Abstract
Four randomized, double-blind, placebo-controlled studies in 6090 children that investigated the efficacy of live attenuated influenza vaccine (LAIV) upon revaccination of children against laboratory-confirmed cases of influenza in consecutive seasons were reviewed.
The efficacy in season 2 of LAIV administered over 2 consecutive seasons was 86.7% (95 % CI: 76.8%, 92.4%) against strains antigenically similar to those contained in the vaccine. The additional efficacy of LAIV administered in season 2 compared to LAIV recipients in season 1 only was 58.4% (28.3%, 75.9%). LAIV administered over 2 consecutive seasons also was more efficacious than was LAIV administered in season 2 only (relative efficacy: 53.9% [17.4%, 74.3%]). Residual efficacy of LAIV administered in season 1 only compared to placebo administered in two consecutive seasons was 56.4% (37.0%, 69.8%). This review did not find any evidence of decreasing efficacy of LAIV when administered during 2 consecutive seasons.
Introduction
Recommendations for annual vaccination against influenza have become more inclusive in recent years, with significant expansions in the United States, Canada, and United Kingdom. In parallel, public health agencies have supported evaluations of influenza vaccine effectiveness. This monitoring, which is critical to inform the medical community and maintain public confidence in influenza vaccination, has shown results that vary from year to year, as can be expected from varying degrees of match between wild-type circulating strains and vaccine strains.Citation1 Results from observational studies describing the effectiveness of repeat annual influenza vaccination have been conflicting; some studies suggest decreasing effectiveness,Citation2,3 whereas others show no difference with revaccination.Citation4
The aim of this systematic literature review was to assess data from double-blind, randomized clinical trials to evaluate the efficacy of live attenuated influenza vaccine (LAIV) upon revaccination of children and to analyze consolidated efficacy estimates of LAIV in a second season.
Results
A total of 6090 children were retained in the analysis. Enrollment and disposition of children in season 2 are presented in . All studies contributed children who were administered either LAIV or placebo over 2 consecutive seasons (n=2497 and n=1648, respectively). Studies 3 and 4 only contributed children who were administered LAIV in season 1 and placebo in season 2 (n=1105) or placebo in season 1 and LAIV in season 2 (n=840).
Table 1. Enrollment and Disposition of Children in Season 2.
The proportions of laboratory-confirmed cases of influenza in season 2 in children who were administered placebo during 2 consecutive seasons ranged among studies from 1.1% to 29.1% for influenza strains antigenically similar to those contained in the vaccines and 14.4% to 30.9% for all wild-type influenza strains (). The most frequent circulating strains were A/H3N2 (199 cases), but B and A/H1N1 strains were also identified (86 and 35 cases, respectively; ).
Table 2. Influenza Attack Rates in Season 2.
Table 3. Influenza Attack Rates in Season 2 and Efficacy of Repeated Administration of LAIV by Strain.
The attack rates in season 2 were consistently lower in each study among children who were administered LAIV versus those who were administered a placebo over 2 consecutive seasons, resulting in consolidated efficacy estimates of 86.7% (95% confidence interval [CI]: 76.8%, 92.4%) against antigenically similar influenza strains and 76.6% (95% CI: 66.3%, 83.7%) against all influenza strains (, ).
Figure 1. Efficacy in Season 2 of LAIV Administered Over 2 Consecutive Seasons. (A) Against Influenza Strains Antigenically Similar to Those Contained in the Vaccine. (B) Against All Influenza Strains; LAIV=live attenuated influenza vaccine.
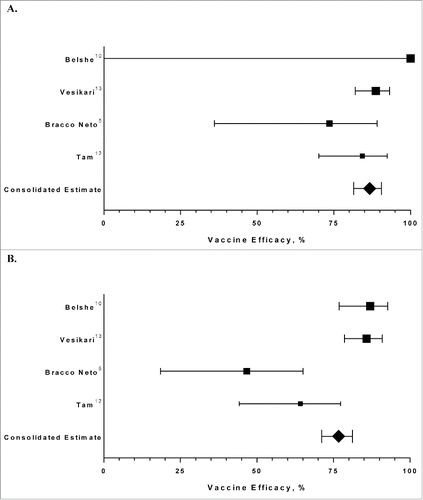
Table 4. Efficacy Estimates Against Any Influenza Strains.
Among LAIV recipients in season 1, the efficacy (95% CI) of a repeat dose of LAIV in season 2 was also statistically significant: 58.4% (28.3%, 75.9%) against antigenically similar influenza strains and 27.6% (0.8%, 47.2%) against all influenza strains (). Conversely, among LAIV recipients in season 2, administration of LAIV in the previous season was associated with a lower attack rate by antigenically similar influenza strains in season 2 resulting in a relative efficacy of 53.9% (17.4%, 74.3%). The attack rates by all influenza strains did not differ significantly (relative efficacy: −3.2% [−49.1%, 28.5%]; ).
Of note, the administration of 2 doses of LAIV in season 1 only was also associated with significantly lower attack rates in season 2 when compared to no vaccination during 2 consecutive seasons. The resulting residual efficacy (95% CI) estimates were 56.4% (37.0%, 69.8%) against influenza strains antigenically similar to those contained in the vaccines, and 40.7% (22.6%, 54.6%) against all influenza strains ().
presents the efficacy in season 2 of repeated administration of LAIV against A/H1N1 strains, A/H3N2 strains, and B strains versus no vaccination. Consolidated efficacy (95% CI) estimates against antigenically similar influenza strains were similar for A/H1N1 and A/H3N2 (93.2% [53.4%, 99.0%] and 88.6% [77.3%, 94.3%], respectively). Efficacy estimates were 93.2% (53.4%, 99.0%) against all A/H1N1 strains, 87.5% (78.2%, 92.8%) against all A/H3N2 strains, and 41.1% (0.0%, 65.2%) against all B strains, with a majority of B strains identified in the Bracco Neto et al studyCitation5 from an opposite lineage or not matching the B strain contained in the vaccine. Efficacy against influenza B strains was higher when the analysis was restricted to B strains from the same lineage as the vaccine B strain, whether or not drifted variant strains were considered antigenically similar (70.3% [14.8%, 89.7%]) or dissimilar (71.0% [4.5%, 91.2%]).
Discussion
The first publication suggesting decreasing vaccine effectiveness with repeated influenza vaccination may be an investigation by Hoskins et alCitation6 of an influenza outbreak that occurred in 1976 in an English boys' boarding school. An analysis of 375 boys present at school during the 1976 outbreak as well as in 2 previous influenza outbreaks in 1972 and 1974 demonstrated a higher attack rate in those vaccinated with inactivated vaccine in 2 prior seasons relative to those vaccinated in 1976 alone.
More recently, several observational studies investigated the effectiveness of repeated vaccinations in the general population—mostly adults and the elderly—with a large majority of vaccine recipients being inactivated influenza vaccine (IIV) recipients. During season 2010–2011, Ohmit et alCitation2 observed that the effectiveness of influenza vaccines administered in individuals already vaccinated in season 2009–2010 was low, with a negative point estimate of −45% (95% CI: −226%, 35%). Another study led by the same author during the next season estimated the influenza vaccines effectiveness at 56% (95% CI: 37%, 69%) in subjects vaccinated in season 2011–2012 only and 45% (95% CI: 27%, 58%) in subjects vaccinated during 2 consecutive seasons, suggesting again a negative association between vaccination in 2011–2012 and vaccination in the prior season.Citation3 However, Skowronski et al,Citation4 using a similar study design in Canada during the same season, found a different trend, with higher effectiveness in subjects vaccinated during 2 consecutive seasons (60% [95% CI: 50%, 77%]) than in subjects vaccinated in season 2011–2012 only (53% [95% CI: 12%, 77%]).
Smith et alCitation7 suggested that variations in repeat vaccine efficacy could be explained by the antigenic distances between influenza vaccine strains and wild-type influenza strains, with lower vaccine efficacy in subjects who had been vaccinated in previous seasons with strains close to the wild-type strains circulating in the current season. Another biological rationale for decreasing effectiveness with repeated vaccinations was recently suggested by Bodewes et al, who investigated anti-influenza immunity in children with cystic fibrosis. These children received influenza vaccine annually, compared with previously unvaccinated healthy children.Citation8 Annual influenza vaccination with IIV was shown to hamper the development of virus-specific CD8+ T cell responses. However, a review article by Belshe et alCitation9 showed that LAIV efficacy did not decrease with increasing pre-existing immunity to influenza in children 15 to 84 months of age compared with placebo or in children 6 months to 17 years of age compared with trivalent IIV. Additionally, LAIV efficacy did not decrease with prior vaccination with IIV. In a large double-blind, randomized clinical trial, the relative efficacy of LAIV compared with IIV against all strains regardless of antigenic match was similar in children 6 to 59 months of age who were previously vaccinated with IIV (51% fewer cases with LAIV) and those previously unvaccinated (57% fewer cases of with LAIV).Citation9 Of note, LAIV is approved for use only in children 2 years of age and older.
