ABSTRACT
Anflu® is a seasonal trivalent inactivated split-virion influenza vaccine manufactured by Sinovac Biotech Co., Ltd. The objectives of this study were to evaluate the safety of Anflu® (2013–14 formulation: H1N1, H3N2 and BYAM) in infants and adults and its immunogenicity and cross-reactivity against mismatched influenza B lineage and avian influenza A(H7N9) viruses (hereafter BVIC and H7N9, respectively) in adults. In this phase IV open label trial, infants 6–35 months old (n=61) each received two injections with 28 days apart; adults 18–60 yrs old (n=60) and elderly >60 yrs old (n=61) each received one injection. Information of adverse events was collected through safety observation and follow-up visits. Pre- and post-immune blood samples (day 0 and 21) were collected from subjects ≥18 yrs old to detect hemagglutination inhibition antibody titers and calculate seroprotection rates (SPRs) and seroconversion rates (SCRs). The overall adverse reaction incidence was 1.6% (3/182), and no serious adverse event was reported during the study period. For subjects ≥18 yrs old, the SCRs, SPRs, and the geometric mean titers (GMTs) met the European criteria for all three strains. In addition, the point estimations of SCR, SPR and GMT for BVIC also met the European criteria. Six subjects were seroconverted against H7N9; however the serological results did not meet the European criteria. In conclusion, the results showed a satisfactory safety and immunogenicity profile of Anflu® and cross-reactivity against BVIC, but did not demonstrate cross-reactivity against H7N9 (Clinicaltrials.gov ID: NCT02269852).
Introduction
Susceptible populations such as young children and the elderly, have higher risks of developing severe complications and resulting hospitalization or death when infected. Annual influenza attack rate was estimated to be 5%–10% in adults, and 20%–30% in children according to the 2012 World Health Organization (WHO) position paper.Citation1 In addition, an analysis of eight cities in China during 2003–2008 showed that 86% of the influenza-associated death cases in investigated areas were ≥ 65 yrs old.Citation2
Influenza is caused by Influenza viruses, which are classified as types A, B and C based on their nucleoprotein.Citation1 The subtypes of influenza A viruses are determined based on the combination of hemagglutinin (HA) and neuraminidase (NA). There are 18 known HA subtypes and 11 known NA subtypes.Citation3 In contrast, influenza B viruses are not divided into subtypes but can be further divided into 2 antigenically distinguishable lineages (i.e., the Victoria lineage and Yamagata lineage).Citation1 Since there are only two predominate lineages of influenza B viruses, they are referred to as the opposite influenza B lineage to each other.Citation4 Among these influenza viruses, types A and B are of major concern, whereas type C is less likely to cause severe human diseases.Citation1,5 Thus influenza viruses A and B are included in vaccines. Trivalent influenza vaccine contains 2 influenza A strains and 1 influenza B strain.
The effectiveness of influenza vaccines varies with the match between the vaccine strains and prevailing influenza strains.Citation1 In addition, the duration of protection of influenza vaccines is relatively short.Citation1 WHO adjusts the recommended strains for the vaccine formulation twice per year (one for the Northern hemisphere and one for the Southern hemisphere) to optimize the vaccine efficacy. Therefore it is recommended to get influenza vaccination annually for optimal protection.
Anflu®, the 2013–2014 Northern hemisphere seasonal trivalent inactivated split influenza virus vaccine (hereafter Anflu®) was manufactured by Sinovac Biotech Co., Ltd according to the WHO recommended composition (H1N1, H3N2 and BYAM). This phase IV clinical trial was initiated in December 2013 with the primary objectives of assessing the safety and immunogenicity profile of Anflu®.
