Abstract
Several promising subunit vaccines against ricin toxin (RT) have been developed during the last decade and are now being tested for safety and immunogenicity in humans and for efficacy in nonhuman primates. The incentive to develop a preventive vaccine as a countermeasure against RT use as a bioweapon is based on the high toxicity of RT after aerosol exposure, its environmental stability, abundance, and ease of purification. RT is the second most lethal biological toxin and is considered a “universal toxin” because it can kill all eukaryotic cells through binding to ubiquitous cell surface galactosyl residues. RT has two subunits conjoined by a single disulfide linkage: RTB, which binds galactosyl residues and RTA which enzymatically inactivates ribosomes intracellularly by cleavage ribosomal RNA. Attenuation of toxicity by elimination of the active site or introduction of other structural mutations of RTA has generated two similar clinical subunit vaccine candidates which induce antibodies in both humans and nonhuman primates. In rhesus macaques, inhaled RT causes rapid lung necrosis and fibrosis followed by death. After parenteral vaccination with RTA vaccine, macaques can be protected against aerosol RT exposure, suggesting that circulating antibodies can protect lung mucosa. Vaccination induces RT-neutralizing antibodies, the most likely correlate of protection. Macaques responded to conformational determinants in an RTA vaccine formulation, indicating preservation of RTA structure during initial manufacture. Comparative mapping studies have also demonstrated that macaques and humans recognize the same epitopes, significant in the study of macaques as a model during development of vaccines which cannot be tested for efficacy in humans.
Introduction
Ricin toxin (RT) is a 64 kDa glycoprotein produced by the castor bean plant (Ricinus communis). These plants are prevalent worldwide. Crude RT is easy to make and it is exceedingly toxic to mammals following inhalation, ingestion or injection. RT consists of 2 distinct subunits, RTA and RTB, that are essential for cytotoxicity.Citation1 RTA (267 AA) is a ribosome-inactivating protein (RIP) that triggers inflammatory signaling pathways and programed cell death in mammalian cells. RTB promotes the attachment of the holotoxin to galactosyl residues on cell surfaces as well as internalization and transport of RT from the plasma membrane to the endoplasmic reticulum, where RTA is then delivered into the cytoplasm. RT is considered a “universal toxin” because it can intoxicate all known eukaryotic cell types. Indeed, within hours of aerosol exposure of non-human primates (NHPs) and rodents, RT causes clinical symptoms that are associated with epithelial necrosis, multifocal hemorrhagic edema and death within 24–36 hours.Citation2,3 By inhalation, RT toxicity onset is rapid and irreversible, with a lethal dose in laboratory animals, including rhesus macaques, of ˜5 µg/kg. The lethal dose of aerosolized RT extrapolated to humans is approximately 350 µg of inhaled RT for a 70 kg individual. The dramatic and severe pathologies associated with aerosol exposure are primarily confined to the lung and associated parenchyma although damage to the gut has also been observed after oral ingestion.Citation2 RT is designated by the CDC and NIH as a select agent Class B Biothreat. RT is also a significant concern to the US military because of its potential deployment as a bioweapon.Citation4
The impetus to develop a preventive vaccine against RT has grown over the last decade because of the mounting concern that crude RT powder can be easily made and used in a terrorist attack on civilian or military populations. At this time, a vaccine against RT is primarily aimed at military populations deployed to locations where RT powder might be used; mass immunization of civilian populations is improbable since exposure to RT would likely be rare, unpredictable, and unlikely to be persistent, since RT is not transmissible. Two closely related RTA-based subunit vaccines, RiVax™ and RVEc™, are now under development. RiVax is a full-length non-glycosylated recombinant derivative of RTA with 2 point mutations: one in the active site (Y80A) and the other (V76M) in a motif associated with vascular leak syndrome (VLS).Citation5 X-ray crystallography has revealed that the structure of RiVax is virtually identical to that of native RTA, indicating that point mutations in the molecule have a minimal effect on its tertiary structure.Citation6 RVEc is a truncated derivative of RTA that lacks the hydrophobic carboxy-terminal region (residues 199–267) as well as a small hydrophobic loop in the N-terminus (residues 34–43), resulting in a molecule with increased solubility and thermal stability.Citation7-10 RVEc does not contain mutations that directly inactivate the active site of RTA, but the removal of both segments results in an inactive molecule devoid of enzymatic activity and reduction or elimination of its ability to cause vascular leak as demonstrated in experimental models.Citation10 Both candidate vaccines are under investigation in animal studies, advanced manufacturing and Phase I clinical trials. Both RiVax and RVEc are highly immunogenic in mice and elicit protective immunity against exposure to RT by inhalation, gavage or injection.Citation10,11 Despite structural differences between these 2 subunit vaccines, head-to-head comparisons have indicated that RiVax and RVEc when adsorbed to Alhydrogel or co-administered with LT-II adjuvants have identical capacities to induce neutralizing antibodies and to protect in mice.Citation12,13 No head-to-head comparisons have yet been attempted in non-human primates although intramuscular administration of RiVax adsorbed to aluminum hydroxide adjuvant protects rhesus macaques against the lethality of aerosolized RT.Citation14 RiVax administered by intramuscular injection also protects mice against aerosol exposure, suggesting that RT-specific secretory IgA may not be essential for protection of lung epithelia, although studies in vaccinated mice suggest that high doses of aerosolized RT attenuates but does not eliminate long term lung damage.Citation15 Hence intramuscular vaccination combined with intranasal administration might be more protective. It is also possible that better dose regimens using the intramuscular route might result in better protection of lungs and other mucosal surfaces by increasing total levels of neutralizing antibodies.
RiVax is virtually identical to native RTA, but in direct contrast to RVEc, it unfolds and denatures rapidly in aqueous solutions.Citation7,16 The inherent instability of RTA and the tendency to unfold, denature and aggregate, indicate that techniques to stabilize the structure of RiVax are crucial to its future success. Recent efforts in molecular stabilization technologies have improved cold-chain logistics and could potentially improve efficacy with enhanced maintenance of its native structure. Stabilization of RiVax on the surface of aluminum hydroxide adjuvant was performed in an effort to stabilize the structure and prevent the unfolding and denaturation that occurs in solution.Citation14 This method of stabilization involved the lyophilization of the aluminum adjuvant with the bound RiVax antigen in the presence of trehalose, creating a “glassified” vaccine that was easily hydrated with water.Citation17 Because this formulation of RiVax was effective in mice after many months of incubation at 40°C, it is presumed that the adjuvant-bound antigen was not grossly destabilized. Any unfolding of the antigen that may have taken place had occurred during the aqueous phase processing. It was considered very likely from the efficacy results that epitopes responsible for induction of protective immunity were wholly or in part retained in the vaccine.Citation14 In the case of RVEc, binding to the surface of aluminum adjuvant appeared to stabilize the molecule further.Citation7 However, no direct biophysical comparisons between the stabilized versions of RiVax and RVEc have yet been done.
Despite these promising results, there are several critical impediments that hinder FDA licensure of a RT vaccine. Key among these are the need for well-established animal models of protection against aerosol RT exposure, better definitions of correlates of protection, and identification of additional adjuvants, beyond aluminum salts, that promote the rapid onset of circulating RT-neutralizing antibodies.
Protective efficacy of RiVax in Rhesus Macaques
Because efficacy trials cannot be carried out in humans, the use of appropriate animal models is critical to establish immunological correlates of protection.Citation18,19 However, one of the major difficulties in establishing immunological correlates for RT intoxication is that there is no natural human disease caused by RT with which to compare. Over the past few years, studies of RT aerosols in rhesus macaque (Macaca mulatta) have suggested that these animals are a suitable model for vaccine development.Citation2 Upon aerosol exposure to supra-lethal doses of RT, macaques develop massive pulmonary edema with necrotizing alveolitis that mainly affects the lower respiratory tract from terminal bronchioles to alveoli. Citation2 Supra-lethal doses of RT have been used in recent vaccine protection studies to challenge vaccinated and unvaccinated animals.Citation14 Surprisingly, we found that parenterally vaccinated animals were completely protected against a high dose of aerosolized RT.Citation14 Neutralizing antibodies were present in the blood of vaccinated animals prior to challenge, suggesting that these antibodies transudate into the lung.Citation14 However, the direct demonstration of neutralizing antibodies in the lungs or their role in protection against RT exposure has not yet been reported.
Effects of immunization on intoxication and lung pathology in Macaques
We have studied the lungs of vaccinated macaques 14 days after aerosol challenge with RT. At necropsy, lungs of all of the vaccinated animals were grossly normal with the exception of proliferation of fibroblasts with collagen depositions around terminal bronchioles and infiltration of small numbers of lymphoid cells, suggesting resolving epithelial damage and an ongoing immune response.Citation14 Antibodies also have important proinflammatory activities, primarily mediated by their Fc. These activities include complement activation and Fc receptor binding. Yet we also know that much of the syndrome associated with exposure to RT and other toxins is due to an acute inflammatory response.Citation2,20-22 It is not yet known how antibodies, which are themselves proinflammatory, block the proinflammatory activity of the toxin, rather than enhancing it. We can partially address this question by comparing inflammatory responses in immunized macaques with those observed in the controls. We have observed evidence of both systemic and local pulmonary inflammatory responses in non-immune animals exposed to aerosolized RT. In contrast, immunized macaques demonstrated both pronounced neutrophilia and depletion of lymphocytes, with only transient low grade fever.Citation14 Thus, immunization prevented some aspects of the systemic inflammatory response due RT exposure, but had no effect on others (e.g. neutrophilia). Levels of circulating leukocytes returned to pretreatment values within 6 days of exposure in immunized animals.
It was more difficult to study local inflammatory responses in the immunized animals, because these animals were not sacrificed until 2 weeks following exposure. At that time lung pathology showed only minimal residual disease and fibrosis. However clinical observations and laboratory analyses strongly suggest that pulmonary inflammation was present but blunted. Immunized animals had no evidence of respiratory distress, in marked contrast to controls. Clinical laboratory analyses in control animals showed hemoconcentration, which correlated with fluid shifts from the blood to the lungs and were associated with histologic evidence of pulmonary inflammation.Citation20 In contrast, immunized animals showed no evidence of hemoconcentration or fluid shifts, as exemplified by hematocrits. There were no changes in the numbers of RBCs, hemoglobin concentrations, or blood urea nitrogen such as has been observed in control animals.Citation20 These data suggest that vaccination results in a marked diminution of the proinflammatory effect of RT at the site of exposure and that many but that not all aspects of the systemic inflammatory response are dampened.
Correlates of antibody-mediated protection against RT
Immunity to RT is associated with the production of protective antibodies.Citation23-30 Studies with MAbs in mouse protection experiments have strongly suggested that antibodies that prevent cytotoxicity in vitro, that are by definition neutralizing antibodies, are also responsible for protection against parenteral RT exposure, since only neutralizing antibodies could confer protection on recipient mice by passive transfer.Citation26 However, it is likely that other mechanisms may also contribute to protection. These might include clearance of RT by RT-binding antibodies via the Fc receptor on macrophages or other cells.Citation29 Furthermore, passive transfer of at least one particular humanized neutralizing monoclonal antibody produced in plants (MAb PB10), conferred lung protection in mice further implicating neutralizing antibodies as the key mediators of protective immunity.Citation31
Although some anti-RTA antibodies are able to neutralize RT, it is only in the past several years that structural details regarding the nature of the interactions between RT and anti-RTA antibodies have emerged.Citation32-34 Only in the past several years have structural details regarding the nature of the interactions between RT and anti-RTA antibodies emerged. In general, immunization with RTA induces better protection than immunization with RTB, justifying the development of RTA-based vaccines.Citation28 With regard to MAbs induced by RTA, the frequency of RT neutralizing antibodies after vaccination of mice show that only a minority neutralize RT in vitro. In addition, of those MAbs that neutralize RT, the majority appear to recognize discontinuous epitopes, while Mabs recognizing linear epitopes are rare.Citation26 Importantly, RTA consists of 3 distinct structural domains (): domain I (residues 1–117) is dominated by a 6-stranded β sheet that terminates in a solvent exposed loop-helix-loop; domain II (residues 118–210) is dominated by 5 α-helices that run through the center of RTA; and domain III (residues 211–267) forms a protruding element that slides into the cleft between RT-B's 2 domains.Citation1 RTA's active site constitutes a shallow pocket in the central portion of the protein. The two candidate RT vaccines, RiVax and RVEc, are structurally distinct in that RiVax is a full-length derivative of RTA, while RVEc is a truncated variant lacking folding domain 3 (residues 199–267) and a small hydrophobic loop in the N-terminus (residues 34–43).
Figure 1. Depiction of the Structures of RiVax and RVEc. A front and back surface projection of RiVax (PDB: 3SRP) with the color coded with attenuating point mutations (yellow), active site (red), epitope cluster I (blue), and epitope cluster II (cyan). For comparison purposes, the regions of RiVax that have been omitted in RVEc (residues 34–43 and 199–267) are colored in white.
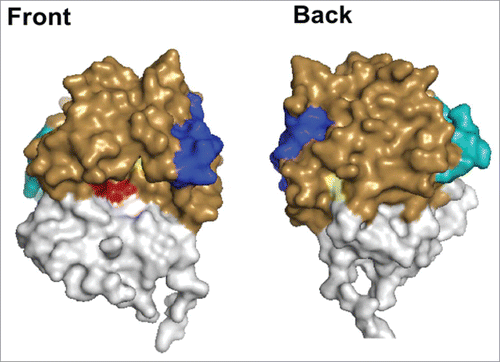
To date, 4 putative toxin-neutralizing hotspots or epitope clusters have been identified on RTA based on competition ELISA and pepscan analysis of murine MAbs ().Citation26,35 The four clusters are scattered across the surface of RTA but restricted to domains I and II. None of the hotspots directly occludes the active site of RTA. Cluster I is centered on a surface exposed α-helix (residues 97–109), which is structurally conserved among all RIPs including abrin and Shiga toxins.Citation26,34,36,37 Monoclonal antibodies PB10 and R70 are the prototype neutralizing antibodies for linear determinants of Cluster I and a plant expressed PB10 chimera is being evaluated as a candidate for post-exposure ricin therapy.Citation31,37 Rudolph et al have recently solved the X-ray crystal structures of 5 different single chain camelid antibodies complexed with RTA.Citation38 Cluster II resides within α-helix F (residues 187–194), which is adjacent to and overlapping with an arginine rich region of RTA that is postulated to interact with one or more ribosomal proteins.Citation39 Cluster III is located in α-helix E (residues 163–174) and corresponds to a previously reported human B cell epitope identified by peptide array analysis of sera from 15 Hodgkin's lymphoma patients who had been treated with an anti-CD25-RTA-Immunotoxin.Citation40 Finally, cluster IV is defined by a single neutralizing MAb (“IB2”) that was postulated to recognize a discontinuous epitope located near the RTA-RTB interface. Recent epitope mapping studies using hydrogen/deuterium exchange challenge that original prediction (R. Toth, D. Weis, D. Volkin and N. Mantis, manuscript in preparation). We have estimated that the panel of neutralizing and non-neutralizing MAbs define epitopes that span roughly 50% of the surface of RTA and ∼75% of the total number of predicted B-cell epitopes.
We have used a subset of toxin-neutralizing and non-neutralizing MAbs in competition ELISAs with sera from immunized macaques to gain insights into the epitopes on RTA recognized in RiVax-protected animalsCitation14 We determined that vaccinated macaques responded to 2 neutralizing conformational determinants recognizing cluster 1 and cluster 2 (defined by MAbs WECB2, and PA1), implying that the conformational regions were preserved and that they elicited protective antibodies. We also found that sera obtained from human volunteers vaccinated with RiVax had MAb inhibition profiles remarkably similar to those from vaccinated macaques, suggesting that antibody responses and B cell epitope utilization are conserved. Human sera and macaque sera recognized conformational and linear determinants in Clusters I and II. This information is invaluable in terms of using animal models to assess the efficacy of RT vaccines. We are currently investigating the mechanism by which antibodies against RTA neutralize RT, with considerable evidence suggesting that the anti-RTA antibodies alter intracellular trafficking rather than inhibiting the binding of RT to cells.Citation30,41 Indeed, it might be predicted that immune complexes would be routed to lysosomes rather than endosomes, and hence be destroyed. Since retrograde routing to endosomes is key for RTA to translocate into the cytosol and exert its ribotoxic effect.
RTA vaccines in humans
RiVax has been tested for safety and immunogenicity in 2 small human Phase I studies. In one case RiVax was formulated without adjuvant and in the other it was adsorbed to aluminum hydroxide.Citation42,43 In both trials, 3 doses of 1–100 µg were used. Volunteers vaccinated on days 0, 6, and 26 weeks developed antibodies except at the lowest antigen dose, some of which were neutralizing. Anti-RT antibody responses were dose dependent and higher and longer lasting in the volunteers receiving the aluminum-adsorbed vaccine, with all volunteers developing detectable neutralizing antibodies 2 weeks after the third vaccination. However, neutralizing antibodies appeared with slower kinetics than total antibodies and had declined at 1 year after the first vaccination. USAMRIID has also completed a Phase 1 clinical study in which RVEc (rRTA 1–33/44–198 protein antigen) adsorbed to aluminum adjuvant was shown to be safe and immunogenic at 20 µg and 50 µg doses given on days 0, 28 and 56. Two weeks after the third vaccination, all of the volunteers developed antibodies, but only 50% developed neutralizing antibodies with titers >1:50. Four (4) subjects in the 50 µg dose group received a boost ˜20 months after the initial prime vaccination. The boost was safe and well-tolerated, and the 4 subjects mounted a robust anamnestic response and high neutralizing titers (>1:1000) which persisted for at least 6 months after the boost. Antibodies (IgG) from the vaccinated subjects passively transferred to mice that were subsequently challenged with lethal doses of RT protected animals from death.Citation44
Future clinical studies with RiVax or RVEc will be carried out to further evaluate safety, immunogenicity and antibody profiles using different dose regimens and possibly the use of alternate adjuvants and delivery systems. We will focus on inducing an earlier and more robust neutralizing antibody response. Archived sera from protected macaques will also facilitate a direct comparison of protective antibody profiles in human vs macaques. Finally the issue of which antibodies transudate into the lung will be studied in macaques using both active and passive vaccination.
Conclusions
Although protective immunity against RT has been demonstrated to be mediated by antibody, the exact species, epitope specificity and anatomical location of antibodies involved in protective immunity remain to be established. The definitive correlation between the structure of RTA and the induction of protective immunity must be more firmly established. The extent to which immunogenic structures, especially conformational “neutralizing” epitopes, are preserved in the vaccine candidates and their formulations will probably influence the generation of protective antibodies and the frequency and dose for vaccination. Rhesus macaques are now the species of choice to examine the efficacy of RT vaccine candidates, since they are thought to develop similar antibodies. It is likely that definitive trials for the licensure of an RT vaccine will utilize safety and efficacy results generated in rhesus macaques along with safety and immunogenicity studies of different dose regimens in humans. These trials will establish the relationship between protective immunity in the animal models and the level and species of antibodies generated in humans using the most effective dose regimens.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
CJR is supported in part by the National Center for Research Resources and the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health through Grant Number OD011104 to the Tulane National Primate Research Center. NJM is supported through NIAID Contract No. HHSN272201400021C. EV is supported by the Simmons Patigian Chair and the Horchow Foundation.
References
- Montfort W, Villafranca JE, Monzingo AF, Ernst SR, Katzin B, Rutenber E, Xuong NH, Hamlin R, Robertus JD. The three-dimensional structure of ricin at 2.8 A. J Biol Chem 1987; 262:5398-403; PMID:3558397
- Bhaskaran M, Didier PJ, Sivasubramani SK, Doyle LA, Holley J, Roy CJ. Pathology of lethal and sublethal doses of aerosolized ricin in rhesus macaques. Toxicol Pathol 2014; 42:573-81; PMID:23761937; http://dx.doi.org/10.1177/0192623313492248
- Roy CJ, Song K, Sivasubramani SK, Gardner DJ, Pincus SH. Animal models of ricin toxicosis. Curr Top Microbiol Immunol 2012; 357:243-57; PMID:21956160; http://dx.doi.org/10.1007/82_2011_173
- Wolfe DN, Florence W, Bryant P. Current biodefense vaccine programs and challenges. Hum Vaccin Immunother 2013; 9:1591-7; PMID:23428906; http://dx.doi.org/10.4161/hv.24063
- Smallshaw JE, Firan A, Fulmer JR, Ruback SL, Ghetie V, Vitetta ES. A novel recombinant vaccine which protects mice against ricin intoxication. Vaccine 2002; 20:3422-7; PMID:12213413; http://dx.doi.org/10.1016/S0264-410X(02)00312-2
- Legler PM, Brey RN, Smallshaw JE, Vitetta ES, Millard CB. Structure of RiVax: a recombinant ricin vaccine. Acta Crystallogr D Biol Crystallogr 2011; 67:826-30; PMID:21904036; http://dx.doi.org/10.1107/S0907444911026771
- Carra JH, Wannemacher RW, Tammariello RF, Lindsey CY, Dinterman RE, Schokman RD, Smith LA. Improved formulation of a recombinant ricin A-chain vaccine increases its stability and effective antigenicity. Vaccine 2007; 25:4149-58; PMID:17408819; http://dx.doi.org/10.1016/j.vaccine.2007.03.011
- McLain DE, Horn TL, Detrisac CJ, Lindsey CY, Smith LA. Progress in biological threat agent vaccine development: a repeat-dose toxicity study of a recombinant ricin toxin A-chain (rRTA) 1-33/44-198 vaccine (RVEc) in male and female New Zealand white rabbits. Int J Toxicol 2011; 30:143-52; PMID:21378370; http://dx.doi.org/10.1177/1091581810396730
- McLain DE, Lewis BS, Chapman JL, Wannemacher RW, Lindsey CY, Smith LA. Protective effect of two recombinant ricin subunit vaccines in the New Zealand white rabbit subjected to a lethal aerosolized ricin challenge: survival, immunological response, and histopathological findings. Toxicol Sci 2012; 126:72-83; PMID:21987460; http://dx.doi.org/10.1093/toxsci/kfr274
- Porter A, Phillips G, Smith L, Erwin-Cohen R, Tammariello R, Hale M, DaSilva L. Evaluation of a ricin vaccine candidate (RVEc) for human toxicity using an in vitro vascular leak assay. Toxicon 2011; 58:68-75; PMID:21616091; http://dx.doi.org/10.1016/j.toxicon.2011.05.005
- Marconescu PS, Smallshaw JE, Pop LM, Ruback SL, Vitetta ES. Intradermal administration of RiVax protects mice from mucosal and systemic ricin intoxication. Vaccine 2010; 28:5315-22; PMID:20562013; http://dx.doi.org/10.1016/j.vaccine.2010.05.045
- O'Hara JM, Brey RN 3rd, Mantis NJ. Comparative efficacy of two leading candidate ricin toxin a subunit vaccines in mice. Clin Vaccine Immunol 2013; 20:789-94; PMID:23515013; http://dx.doi.org/10.1128/CVI.00098-13
- Vance DJ, Greene CJ, Rong Y, Mandell LM, Connell TD, Mantis NJ. Comparative adjuvant effects of type II heat-labile enterotoxins in combination with two different candidate ricin toxin vaccine antigens. Clin Vaccine Immunol 2015; PMID:26491037; http://dx.doi.org/10.1128/CVI.00402-15
- Roy CJ, Brey RN, Mantis NJ, Mapes K, Pop IV, Pop LM, Ruback S, Killeen SZ, Doyle-Meyers L, Vinet-Oliphant HS, et al. Thermostable ricin vaccine protects rhesus macaques against aerosolized ricin: Epitope-specific neutralizing antibodies correlate with protection. Proc Natl Acad Sci U S A 2015; 112:3782-7; PMID:25775591; ttp://dx.doi.org/10.1073/pnas.1502585112
- Smallshaw JE, Richardson JA, Vitetta ES. RiVax, a recombinant ricin subunit vaccine, protects mice against ricin delivered by gavage or aerosol. Vaccine 2007; 25:7459-69; PMID:17875350; http://dx.doi.org/10.1016/j.vaccine.2007.08.018
- Peek LJ, Brey RN, Middaugh CR. A rapid, three-step process for the preformulation of a recombinant ricin toxin A-chain vaccine. J Pharm Sci 2007; 96:44-60; PMID:16998874; http://dx.doi.org/10.1002/jps.20675
- Hassett KJ, Cousins MC, Rabia LA, Chadwick CM, O'Hara JM, Nandi P, Brey RN, Mantis NJ, Carpenter JF, Randolph TW. Stabilization of a recombinant ricin toxin A subunit vaccine through lyophilization. Eur J Pharm Biopharm 2013; 85:279-86; PMID:23583494; http://dx.doi.org/10.1016/j.ejpb.2013.03.029
- Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis 2008; 47:401-9; PMID:18558875; http://dx.doi.org/10.1086/589862
- Plotkin SA. Complex correlates of protection after vaccination. Clin Infect Dis 2013; 56:1458-65; PMID:23386629; http://dx.doi.org/10.1093/cid/cit048
- Pincus SH, Bhaskaran M, Brey RN 3rd, Didier PJ, Doyle-Meyers LA, Roy CJ. Clinical and pathological findings associated with aerosol exposure of macaques to ricin toxin. Toxins (Basel) 2015; 7:2121-33; PMID:26067369; http://dx.doi.org/10.3390/toxins7062121
- David J, Wilkinson LJ, Griffiths GD. Inflammatory gene expression in response to sub-lethal ricin exposure in Balb/c mice. Toxicology 2009; 264:119-30; PMID:19682533; http://dx.doi.org/10.1016/j.tox.2009.08.003
- Wong J, Korcheva V, Jacoby DB, Magun B. Intrapulmonary delivery of ricin at high dosage triggers a systemic inflammatory response and glomerular damage. Am J Pathol 2007; 170:1497-510; PMID:17456757
- Foxwell BM, Detre SI, Donovan TA, Thorpe PE. The use of anti-ricin antibodies to protect mice intoxicated with ricin. Toxicology 1985; 34:79-88; PMID:3969682
- Neal LM, O'Hara J, Brey RN 3rd, Mantis NJ. A monoclonal immunoglobulin G antibody directed against an immunodominant linear epitope on the ricin A chain confers systemic and mucosal immunity to ricin. Infect Immun 2010; 78:552-61; PMID:19858297; http://dx.doi.org/10.1128/IAI.00796-09
- O'Hara JM, Mantis NJ. Neutralizing monoclonal antibodies against ricin's enzymatic subunit interfere with protein disulfide isomerase-mediated reduction of ricin holotoxin in vitro. J Immunol Methods 2013; 395:71-8; PMID:23774033; http://dx.doi.org/10.1016/j.jim.2013.06.004
- O'Hara JM, Neal LM, McCarthy EA, Kasten-Jolly JA, Brey RN 3rd, Mantis NJ. Folding domains within the ricin toxin A subunit as targets of protective antibodies. Vaccine 2010; 28:7035-46; PMID:20727394; http://dx.doi.org/10.1016/j.vaccine.2010.08.020
- O'Hara JM, Yermakova A, Mantis NJ. Immunity to ricin: fundamental insights into toxin-antibody interactions. Curr Top Microbiol Immunol 2012; 357:209-41; PMID:22113742; http://dx.doi.org/10.1007/82_2011_193
- Maddaloni M, Cooke C, Wilkinson R, Stout AV, Eng L, Pincus SH. Immunological characteristics associated with the protective efficacy of antibodies to ricin. J Immunol 2004; 172:6221-8; PMID:15128810; http://dx.doi.org/10.4049/jimmunol.172.10.622
- Pincus SH, Das A, Song K, Maresh GA, Corti M, Berry J. Role of Fc in antibody-mediated protection from ricin toxin. Toxins (Basel) 2014; 6:1512-25; PMID:24811206; http://dx.doi.org/10.3390/toxins6051512
- Song K, Mize RR, Marrero L, Corti M, Kirk JM, Pincus SH. Antibody to ricin a chain hinders intracellular routing of toxin and protects cells even after toxin has been internalized. PLoS One 2013; 8:e62417; PMID:23638075; http://dx.doi.org/10.1371/journal.pone.0062417
- Sully EK, Whaley KJ, Bohorova N, Bohorov O, Goodman C, Kim do H, Pauly MH, Velasco J, Hiatt E, Morton J, et al. Chimeric plantibody passively protects mice against aerosolized ricin challenge. Clin Vaccine Immunol 2014; 21:777-82; PMID:24574537; http://dx.doi.org/10.1128/CVI.00003-14
- Colombatti M, Johnson VG, Skopicki HA, Fendley B, Lewis MS, Youle RJ. Identification and characterization of a monoclonal antibody recognizing a galactose-binding domain of the toxin ricin. J Immunol 1987; 138:3339-44; PMID:2437188
- Colombatti M, Pezzini A, Colombatti A. Monoclonal antibodies against ricin: effects on toxin function. Hybridoma 1986; 5:9-19; PMID:3957360
- Lemley PV, Amanatides P, Wright DC. Identification and characterization of a monoclonal antibody that neutralizes ricin toxicity in vitro and in vivo. Hybridoma 1994; 13:417-21; PMID:7860097
- O'Hara JM, Kasten-Jolly JC, Reynolds CE, Mantis NJ. Localization of non-linear neutralizing B cell epitopes on ricin toxin's enzymatic subunit (RTA). Immunol Lett 2014; 158:7-13; PMID:24269767; http://dx.doi.org/10.1016/j.imlet.2013.11.009
- Lebeda FJ, Olson MA. Prediction of a conserved, neutralizing epitope in ribosome-inactivating proteins. Int J Biol Macromol 1999; 24:19-26; PMID:10077268
- Vance DJ, Mantis NJ. Resolution of two overlapping neutralizing B cell epitopes within a solvent exposed, immunodominant alpha-helix in ricin toxin's enzymatic subunit. Toxicon 2012; 60:874-7; PMID:22750533; http://dx.doi.org/10.1016/j.toxicon.2012.06.014
- Rudolph MJ, Vance DJ, Cheung J, Franklin MC, Burshteyn F, Cassidy MS, Gary EN, Herrera C, Shoemaker CB, Mantis NJ. Crystal structures of ricin toxin's enzymatic subunit (RTA) in complex with neutralizing and non-neutralizing single-chain antibodies. J Mol Biol 2014; 426:3057-68; PMID:24907552; http://dx.doi.org/10.1016/j.jmb.2014.05.026
- Li XP, Kahn PC, Kahn JN, Grela P, Tumer NE. Arginine residues on the opposite side of the active site stimulate the catalysis of ribosome depurination by ricin A chain by interacting with the P-protein stalk. J Biol Chem 2013; 288:30270-84; PMID:24003229; http://dx.doi.org/10.1074/jbc.M113.510966
- Castelletti D, Fracasso G, Righetti S, Tridente G, Schnell R, Engert A, Colombatti M. A dominant linear B-cell epitope of ricin A-chain is the target of a neutralizing antibody response in Hodgkin's lymphoma patients treated with an anti-CD25 immunotoxin. Clin Exp Immunol 2004; 136:365-72; PMID:15086403
- Yermakova A, Klokk TI, Cole R, Sandvig K, Mantis NJ. Antibody-mediated inhibition of ricin toxin retrograde transport. MBio 2014; 5:e00995; PMID:24713323; http://dx.doi.org/10.1128/mBio.00995-13
- Vitetta ES, Smallshaw JE, Coleman E, Jafri H, Foster C, Munford R, Schindler J. A pilot clinical trial of a recombinant ricin vaccine in normal humans. Proc Natl Acad Sci U S A 2006; 103:2268-73; PMID:16461456; http://dx.doi.org/10.1073/pnas.0510893103
- Vitetta ES, Smallshaw JE, Schindler J. Pilot phase IB clinical trial of an alhydrogel-adsorbed recombinant ricin vaccine. Clin Vaccine Immunol 2012; 19:1697-9; PMID:22914366; http://dx.doi.org/10.1128/CVI.00381-12
- Pittman PR, Reisler RB, Lindsey CY, Güereña F, Rivard R, Clizbe DP, Chambers M, Norris S, