneumococcal conjugate vaccine trial and in their younger siblings. Pediat Infect Dis J 2009; 28:990-5; PMID:19536041; http://dx.doi.org/10.1097/INF.0b013e3181a78185
- Hicks LA, Harrison LH, Flannery B, Hadler JL, Schaffner W, Craig AS, Jackson D, Thomas A, Beall B, Lynfield R, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J Infect Dis 2007; 196:1346-54; PMID:17922399; http://dx.doi.org/10.1086/521626
- Panina EM, Mironov AA, Gelfand MS. Comparative genomics of bacterial zinc regulons: enhanced ion transport, pathogenesis, and rearrangement of ribosomal proteins. Proc Natl Acad Sci U S A 2003; 100:9912-7; PMID:12904577; http://dx.doi.org/10.1073/pnas.1733691100
- Ogunniyi AD, Grabowicz M, Mahdi LK, Cook J, Gordon DL, Sadlon TA, Paton JC. Pneumococcal histidine triad proteins are regulated by the Zn2+-dependent repressor AdcR and inhibit complement deposition through the recruitment of complement factor H. FASEB J 2009; 23:731-8; PMID:18971260; http://dx.doi.org/10.1096/fj.08-119537
- Plumptre CD, Ogunniyi AD, Paton JC. Polyhistidine triad proteins of pathogenic streptococci. Trends Microbiol 2012; 20:485-93; PMID:22819099; http://dx.doi.org/10.1016/j.tim.2012.06.004
- Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011; 378:1962-73; PMID:21492929; http://dx.doi.org/10.1016/S0140-6736(10)62225-8
- Jedrzejas MJ. Pneumococcal virulence factors: structure and function. Microbiol Mol Biol Rev 2001; 65:187-207; first page, table of contents; PMID:11381099; http://dx.doi.org/10.1128/MMBR.65.2.187-207.2001
- Godfroid F, Hermand P, Verlant V, Denoel P, Poolman JT. Preclinical evaluation of the Pht proteins as potential cross-protective pneumococcal vaccine antigens. Infect Immun 2011; 79:238-45; PMID:20956575; http://dx.doi.org/10.1128/IAI.00378-10
- Denoel P, Philipp MT, Doyle L, Martin D, Carletti G, Poolman JT. A protein-based pneumococcal vaccine protects rhesus macaques from pneumonia after experimental infection with Streptococcus pneumoniae. Vaccine 2011; 29:5495-501; PMID:21624422; http://dx.doi.org/10.1016/j.vaccine.2011.05.051
- Garcia-Suarez Mdel M, Cima-Cabal MD, Florez N, Garcia P, Cernuda-Cernuda R, Astudillo A, Vazquez F, De los Toyos JR, Mendez FJ. Protection against pneumococcal pneumonia in mice by monoclonal antibodies to pneumolysin. Infect Immun 2004; 72:4534-40; PMID:15271913; http://dx.doi.org/10.1128/IAI.72.8.4534-4540.2004
- Adamou JE HJ, Erwin AL, Walsh W, Gayle T, et al. Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis. Infect Immunol 2001; 69:949-58; PMID:11159990; http://dx.doi.org/10.1128/IAI.69.2.949-958.2001
- Leroux-Roels G, Maes C, De Boever F, Traskine M, Ruggeberg JU, Borys D. Safety, reactogenicity and immunogenicity of a novel pneumococcal protein-based vaccine in adults: a phase I/II randomized clinical study. Vaccine 2014; 32:6838-46; PMID:24607003; http://dx.doi.org/10.1016/j.vaccine.2014.02.052
- Pauksens K, Nilsson AC, Caubet M, Pascal TG, Van Belle P, Poolman JT, Vandepapeliere PG, Verlant V, Vink PE. Randomized controlled study of pneumococcal vaccine formulations containing PhtD and dPly proteins with alum or adjuvant system AS02V in elderly adults: safety and immunogenicity. Clin Vaccine Immunol 2014; 21:651-60; PMID:24599529
- Hoskins J, Alborn WE, Jr., Arnold J, Blaszczak LC, Burgett S, DeHoff BS, Estrem ST, Fritz L, Fu DJ, Fuller W, et al. Genome of the bacterium Streptococcus pneumoniae strain R6. J Bacteriol 2001; 183:5709-17; PMID:11544234; http://dx.doi.org/10.1128/JB.183.19.5709-5717.2001
- Prymula R, Szenborn L, Silfverdal S, Wysocki J, Albrecht P, Francois N, Gardev A, Borys D. Safety and reactogenicity of 2 formulations of an investigational protein-based pneumococcal vaccine in infants in Europe:a phase II trial [Abstract ISPPD - 0167]. The International Symposium on Pneumococci and Pneumococcal Diseases. Hyderabad, India: Pneumonia, 2014.