In contrast with the varying findings of observational studies that were conducted in different age groups and included mostly subjects who were administered inactivated influenza vaccines, this review of 4 randomized clinical trials conducted over 2 consecutive seasons provides consistent evidence that LAIV efficacy in children did not decline after LAIV vaccination in the previous season. The studies were conducted according to a methodology that controlled for several sources of bias that could have occurred in the observational studies discussed: LAIV or placebo was allocated at random, and the surveillance for laboratory-confirmed cases of influenza was prospective and independent from the treatment groups. However, study 1 was conducted 4 years earlier than studies 2, 3, and 4, with a formulation of LAIV that included different strains. There were also differences in the influenza epidemics with different degree of match between circulating and vaccine strains and different levels of severity that may have contributed to the heterogeneity of the results. Of note, subjects in season 2 were limited to children 18 months to 7 years of age, and they were followed up for only 2 consecutive seasons. More follow-up is needed to assess the long-term effectiveness of expanded vaccination programs of the pediatric population.
In conclusion, LAIV administered during 2 consecutive seasons was highly efficacious in season 2 versus administration of placebo in the 2 seasons. Further analyses show that when LAIV is administered in season 1, there is residual efficacy in season 2 and significant additional efficacy when LAIV is also administered in season 2. The efficacy of LAIV over 2 consecutive seasons was also similar to or greater than the efficacy of LAIV administered in season 2 only. Data describing the efficacy of LAIV beyond 2 consecutive seasons would be valuable.
Methods
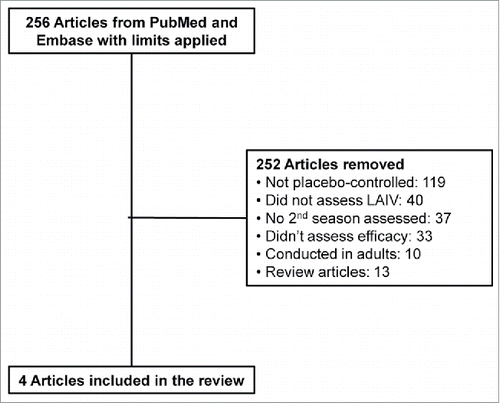
Original published studies on LAIV were identified by a literature search on PubMed and Embase for the period from January 1, 1995 to July 1, 2015. The following search term was used: “live attenuated influenza vaccine” or “LAIV.” The following limits also were applied: “English,” “randomized controlled trial,” “humans,” and “child: birth-18 years.” This literature search resulted in 256 studies being retrieved.
A manual review of the title and abstracts of the search results was performed. Studies were excluded if they were not randomized, double-blind, placebo-controlled studies. Articles also were excluded if the efficacy of LAIV was not assessed over 2 consecutive seasons ().
In total, 4 randomized, double-blind, controlled studies in children investigated LAIV efficacy versus placebo against laboratory-confirmed cases of influenza in 2 consecutive influenza seasons were used in the present analysis.Citation5,10-15 The quality of the studies were evaluated using the GRADE process.Citation16 All 4 studies had a GRADE score of 4, because they were randomized, controlled trials and enrolled at least 1000 patients. Studies 1 and 2 randomized children at enrollment only and, therefore, compared children revaccinated with LAIV in season 2 with children who received placebo over 2 consecutive seasons.Citation10,11,13 Study 3 re-randomized children in season 2, so that LAIV and placebo administered in season 2 could be compared among LAIV or placebo recipients in season 1.Citation12 Study 4 randomized children at enrollment only. However, because of a treatment allocation labeling error in season 2, some enrollees received (1) LAIV in season 1 and placebo in season 2 or (2) placebo in season 1 and LAIV in season 2.Citation5
These studies enrolled healthy children who were 6 to 71 months of age in season 1 (18–83 months of age in season 2). Eligibility to participate in the second year required continued good health and completion of the primary dosing series and surveillance in year 1. Children with a history of clinically significant hypersensitivity to eggs were excluded, as were those with underlying chronic illnesses and those who, at any time prior to entry into this study, received a dose of any influenza vaccine (commercial or investigational).
Live attenuated influenza vaccine consisted of 106.5–7.5 median tissue culture infectious doses (TCID50) or fluorescent focus units of each of the 3 influenza strains (A/H1N1, A/H3N2, and B). All children in studies 2 and 3 who were administered LAIV in season 1 received 2 doses. Children enrolled in studies 1 and 4 were administered either 1 or 2 doses. Time between doses was approximately 1 month, with the exception of study 1, in which the interval was 6 to 10 weeks. All children who were administered LAIV in season 2 received 1 dose. Placebo did not differ in appearance, delivery, or taste. The strains contained in the vaccines are presented in .
Parents were contacted every 2 to 3 weeks until the beginning of an influenza outbreak in the community. Thereafter, weekly contact was made with the families to remind parents to notify study personnel if their child had symptoms of respiratory illness. A nasal swab sample was obtained if children exhibited any predefined symptoms of respiratory illness: runny nose or nasal congestion, sore throat, cough, headache, muscle aches, chills, vomiting, suspected or confirmed otitis media, decreased activity, irritability, wheezing, shortness of breath, and pulmonary congestion. A case of influenza was defined consistently across studies as any illness detected by active surveillance, as described above, with a positive culture for wild-type influenza virus.
All children who were administered 2 doses of LAIV or placebo in season 1 and 1 dose of LAIV or placebo in season 2 were retained in the analysis. Consistently with the original study analyses, LAIV efficacy was assessed (1) against influenza strains antigenically similar to those contained in the vaccine and (2) against all influenza strains regardless of antigenic similarity. Efficacy was assessed further by influenza types and subtypes: A/H1N1, A/H3N2, and B strains.
The efficacy in season 2 of the four possible combinations - LAIV in seasons 1 and 2, LAIV in season 1/placebo in season 2, placebo in season 1/LAIV in season 2, and placebo in both seasons - was estimated. A log binomial model was used to calculate the relative risks of laboratory-confirmed influenza, with study taken into account as a fixed effect. Vaccine efficacy was calculated as 1 minus the relative risk. The analysis was conducted with SAS version 9.3 (Cary, NC, USA).
Each original study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Written informed consent was provided by each subject's parent or legal guardian (with age-appropriate assent from the child) after the nature and possible consequences of the study were explained.
Disclosure of potential conflicts of interest
HC and CA are employees of AstraZeneca, the parent company of MedImmune, and own AstraZeneca stock and/or stock options. RB has received grants to his institution from the NIH and MedImmune; consultant fees from MedImmune; payment for lectures including service on speakers' bureaus from MedImmune, Merck, and Sanofi; and payment for the development of educational presentations from MedImmune. TH has received consultant fees from MedImmune, Novartis, GlaxoSmithKline, and Sanofi Pasteur MSD; grants to his institution from GlaxoSmithKline; and payment for lectures including service on speakers' bureaus from AstraZeneca/MedImmune and AbbVie.
Authors' contributions
Study concept and design: Robert Belshe, Terho Heikkinen, Herve Caspard, Christopher Ambrose. Acquisition of data: Herve Caspard, Christopher Ambrose. Analysis and interpretation of data: all authors. Drafting of the manuscript and critical revision of the manuscript for important intellectual content: all authors. Critical review and editing of the manuscript: all authors. Statistical analysis: Herve Caspard. All authors approved the final manuscript for submission.
Funding
This research was funded by MedImmune. Role of the sponsor: Drs Caspard and Ambrose are employees of AstraZeneca, the parent company of MedImmune. MedImmune funded the study; therefore, the role of the sponsor included study design; collection, analysis, and interpretation of data; writing of the report; and the decision to submit the article for publication. Additional contributions: Editorial assistance in the form of formatting the manuscript for submission was provided by John E. Fincke, PhD, Candace Lundin, DVM, MS, and Anny S. Wu, PharmD, of Complete Healthcare Communications, Inc. (Chadds Ford, PA) and funded by AstraZeneca.
References
- Heikkinen T, Heinonen S. Effectiveness and safety of influenza vaccination in children: European perspective. Vaccine 2011; 29:7529–34. PMID:21820481
- Ohmit SE, Petrie JG, Malosh RE, Cowling BJ, Thompson MG, Shay DK, Monto AS. Influenza vaccine effectiveness in the community and the household. Clin Infect Dis 2013; 56:1363–9. PMID:3413420
- Ohmit SE, Thompson MG, Petrie JG, Thaker SN, Jackson ML, Belongia EA, Zimmerman RK, Gaglani M, Lamerato L, Spencer SM, et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis 2014; 58:319–27. PMID:24235265; http://dx.doi.org/10.1093/cid/cit736
- Skowronski DM, Janjua NZ, Sabaiduc S, De Serres G, Winter AL, Gubbay JB, Dickinson JA, Fonseca K, Charest H, Bastien N, et al. Influenza A/Subtype and B/Lineage effectiveness estimates for the 2011–2012 trivalent vaccine: cross-season and cross-lineage protection with unchanged vaccine. J Infect Dis 2014; 210:126–37.
- Bracco Neto H, Farhat CK, Tregnaghi MW, Madhi SA, Razmpour A, Palladino G, Small MG, Gruber WC, Forrest BD. Efficacy and safety of 1 and 2 doses of live attenuated influenza vaccine in vaccine-naive children. Pediatr Infect Dis J 2009; 28:365–71. PMID:19395948
- Hoskins TW, Davies JR, Smith AJ, Miller CL, Allchin A. Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at Christ's Hospital. Lancet 1979; 1:33–35. PMID:83475
- Smith DJ, Forrest S, Ackley DH, Perelson AS. Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci U S A 1999; 96:14001–6. PMID:10570188
- Bodewes R, Fraaij PL, Geelhoed-Mieras MM, van Baalen CA, Tiddens HA, van Rossum AM, van der Klis FR, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. Annual vaccination against influenza virus hampers development of virus-specific CD8(+) T cell immunity in children. J Virol 2011; 85:11995–2000. PMID:21880755; http://dx.doi.org/10.1128/JVI.05213-11
- Belshe RB, Toback SL, Yi T, Ambrose CS. Efficacy of live attenuated influenza vaccine in children 6 months to 17 years of age. Influenza Other Respi Viruses 2010; 4:141–5. PMID:20409210; http://dx.doi.org/10.1111/j.1750-2659.2009.00124.x
- Belshe RB, Gruber WC, Mendelman PM, Cho I, Reisinger K, Block SL, Wittes J, Iacuzio D, Piedra P, Treanor J, et al. Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J Pediatr 2000; 136:168–75. PMID:10657821
- Belshe RB, Coelingh K, Ambrose CS, Woo JC, Wu X. Efficacy of live attenuated influenza vaccine in children against influenza B viruses by lineage and antigenic similarity. Vaccine 2010; 28:2149–56. PMID:20003926; http://dx.doi.org/10.1016/j.vaccine.2009.11.068
- Tam JS, Capeding MR, Lum LC, Chotpitayasunondh T, Jiang Z, Huang LM, Lee BW, Qian Y, Samakoses R, Lolekha S, et al. Efficacy and safety of a live attenuated, cold-adapted influenza vaccine, trivalent against culture-confirmed influenza in young children in Asia. Pediatr Infect Dis J 2007; 26:619–28. PMID:17596805
- Vesikari T, Fleming DM, Aristegui JF, Vertruyen A, Ashkenazi S, Rappaport R, Skinner J, Saville MK, Gruber WC, Forrest BD. Safety, efficacy, and effectiveness of cold-adapted influenza vaccine-trivalent against community-acquired, culture-confirmed influenza in young children attending day care. Pediatrics 2006; 118:2298–12. PMID:17142512
- Ambrose CS, Wu X, Knuf M, Wutzler P. The efficacy of intranasal live attenuated influenza vaccine in children 2 through 17 years of age: a meta-analysis of 8 randomized controlled studies. Vaccine 2012; 30:886–92. PMID:22155144
- Rhorer J, Ambrose CS, Dickinson S, Hamilton H, Oleka NA, Malinoski FJ, Wittes J. Efficacy of live attenuated influenza vaccine in children: a meta-analysis of nine randomized clinical trials. Vaccine 2009; 27:1101–10. PMID:19095024
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64:401–6. PMID:21208779