On the other hand, pandemics caused by avian viruses have given rise to public health concern in recent years. To be specific, the circulation of the avian influenza A(H7N9) viruses (hereafter H7N9) in the Southeastern and middle region of China since March 2013 has given rise to increasing concerns in China, and the potential of cross-reactivity of seasonal influenza vaccine against H7N9 is of particular interest to us and was also investigated in this study.Citation6 In addition, previous studies have reported cross-reactivity of influenza vaccines against mismatched strains. Hence preliminary exploration of the cross-reactivity of Anflu® against influenza B Victoria lineage, the opposite influenza B lineage of the one in the formulation, and H7N9 were the secondary objectives of this study.Citation7,8
Results
Participants
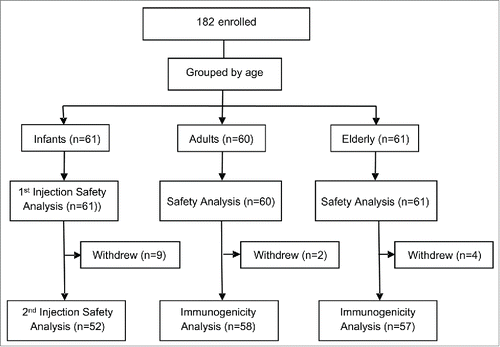
A total of 182 subjects were enrolled in this study: 61 healthy infants 6–35 months old, 60 adults 18–60 yrs old and 61 elderly >60 yrs old. The demographic characteristics of the subjects are shown below ().
Table 1. Demographic characteristics of the subjects by group.
Fifteen subjects withdrew during the course of this clinical trial, and the overall withdrawal rate was 8.2%. None of the subjects withdrew because of adverse event. Among these 15 subjects, 8 infant subjects voluntarily withdrew and did not receive the second injection, 1 infant subject was out of town during the study and did not receive the second injection; therefore safety data of the second injection of these 9 subjects were unavailable for the safety analysis. The other 6 subjects are 2 adults and 4 elderly subjects who voluntarily withdrew. The post-immune blood samples of these 6 subjects were not collected, therefore they were excluded from the per protocol set (PPS) of immunogenicity. Thus, the whole safety analysis set contains data from 182 subjects, the full analysis set of immunogenicity contains 121 subjects (60 adults and 61 elderly), and the PPS of immunogenicity contains 115 subjects (58 adults and 57 elderly), as shown below ().
Safety
Safety study results are summarized by age group. The total adverse reaction (AR) incidence was 1.6% (3/182). There was no serious adverse event (SAE) reported during this study. In the infant group, 61 subjects received at least 1 injection and entered the safety analysis set. One AR was reported, which was grade 2 fever after the first injection. Two ARs of adult subjects were reported, and both were grade 1 local pain; the AR incidence of the adult group was 3.3%. There was no AR observed in the elderly group.
Immunogenicity and cross-reactivity
The pre- and post-immune serum samples were collected from 115 subjects to measure the hemagglutination inhibition (HI) antibody levels against the three strains in Anflu® formulation (H1N1, H3N2, and BYAM) as well as BVIC and H7N9.
The immunogenicity results of Anflu® against the three strains in the formulation (H1N1, H3N2, and BYAM) met the criteria of the Note for Guidance on Harmonisation of Requirements for Influenza Vaccines of the European Agency for the Evaluation of Medicinal Products (the European criteria). Before immunization, none of the geometric mean titers (GMTs) or Seroprotection rates (SPRs) showed significant difference between the adults and the elderly. 21 days after vaccination, in the adult group the Seroconversion rates (SCRs) against H1N1, H3N2 and BYAM strain ranged in 75.9%–91.4%, SPRs against these strains ranged in 96.6%–100.0%, and the geometric mean increase (GMI) of antibody against these strains increased 6.4–35.2 folds. In the elderly group, the SCRs ranged in 80.7%–96.5%, SPRs ranged in 89.5%–100%, and the GMIs increased 5.8–40.3 folds, as shown in the .
Table 2. The serological test results of Anflu® against the strains of the vaccine formulation (H1N1, N3N2, and BYAM strains) as well as the opposite B lineage (BVIC strain) and H7N9 strain.