- Prymula R, Szenborn L, Silfverdal S, Wysocki J, Albrecht P, Francois N, Gardev A, Borys D. Immunogenicity of primary vaccination with an investigational protein-based pneumococcal vaccine in infants in Europe: a phase II randomization trial [Abstract - 0551]. The International Symposium on Pneumococci and Pneumococcal Diseases. Hyderabad, India: Pneumonia, 2014.
- Salha D, Szeto J, Myers L, Claus C, Sheung A, Tang M, Ljutic B, Hanwell D, Ogilvie K, Ming M, et al. Neutralizing antibodies elicited by a novel detoxified pneumolysin derivative, PlyD1, provide protection against both pneumococcal infection and lung injury. Infect Immun 2012; 80:2212-20; PMID:22473606; http://dx.doi.org/10.1128/IAI.06348-11
- Hill PC, Akisanya A, Sankareh K, Cheung YB, Saaka M, Lahai G, Greenwood BM, Adegbola RA. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian villagers. Clin Infect Dis 2006; 43:673-9; PMID:16912937; http://dx.doi.org/10.1086/506941
- Rapola S, Jantti V, Haikala R, Syrjanen R, Carlone GM, Sampson JS, Briles DE, Paton JC, Takala AK, Kilpi TM, et al. Natural development of antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A, and pneumolysin in relation to pneumococcal carriage and acute otitis media. J Infect Dis 2000; 182:1146-52; PMID:10979911; http://dx.doi.org/10.1086/315822
- Musher DM, Phan HM, Baughn RE. Protection against bacteremic pneumococcal infection by antibody to pneumolysin. J Infect Dis 2001; 183:827-30; PMID:11181163; http://dx.doi.org/10.1086/318833
- Dicko A, Odusanya OO, Diallo AI, Santara G, Barry A, Dolo A, Diallo A, Kuyinu YA, Kehinde OA, Francois N, et al. Primary vaccination with the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in infants in Mali and Nigeria: a randomized controlled trial. BMC Public Health 2011; 11:882; PMID:22112189; http://dx.doi.org/10.1186/1471-2458-11-882
- Prymula R, Peeters P, Chrobok V, Kriz P, Novakova E, Kaliskova E, Kohl I, Lommel P, Poolman J, Prieels JP, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet 2006; 367:740-8; PMID:16517274; http://dx.doi.org/10.1016/S0140-6736(06)68304-9
- Eskola J, Kilpi T, Palmu A, Jokinen J, Haapakoski J, Herva E, Takala A, Kayhty H, Karma P, Kohberger R, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med 2001; 344:403-9; PMID:11172176; http://dx.doi.org/10.1056/NEJM200102083440602
- Odusanya OO, Kuyinu YA, Kehinde OA, Shafi F, Francois N, Yarzabal JP, Dobbelaere K, Ruggeberg JU, Borys D, Schuerman L. Safety and immunogenicity of 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in Nigerian children: booster dose and 2-dose catch-up regimens in the second year of life. Hum Vaccines Immunother 2014; 10:757-66; PMID:24356787; http://dx.doi.org/10.4161/hv.27276
- Dicko A, Santara G, Mahamar A, Sidibe Y, Barry A, Dicko Y, Diallo A, Dolo A, Doumbo O, Shafi F, et al. Safety, reactogenicity and immunogenicity of a booster dose of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in Malian children. Hum Vaccines Immunother 2013; 9:382-8; PMID:23291945; http://dx.doi.org/10.4161/hv.22692
- Palmu AA, Jokinen J, Borys D, Nieminen H, Ruokokoski E, Siira L, Puumalainen T, Lommel P, Hezareh M, Moreira M, et al. Effectiveness of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against invasive pneumococcal disease: a cluster randomised trial. Lancet 2013; 381:214-22; PMID:23158882; http://dx.doi.org/10.1016/S0140-6736(12)61854-6
- Tregnaghi MW, Saez-Llorens X, Lopez P, Abate H, Smith E, Posleman A, Calvo A, Wong D, Cortes-Barbosa C, Ceballos A, et al. Efficacy of pneumococcal nontypable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in young Latin American children: a double-blind randomized controlled trial. PLoS Med 2014; 11:e1001657.
- Dagan R, Givon-Lavi N, Greenberg D, Fritzell B, Siegrist CA. Nasopharyngeal carriage of Streptococcus pneumoniae shortly before vaccination with a pneumococcal conjugate vaccine causes serotype-specific hyporesponsiveness in early infancy. J Infect Dis 2010; 201:1570-9; PMID:20384496; http://dx.doi.org/10.1086/652006
- Vesikari T, Karvonen A, Korhonen T, Karppa T, Sadeharju K, Fanic A, Dieussaert I, Schuerman L. Immunogenicity of 10-valent pneumococcal nontypeable Haemophilus Influenzae Protein D Conjugate Vaccine when administered as catch-up vaccination to children 7 months to 5 years of age. Pediatr Infect Dis J 2011; 30:e130-41; PMID:21540760; http://dx.doi.org/10.1097/INF.0b013e31821d1790
- Garcia-Rodriguez JA, Fresnadillo Martinez MJ. Dynamics of nasopharyngeal colonization by potential respiratory pathogens. J Antimicrobial Chemother 2002; 50 Suppl S2:59-73; PMID:12556435; http://dx.doi.org/10.1093/jac/dkf506
- Odutola AA, Afolabi MO, Jafali J, Baldeh I, Owolabi OA, Owiafe P, Bah G, Jaiteh B, Mohammed NI, Donkor SA, et al. Haematological and biochemical reference values of Gambian infants. Trop Med Int Health 2014; 19:275-83; PMID:24393095; http://dx.doi.org/10.1111/tmi.12245
- Concepcion NF, Frasch CE. Pneumococcal type 22f polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol 2001; 8:266-72; PMID:11238206
- Henckaerts I, Goldblatt D, Ashton L, Poolman J. Critical differences between pneumococcal polysaccharide enzyme-linked immunosorbent assays with and without 22F inhibition at low antibody concentrations in pediatric sera. Clin Vaccine Immunol 2006; 13:356-60; PMID:16522777; http://dx.doi.org/10.1128/CVI.13.3.356-360.2006
- Poolman JT, Frasch CE, Kayhty H, Lestrate P, Madhi SA, Henckaerts I. Evaluation of pneumococcal polysaccharide immunoassays using a 22F adsorption step with serum samples from infants vaccinated with conjugate vaccines. Clin Vaccine Immunol 2010; 17:134-42; PMID:19889940; http://dx.doi.org/10.1128/CVI.00289-09
- Romero-Steiner S, Libutti D, Pais LB, Dykes J, Anderson P, Whitin JC, Keyserling HL, Carlone GM. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol 1997; 4:415-22; PMID:9220157
- Henckaerts I, Durant N, De Grave D, Schuerman L, Poolman J. Validation of a routine opsonophagocytosis assay to predict invasive pneumococcal disease efficacy of conjugate vaccine in children. Vaccine 2007; 25:2518-27; PMID:17034907; http://dx.doi.org/10.1016/j.vaccine.2006.09.029
- Findlay JW, Smith WC, Lee JW, Nordblom GD, Das I, DeSilva BS, Khan MN, Bowsher RR. Validation of immunoassays for bioanalysis: a pharmaceutical industry perspective. J Pharm Biomed Analysis 2000; 21:1249-73; PMID:10708409; http://dx.doi.org/10.1016/S0731-7085(99)00244-7
