ABSTRACT
In the Netherlands, people indicated for seasonal influenza vaccination are divided in 3 risk groups, i.e. those less than 60 y (y) with comorbidity and those 60 y and over with and without comorbidity. Those risk groups were also eligible for pandemic vaccination during the 2009 influenza A(H1N1) pandemic.
We assessed tolerability of seasonal influenza vaccination and 2 doses of pandemic influenza A(H1N1) vaccine, adjuvanted with MF-59, administered 2 and 5 weeks after seasonal 2009–2010 vaccination among adults.
Vaccinees were asked to return questionnaires on local and systemic adverse events (AEs) after each of 3 consecutive vaccinations given at the office of their General Practitioner. Sex- and risk group-specific AE-frequencies were calculated. Generalized Linear Mixed Model with seasonal vaccination as reference was used to calculate odds ratios (ORs) for AEs of the 2 pandemic doses.
5553 questionnaires (3251 vaccinees) were returned. Vaccinees reported any local AE after seasonal vaccination and both pandemic doses in 34%, 23%, and 18%, respectively. These percentages were 29%, 25%, and 16% for any systemic AE. Men reported fewer local and systemic AEs then women (p<0.0001). The risk of local (OR range 0.34-0.63) and systemic (OR range 0.39–0.99) AEs (overall, stratified by risk group and by sex) was lower after both pandemic doses compared to seasonal vaccination. This decreased risk was more pronounced after the second pandemic dose than after the first.
Therefore, we conclude that MF59-adjuvanted pandemic vaccine given after seasonal vaccination was well tolerated.
Introduction
Annual influenza epidemics occur worldwide, resulting in considerable morbidity, mortality and economic burden.Citation1 Morbidity and mortality are generally associated with vulnerable populations at risk of complications of infection, like pneumonia.Citation2-5
In 2009, the World Health Organization (WHO) declared a pandemic, caused by an influenza A(H1N1) strain. In response, the Dutch Health Council advised that all people in the Netherlands, eligible for routine seasonal influenza vaccination should be offered vaccination against this pandemic strain.Citation6 Several additional groups were defined for vaccination by their General Practitioner (GP), e.g. pregnant women in their second and third trimester and household members of high-risk patients. Health care workers were offered vaccination by their employers whereas children between 6 months and 5 y of age and household members of infants below 6 months of age could get the vaccinations from the municipal health services.Citation6
Given the urgency of availability, extensive information on tolerability of these new pandemic influenza vaccines lacked.Citation7 At the time the vaccines became available, a stern public debate about its safety started worldwide. In the Netherlands, safety of pandemic vaccinations was monitored by passive surveillance and by several active questionnaire surveys. Here we report on the tolerability of the pandemic vaccine administered after the seasonal vaccination in the Netherlands among adults vaccinated at GP office.
Results
Response and demographics
The overall response rate was an estimated 40% with approximately 14,000 questionnaires distributed. No exact numbers distributed per predefined risk group are available, precluding calculation of category specific response rates.
In total, 5553 questionnaires were returned by 3251 participants: 642 (19.7%) vaccinees returned all 3 questionnaires; 1018 (31.3%) and 1591 (48.9%) vaccinees returned 2 or just one questionnaire, respectively (). There was slight predominance (52.5–54.3%) of female respondents, which is comparable to the sex distribution for the adult population in the Utrecht province (52% females). Reported comorbidity varied by vaccination (69.7%–74.8%). In less than 3% of the respondents, sex and comorbidity were unknown. Participants mean age for the 3 vaccinations varied between 63.8y-65.2y. With respect to administered vaccine, women were statistically significantly younger than men (1.3 y, 1.7 y and 1.4 y for the respective doses).
Table 1. Demographics of the study population.
Local adverse events
Participants reported a significant higher proportion of any local AE (redness, swelling and/or pain at the injection site) following the seasonal influenza vaccination compared with both pandemic vaccine doses (). For redness percentages were 17.9%, 4.9%, and 3.6% for the respective doses, for swelling 17.3%, 5.1%, and 3.9% and for pain at the injection site 28.9%, 20.9%, and 17.2%. The majority concerned reports of mild or moderate events. Over the 3 doses together, for redness 20%–28% of the reports concerned pronounced local AEs. Likewise, 18%–19% and 12%–13% of reports on respectively swelling and pain were considered as pronounced.
Table 2. Numbers and frequencies of severities of local adverse events by sex. Differences between men and women are tested. Significant p-values are in bold.
For any local AE the reported frequency in women was statistically significant higher than in men.
Systemic adverse events
Vaccinees reported at least one systemic AE in 29.4% (n = 576), 25.3% (n=535) and 16.4% (n = 242) following the 3 respective vaccinations. Listlessness, fatigue, headache and myalgia were reported most frequently (). After seasonal vaccination, 4.5% (n = 88) of vaccinees reported fever, compared with 4.6% (n = 98) and 1.9% (n = 28) following the first and second pandemic doses, respectively. For fever following seasonal vaccination, 51 participants (2.6%) reported a ‘the highest temperature measured’. Twenty-five Participants (1.3%) reported a temperature ≥38°C (median 38.5°C; range 38-39.6°C). For the first pandemic dose, 68 participants (3.2%) reported a highest temperature (41(1.9%) ≥38°C; median 38.0°C; range 38-40°C). For the second pandemic dose 11 (0.7%) out of 17 (1.2%) vaccinees reported a temperature ≥38°C (median 38.0°C; range 38–39°C). Proportions for all reported systemic AEs, except itch, were not statistically significantly different between the seasonal vaccination and the first pandemic dose. Proportions of reported fever, listlessness, fatigue, headache, dizziness and myalgia were all statistically significantly higher after the first pandemic dose than after de second pandemic dose.
Figure 1. Sex specific proportions (%) of systemic adverse events' after seasonal influenza vaccination and 2 doses of pandemic influenza vaccine.
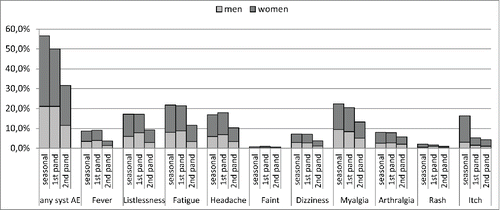
Frequencies of reported systemic AEs in women were higher than in men, for all 3 vaccinations except for rash after both pandemic doses. These differences were statistically significant except for fever and fainting (all doses), dizziness (seasonal vaccination and second pandemic dose), listlessness (first pandemic dose) and arthralgia (second pandemic dose).
Risk groups
For all 3 vaccine doses, the risk group <60 y with comorbidity showed higher frequencies of local and systemic AEs compared with both other risk groups ≥ 60 y (, ). This difference was statistically significant for any local AE, any redness, any swelling, any pain, any systemic AE, fever, fatigue, headache, myalgia (all doses), grades of redness and swelling (seasonal vaccination), listlessness (seasonal vaccination and first pandemic dose), grades of pain, dizziness (both pandemic doses), arthralgia, itch (seasonal vaccination and second pandemic dose) and for fainting (second pandemic dose).
Figure 2. Risk group specific proportions (%) of systemic adverse events' after seasonal influenza vaccination and 2 doses of pandemic influenza vaccine.
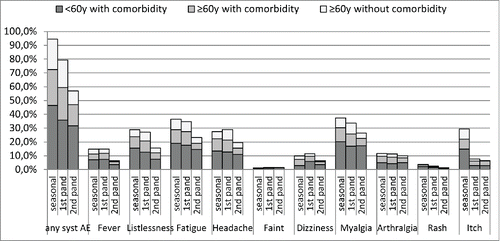
Table 3. Number and frequencies of local and systemic adverse events by risk group and vaccination. Differences between risk groups are tested. Statistical significant p-values are in bold.
Generalized linear mixed model
GLMM, with seasonal influenza vaccine set as reference, showed a decreased risk for local and systemic AEs for both pandemic doses, overall (adjusted for age) and for separate risk groups (adjusted for sex) (). These differences were statistically significant, except for systemic AEs in both risk groups ≥ 60 y and the male stratum in the overall analysis.
Table 4. Multivariable Generalized Linear Mixed Model analysis of the risk for local and systemic AEs after both pandemic doses with seasonal influenza vaccination set as reference, stratified by risk group.
AEs in respondents with 3 vs less than 3 questionnaires (data not shown)
We found no statistically significant differences for any of the reported local AEs between the participants who returned all 3 or fewer questionnaires (all p-values ≥ 0.1).
However, vaccinees who returned all 3 questionnaires, reported statistically significantly lower frequencies of any systemic AE after seasonal vaccination and the second pandemic dose compared with those who filled in fewer questionnaires. Likewise, this holds for some specific systemic AEs.
Regarding reported local and systemic AEs, in general no statistically significant differences were observed between risk groups with participants returning all 3 questionnaires and risk groups returning fewer questionnaires.
GLMM, for participants returning 3 questionnaires, also showed decreased risks after both pandemic doses, but with the smaller numbers, more ORs were statistically non-significant (data not shown).
Discussion
We compared and presented observational tolerability data of seasonal and pandemic influenza vaccination, consecutively administered in late 2009. Data are partly cross-sectional, but 20% of participants provided tolerability data on all 3 vaccinations, offering the possibility of a cohort-based analysis.
In general, results show lower frequencies of both local and systemic AEs following the 2-dose pandemic vaccine compared with the seasonal influenza vaccine, although not all differences are statistically significant. In addition, women reported higher frequencies of AEs than men did and people <60 y with comorbidity reported the highest frequencies of AEs compared to vaccinees ≥60 y with and without comorbidity.
We found higher proportions for pain at the injection site as well as for systemic AEs compared with findings of Harmark et al. who also studied the tolerability of Focetria®Citation8 in the Netherlands. Harmark et al. used Dutch inhabitants in their survey who received their pandemic vaccinations through GPs. However, they did not restrict inclusion to people who were eligible for seasonal vaccination, as we did. However, coverage data show that most Dutch people eligible for the pandemic vaccination were also vaccinated with seasonal influenza vaccine.Citation9 This implies that most participants in Harmarks study probably also received seasonal influenza vaccine prior to the pandemic doses but they only assessed AEs after pandemic vaccinations. The differences found may be caused by inclusion of other groups, e.g., pregnant women and household members of high-risk patients, and by recall bias. Harmark et al. collected data between November 16, 2009 and March 3, 2010 and participants could register online within this entire period. However, nearly all GPs finished their pandemic vaccination campaigns before Christmas 2009. In our study, questionnaires were handed out directly after immunization and we requested to send them back after one week. We did find equal frequencies of local redness and swelling, possibly indicating that the source population in both surveys shows much resemblance.
Within the different risk groups, the proportion AEs was statistically significant higher in the <60 y with comorbidity group compared with the other older age risk groups. A similar result was found in 2 other studies on tolerability of influenza vaccination and in a study on tolerability of Q-fever, all showing that the odds of AEs decrease with increasing age.Citation8,10,11 This decreasing risk for AEs with increasing age may be caused by immunosenescence and/or comorbidity or medication.Citation12
Furthermore, the sex dependency of AEs we found is also found in the Q-fever studyCitation11 and similar to the findings of Harmark et al.Citation8
Several studies comparing MF59 adjuvanted vaccines with non-adjuvanted vaccines, have found that adjuvanted vaccines resulted in slightly more AEsCitation2,13 as is also known for other adjuvants. Interestingly however, we found lower rates of AEs after the pandemic doses compared with the non-adjuvanted seasonal influenza vaccines. Several explanations for these findings may be possible. First, the seasonal 2009-2010 influenza vaccine did contain a H1N1 strain, although it differed from the circulating pandemic H1N1 strain. Perhaps this influenced the reactions to the subsequent pandemic doses, because of some cross-immunity.
A second explanation for our results could be that although an adjuvant was added to the pandemic vaccine, the lower amount of virus antigen may have resulted in fewer AEs. One pandemic dose contained 7.5 mcg virus antigen compared with in total 45 mcg virus in the seasonal influenza vaccines.
Furthermore, the seasonal vaccines contained 3 different influenza strains, which could trigger a stronger and broader response of the immune system by multiple epitopes and by interaction, resulting in more AEs.
Finally, there is the possibility of assessment bias due to the decreasing attention for experienced AEs in booster doses.
Strengths and limitations
Through our study we gained insight in the occurrence of adverse events following immunization (AEFI) after subsequent doses of different influenza vaccines. This resembles real life, because people eligible for seasonal influenza vaccination often also are eligible for pandemic vaccines. Data as in our study provide important information on within and between variance of the participants regarding the occurrence of AEFI. However, our study also has several limitations. We found lower frequencies of some systemic AEs between participants returning 3 questionnaires and participants, returning fewer, although most of the differences were non-significant. Therefore, we think this influence will be limited. However, questionnaire surveys on AEs are prone to selection bias, as participants tend to return the questionnaire only in case of AE-occurrence, usually resulting in an overestimation of frequencies.
We also had to rely on self-reported comorbidity and AEs. Our classification was not validated by the GP, which could have led to misclassification. For comorbidity this is probably non-differential, because coding was performed independent of the knowledge on AEs. Therefore, we think the influence of misclassification regarding risk groups will be limited. However, differences in interpretation of solicited AEs could have influenced the frequencies found. Furthermore, the respective risk groups could have different reply attitudes, influencing the results found.
In addition, information on date and time of onset and duration of each AE contained a lot of missing values. Therefore, no analysis could be performed on these variables and likelihood of causality could not be assessed. For local AEs a causal relation with the vaccination is highly likely. However, for systemic AE other coincidental influences could be the cause. Furthermore, we did not include an unvaccinated control group and therefore, even reported systemic AE frequencies with a short lag time include the background rate.
Our data could also be influenced by the so called ‘healthy vaccinee’ effect, i.e., people who are willing or able to come to the GPs office for a vaccination may be healthier than people who are not able to come.Citation14 This might influence the generalizability of our results. However, we believe these frequencies are useful for monitoring variations in AE rates in the general population under real life circumstances and our questionnaire surveys are an appropriate method for surveillance purposes in view of costs and efficiency.
Conclusion
The MF59-adjuvanted pandemic influenza vaccine was well tolerated with lower reported frequencies of local and systemic AEs compared with the prior seasonal influenza vaccination, administered 2 to 5 weeks earlier. A possible ‘prime-boost’ relation with the seasonal influenza vaccine may explain the lower frequencies both of the first and second pandemic dose. As is seen in other vaccination campaigns women had higher rates of reported AE compared with men, as have younger people than the elder. Further research is necessary in understanding AEs after consecutive doses of influenza vaccine. Effective vaccination strategies and education are required to combat forthcoming influenza pandemics.
Materials and methods
Setting
GPs located in the Utrecht province (n = 15) were approached by telephone to ask for cooperation. Five GP practices consented and participated, located in different districts and villages to address variation in degree of urbanization and socio-economic status. GPs organized the vaccinations mainly in mass vaccination sessions at their office. Seasonal vaccination was given first, after 2 weeks followed by 2 consecutive doses of pandemic vaccine, 3 weeks apart as stipulated in the guidelines.Citation6 At each of these mass vaccination sessions, staff of the Dutch Centre for Infectious Disease Control (Cib) of the National Institute for Public Health and the Environment (RIVM) asked all vaccinees to participate in this tolerability survey when leaving the GP office. After consent, vaccinees were handed a questionnaire to fill in and return to CIb in a pre-stamped envelope. Thus, a participant could fill in 3 questionnaires, one for each vaccination. Besides an oral reminder at the next vaccination rounds to send in all questionnaires, no reminders were sent. People vaccinated on occasions other than at mass sessions were not recruited. Medical ethical approval of this study was not necessary because only questionnaire data were used and participants were not subjected to imposed rules or acts.
Inclusion criteria
Adults of 18years (y) and older who received any of the influenza vaccinations at the GP could participate. The returned questionnaires were categorized in 3 study groups: people <60 y with comorbidity, those aged ≥60 y with comorbidity and people aged ≥60 y without comorbidity. These three categories are in line with the Dutch General Practitioners Association (NHG) criteria for eligibility for seasonal influenza vaccination, based on the annual HC advice.
Vaccines
The two seasonal influenza vaccines, used in the Netherlands in 2009, i.e. Vaxigrip® (split virion; Sanofi Pasteur MSD) and Influvac® (subunit surface antigens; Abbott), are both trivalent inactivated vaccines without adjuvants or thiomersal, given intramuscularly or subcutaneously. The composition of the vaccines for the season 2009/2010 was: A/Brisbane/59/2007 (H1N1)-like virus (15mcg); A/Brisbane/10/2007 (H3N2)-like virus(15mcg); and B/Brisbane/60/2008-like virus (15mcg). Seasonal vaccines were supplied in single-dose syringes.
The pandemic vaccine, used by all GPs was Focetria® (Novartis) and had 7.5 mcg influenza virus surface antigens of A/California/7/2009 (H1N1) like virus per dose. The vaccine contained MF59C.1 as adjuvant. It was presented in multi-dose containers with thiomersal as preservative. The two-dose pandemic vaccination campaign started on November 2, 2009. The seasonal vaccination campaign started in the month before. The 2009 vaccination campaign ended before Christmas. All questionnaires were received before the end of January 2010.
Data collection
With the questionnaires information on age, sex, comorbidity, medication, vaccine type and dose number was acquired. In addition, information was collected on local adverse events (AEs) like redness, swelling and/or pain at the injection site (4 categories to tick by the respondent: none, mild, moderate or pronounced) and systemic AEs, including fever, lethargy, fatigue, headache, fainting, dizziness, myalgia, arthralgia, rash and itch (yes/no). In case of fever, we asked for the highest temperature measured and calculated the median temperature. For each local or systemic AE, additional information on the date and time of onset and the duration of each AE was asked, until one week after immunization.
We computed 2 dichotomous (yes/no) variables for any reported local AE and any reported systemic AE, respectively.
Statistics
The proportions of reported local and systemic AEs were calculated with 95% confidence intervals (95%CI), for each risk group and sex. For fever the mean, median and range of the ‘highest temperature measured’ was determined.
To check for selection bias, we used chi square test to assess differences in AE frequencies between groups who returned all 3 questionnaires and fewer than 3 questionnaires, stratified for the 3 vaccinations and risk groups.
Data were also analyzed by means of a Generalized Linear Mixed Model (GLMM), to address dependency of data. Possible confounders studied were age, sex and comorbidity. Variables with statistically significant influence on the outcome were left in the multivariable model. To address possible selection bias, we performed the GLMM on participants, who returned all 3 questionnaires, similar to the above mentioned chi square test.
P-values <0.05 were considered statistically significant. Data were analyzed using SPSS version 22 and SAS 9.3.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Simonsen L. The global impact of influenza on morbidity and mortality. Vaccine 1999; 17 Suppl 1:S3–10; PMID:10471173; http://dx.doi.org/10.1016/S0264-410X(99)00099-7
- Fukase H, Furuie H, Yasuda Y, Komatsu R, Matsushita K, Minami T, Suehiro Y, Yotsuyanagi H, Kusadokoro H, Sawata H, et al. Assessment of the immunogenicity and safety of varying doses of an MF59(R)-adjuvanted cell culture-derived A/H1N1 pandemic influenza vaccine in Japanese paediatric, adult and elderly subjects. Vaccine 2012; 30:5030–7; PMID:22472791; http://dx.doi.org/10.1016/j.vaccine.2012.03.053
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, Iskander JK, Wortley PM, Shay DK, Bresee JS, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1–62; PMID:20689501
- Gasparini R, Lai P, Panatto D. Today's influenza vaccines-why an adjuvant is needed and how it works. European Infectious Disease 2010; 4:36–40
- WHO. Influenza vaccines-WHO position paper. 2005
- Stein ML, Van Vliet H, Timen A. Chronological overview of the 2009 H1N1 pandemic and the response of the Centre for Infectious Disease Control (Cib) of the National Institute for Public Health and the Environment (RIVM). Bilthoven: National Institute for Public Health and the Environment, 2010.
- van Puijenbroek EP, van Grootheest AC. Monitoring adverse events of vaccines against Mexican flu. Int J Risk Saf Med 2011; 23:81–7; PMID:21673415
- Harmark L, van Hunsel F, Hak E, van Grootheest K. Monitoring the safety of influenza A (H1N1) vaccine using web-based intensive monitoring. Vaccine 2011; 29:1941–7; PMID:21236235; http://dx.doi.org/10.1016/j.vaccine.2010.12.123
- Tacken M, Mulder J, Visscher S, Tiersma W, Donkers J, Verheij R, et al. Monitoring vaccinatiegraad Nationaal Programma Grieppreventie 2009. Nijmegen: LINH, 2010
- Gasparini R, Schioppa F, Lattanzi M, Barone M, Casula D, Pellegrini M, Veitch K, Gaitatzis N. Impact of prior or concomitant seasonal influenza vaccination on MF59-adjuvanted H1N1v vaccine (Focetria) in adult and elderly subjects. Int J Clin Pract 2010; 64:432–8; PMID:20039974; http://dx.doi.org/10.1111/j.1742-1241.2009.02309.x
- Schoffelen T, Wong A, Rumke HC, Netea MG, Timen A, van Deuren M, Vermeer-de Bondt PE. Adverse events and association with age, sex and immunological parameters of Q fever vaccination in patients at risk for chronic Q fever in the Netherlands 2011. Vaccine 2014; 32:6622–30; PMID:25446824; http://dx.doi.org/10.1016/j.vaccine.2014.09.061
- McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Curr Opin Immunol 2009; 21:418–24; PMID:19570667; http://dx.doi.org/10.1016/j.coi.2009.05.023
- Pellegrini M, Nicolay U, Lindert K, Groth N, Della Cioppa G. MF59-adjuvanted versus non-adjuvanted influenza vaccines: integrated analysis from a large safety database. Vaccine 2009; 27:6959–65; PMID:19751689; http://dx.doi.org/10.1016/j.vaccine.2009.08.101
- Simonsen L, Viboud C, Taylor RJ, Miller MA, Jackson L. Influenza vaccination and mortality benefits: new insights, new opportunities. Vaccine 2009; 27:6300–4; PMID:19840664; http://dx.doi.org/10.1016/j.vaccine.2009.07.008