According to the European criteria, Anflu® also showed a cross-reactivity against BVIC strain. In the adult group, the SCR against BVIC is 48.3%, GMT increased 3.1 folds, and the pre- and post-immune protection rates were 70.7% and 100%, respectively. In the elderly group, the SCR against BVIC is 35.1%, GMT increased 2.4 folds, and the pre- and post-immune protection rates were 70.2% and 91.2%, respectively. In addition, the SCRs and SPRs against each of the three strains of the formulation and the BVIC strain showed no statistically significant difference between the adult and elderly groups, as shown in the .
Serological tests results showed that the protection of Anflu® against H7N9 did not met the European criteria for the adult or elderly, as shown in . Before vaccination, HI antibody levels against H7N9 in both adult and elderly groups were below detection limit, hence all the subjects were considered seronegative at day 0. 21 days after vaccination, 6 subjects were seropositive against H7N9, and their post-immune antibody titers were all 1:40. The detailed demographical information and serological results of these six subjects are listed in Table S1 as supplementary material.
Discussion
In this study, AR incidences were low in all three groups, and there was no grade 3 AR, SAE, or serious adverse reaction (SAR) case reported during the observation period; the immunogenicity results of Anflu® against all three influenza viruses in the formulation met the European criteria. Thus, Anflu® (2013–2014 formulation) showed good safe and immunogenicity profile.
In addition, since the cases of human infection with H7N9 virus was first found in China at March 2013 and there was no H7N9 vaccine available on the market till the initiation of this study, we regarded this trial as a good opportunity of exploring the cross-reactivity of seasonal influenza vaccine against H7N9. Hence preliminary study of the cross-reactivity of Anflu® against the opposite influenza B lineage (BVIC) and H7N9 were conducted in this study. Immunogenicity of Anflu® against the opposite B lineage also met the European criteria. In contrast, the serological results did not indicated cross-reactivity against H7N9, though six cases of seroconversion against H7N9 were found in this study.
In this study, in both adult and elderly groups the lower limit of 95% confidence interval (CI) of the SCR against BVIC strain were slightly below the European criteria, however, it is notable that the pre-immune SPRs against BVIC strain of both groups were 70.7% and 70.2%, respectively; these high pre-immune SPRs may have constrained the SCRs. These results are consistent with the results of a TIV versus placebo meta-analysis, in which cross-reactivity of the TIV against mismatched influenza B strains among adults were found in 8 RCTs and the point estimates of vaccine efficacy against mismatched strains were slightly lower than those of the matched strains.Citation8 In addition, Belshe et al reported cross-reactivity of live attenuated influenza vaccines against mismatched influenza B lineage, and suggested that previously circulated naturally occurring reassortant influenza B viruses (e.g., an influenza B strain that contains a Victoria lineage hemagglutinin (HA) and a Yamagata lineage neuraminidase (NA)) may led to the cross-reactivity, though the mechanism was unknown.Citation4 Likewise, such reassortant viruses may also contribute to the cross-reactivity detected in this study; however investigation of the locally circulated influenza B viruses in recent years is needed to determine the possibility of this postulation.
On the other hand, Anflu® did not demonstrate cross-reactivity against H7N9, but six subjects were seroconverted against H7N9. It is noteworthy that cases of human infection with H7N9 were initially reported in March 2013 in Anhui province, where this study was conducted.Citation6 Hence these seroconversion cases were more likely correlated with subclinical infection of H7N9 between the pre- and post-immune blood sampling time points than the vaccination of Anflu®. During November 2012 - December 2012, the phase IV trial of Anflu® (2012–13 formulation) was conducted in Henan province. After the 2013 H7N9 outbreak, the serum samples collected in the 2012 study were used for serological tests regarding H7N9. The results showed that none of the subjects seroconverted against H7N9, and there was no cross-reactivity against H7N9.Citation9 In other words, none of the subjects was seropositive against H7N9 in the study conducted before the emergence of the human infection with H7N9, which also suggests the seroconversion cases in the 2013 study may not correlated with the vaccination of Anflu®.
The strength of this study is that it provided insight into the cross-reactivity of seasonal influenza vaccine against opposite influenza B lineage and pandemic influenza virus.Citation7,9 This study supplemented the known results about cross-reactivity of seasonal influenza vaccine against H7N9.
Our study has at least two limitations. First, the sample size of this trial was small, therefore possible rare AEs may not be observed in this particular trial. However, safety data collected from 13 previous trials of Anflu® and post-market self-reporting safety monitoring system of Sinovac Biotech suggest that Anflu® is well tolerated in all age groups, and SAR rarely occurred.Citation10 Secondly, only 6 subject who seroconverted to anti-H7N9 were found in this study, which provided limited preliminary results of the cross-reactivity against H7N9. This trial was conducted in Anhui, where cases of human infection of H7N9 were reported; however the incidence of such infection was relatively low.Citation11 Study in regions with relatively high prevalence of H7N9 (e.g., Zhejiang province, Shanghai) with larger sample size may provide more information and help us getting a more comprehensive understanding of the cross-reactivity of seasonal influenza vaccine against H7N9 with the baseline of subclinical infections.
To sum up, our findings showed that Anflu® has good safety profile and satisfactory immunogenicity. These findings are consistent with previous studies of Anflu®.Citation10 In addition, Anflu® demonstrated cross-reactivity against the opposite influenza B lineage.
Methods
Study design
This open-label, phase IV trial of Anflu® was conducted in Anhui, China during the 2013 northern hemisphere winter. In this study, the immunogenicity of this vaccine in adults and elderly and safety profile in infants, adults and elderly were evaluated. The immunogenicity assessments focused on H1N1, H3N2 and BYAM strains, which were included in the vaccine formulation. The cross-reactivity of Anflu® against BVIC, the opposite influenza B lineage, and H7N9 were assessed as well.
Study participants
Healthy, full-term infants aged 6–35 months old with vaccination certification or birth certification, and healthy adults aged ≥18 yrs old were eligible for this study. Subjects were assigned into three age groups. Infants were assigned to the infant group, subjects between 18–60 yrs old were assigned to the adult group; subjects aged >60 yrs old were assigned to the elderly group.
The inclusion criteria are as follows: healthy full-term infants between 6–35 months old, or healthy adults ≥18 yrs old without vaccination history of seasonal split influenza vaccine in the recent 3 years. The following are the exclusion criteria: acute infection within the previous week; allergy history; history of SAR to vaccine; autoimmune disease or immune deficiency, or administration of immunosuppressive therapy or cell toxic therapy within the previous 6 months; congenital malformations, developmental disorder or serious chronic disease; coagulation abnormalities or disorders; history/family history of epilepsy, cerebropathy, or mental disease; administration of blood products or investigational drug within the previous month; administration of attenuated live vaccine within the previous 14 days; administration of subunit or inactivated vaccine within the previous 7 days; administration of treatment for allergy within the previous 14 days; pregnant or planning pregnancy; axillary temperature >37.0°.
Treatments
Anflu® was a seasonal trivalent inactivated split influenza virus vaccine manufactured in accordance with the registered procedure. This vaccine is thiomersal free. NYMC X-179A (A/California/7/2009), NYMC X-223A (A/Texas/50/2012) and NYMC BX-51B (B/Massachusetts/02/2012) were used as the A(H1N1), A(H3N2), and BYAM component of the active ingredients of Anflu®, respectively. The concentration of each antigen is 15µg per 0.5ml. The vaccine has two dosage forms: 0.25 ml/dose for vaccinee aged 6–36 months old and 0.5 ml/dose for vaccinee > 3 yrs old. The Infant subjects were administrated two doses with a 28-day interval. The adult and elderly subjects were administrated one dose at day 0.
Safety assessment
After each injection, subjects stayed onsite for a 30-minute safety observation. Follow-up visits were conducted until day 35. After each injection, the solicited local and general adverse events (AEs) and axillary temperatures for day 1–3 were collected, and unsolicited AE cases occurred during day 0–35 were also collected. Then the investigator assessed the relationship between each AE case and injection. If an AE was related, probably related, or possibly related to the vaccination, it was then determined as an AR. The AR data was then used for safety analysis. SAR was determined in the like manner according to the correlation between SAE and vaccination.
AR grades 1–3, which means mild, moderate and severe AR, were determined based on the Preventive Vaccine Clinical Trials Adverse Reaction Grading Standard Guidelines of the China Food and Drug Administration, and the Division of Microbiology and Infectious Diseases Pediatric Toxicity Tables (November 2007 draft) from the National Institute of Allergy and Infectious Diseases of National Institutes of Health.
Immunogenicity and cross-reactivity assessment
In this study, infant blood samples were not collected, and data collected from the infant group was for safety assessment only. Blood samples of the adults and elderly were collected immediately before and 21 days after the injection to detect the levels of HI antibody against the three strains of the vaccine formulation, the influenza BVIC lineage virus (B/Brisbane/60/2008), and the avian influenza A(H7N9) virus NIBRG-268 (containing the genes of HA and NA proteins of A/Anhui/1/2013). The lowest dilution fold of serum sample was 1:5. Thus, for the purpose of calculation, HI antibody titers below 1:5 were treated as 1:2.5.
Serological result interpretations are defined as following:
Seroconversion: subjects with pre-immune HI antibody titer <1:10 and post-immune antibody titer ≥1:40, or subjects with pre-immune HI antibody titer ≥1:10 and post-immune antibody titer increased ≥4 folds;
Seroprotection/seropositive: post-immune HI antibody titer ≥1:40;
The immunogenicity of Anflu® was evaluated according to the European criteria.Citation12 21 days after immunization, at least one of the three criteria below should be satisfied:
SCR of each strain should >40% for adults (18 – 60 yrs old), and >30% for the elderly (>60 yrs old).
GMT of each strain should increase by >2.5 folds for the adults (18–60 yrs old), and increase by >2.0 folds for the elderly (>60 yrs old).
SPR of adults should >70%; SPR of the elderly should > 60%.
Statistical analyses
The statistical analyses of this study were conducted by the data analysis team of Sionvac Biotech Co. Ltd. SPSS statistical software (version 15.0, SPSS, Inc., Chicago, IL) was used for data validation and statistical analysis. P ≤ 0.05 was regarded as a statistically significant difference.
The results of immunogenicity tests were used to calculate the SCR, SPR, GMT, and GMI. The point estimate and 95% CI of these immunogenicity results of the subject ≥18 yrs old were calculated. Safety data was summarized in terms of the number and proportion of the AR cases in each age group. Pearson Chi-Square test or Fisher's exact test were used for comparisons of seroconversion rates (SCRs) and seroprotection rates (SPRs) between age groups. Mann-Whitney-U-test was used for comparisons of GMTs and GMIs between age groups.
Ethical statements
This study was conducted in accordance with the International Conference on Harmonization Guideline for Good Clinical Practice. The study protocol was approved by the Medical Ethics Committee of Anhui Center for Disease Control and Prevention, and conducted according to the local institutional ethics committee guidelines. Before enrollment, written informed consents were obtained from each adult subject and parent/guardian of each infant subject.
Abbreviations
| AEs | = | adverse events |
| AR | = | adverse reaction |
| BVIC | = | influenza, B Victoria lineage |
| BYAM | = | influenza, B Yamagata lineage |
| CI | = | confidence interval |
| GMI | = | geometric mean increase |
| GMTs | = | geometric mean titers |
| H7N9 | = | avian influenza, A(H7N9) virus |
| HA | = | hemagglutinin |
| HI | = | hemagglutination inhibition |
| NA | = | neuraminidase |
| PPS | = | per protocol set |
| SAE | = | serious adverse event |
| SAR | = | serious adverse reaction |
| SCRs | = | seroconversion rates |
| SPRs | = | seroprotection rates |
| TIVs | = | trivalent inactivated vaccines |
| WHO | = | World Health Organization |
Disclosure of potential conflicts of interest
Yuansheng Hu, Yufei Song, Xiaoci Ji, Liqun Huo, Zhenping Fu, and Weidong Yin are employed by Sinovac Biotech Co., Ltd.
Supplemental_Material.zip
Download Zip (14.7 KB)Acknowledgments
We would like to express our thanks to the staffs of Guxian Center for Disease Control and Prevention for their efforts in the implementation on the study site.
Funding
This project was funded by Sinovac Biotech Co., Ltd.
References
- WHO. Vaccines Against Influenza WHO Position Paper. 2012 Nov
- Feng L, Shay DK, Jiang Y, Zhou H, Chen X, Zheng Y, Jiang L, Zhang Q, Lin H, Wang S, et al. Influenza-associated mortality in temperate and subtropical Chinese cities, 2003–2008. Bulletin World Health Organization 2012; 90:279–88B; PMID:22511824; http://dx.doi.org/10.2471/BLT.11.096958
- Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, Yang H, Chen X, Recuenco S, Gomez J, et al. New World Bats Harbor Diverse Influenza A Viruses. PLoS Pathogens 2013; 9:e1003657; PMID:24130481; http://dx.doi.org/10.1371/journal.ppat.1003657
- Belshe RB, Coelingh K, Ambrose CS, Woo JC, Wu X. Efficacy of live attenuated influenza vaccine in children against influenza B viruses by lineage and antigenic similarity. Vaccine 2010; 28:2149–56; PMID:20003926; http://dx.doi.org/10.1016/j.vaccine.2009.11.068
- Matsuzaki Y, Katsushima N, Nagai Y, Shoji M, Itagaki T, Sakamoto M, Kitaoka S, Mizuta K, Nishimura H. Clinical features of influenza C virus infection in children. J Infect Dis 2006; 193:1229–35; PMID:16586359; http://dx.doi.org/10.1086/502973
- WHO. Overview of the Emergence and Characteristics of The Avian Influenza A(H7N9). Virus 2013 May 31
- Bethell D, Saunders D, Jongkaewwattana A, Kramyu J, Thitithayanont A, Wiboon-ut S, Yongvanitchit K, Limsalakpetch A, Kum-Arb U, Uthaimongkol N, et al. Evaluation of in vitro cross-reactivity to avian H5N1 and pandemic H1N1 2009 influenza following prime boost regimens of seasonal influenza vaccination in healthy human subjects: a randomised trial. PloS One 2013; 8:e59674; PMID:23555741; http://dx.doi.org/10.1371/journal.pone.0059674
- Tricco AC, Chit A, Soobiah C, Hallett D, Meier G, Chen MH, Tashkandi M, Bauch CT, Loeb M. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med 2013; 11:153; PMID:23800265; http://dx.doi.org/10.1186/1741-7015-11-153
- Guo W, Xu J, Wu J, Zhao S, He H, Shi W, Yu D, Li J, Gao H, Chen J. [Safety and immunogenicity of seasonal inactivated influenza vaccine (split virion) and cross-reactive antibody responses to the H7N9 avian influenza virus]. Zhonghua Liu Xing Bing Xue Za Zhi=Zhonghua Liuxingbingxue Zazhi 2014; 35:949–52; PMID:25376689
- Liu Y, Wu JY, Wang X, Chen JT, Xia M, Hu W, Zou Y, Yin WD. Review of 10 years of clinical experience with Chinese domestic trivalent influenza vaccine Anflu(R). Human Vaccines Immunotherapeutics 2014; 10:73–82; PMID:24104060; http://dx.doi.org/10.4161/hv.26715
- WHO. Number of confirmed human cases of avian influenza A(H7N9) report to WHO (report 10).25 October 2013
- European Medicines Agency (EMA) Committee for Medicinal Products for Human Use. Note for guidance on harmonization of requirements for influenza vaccines (CPMP/BWP/214/96) In: European Committee for Medicinal Products for Human Use, ed., March 12, 1997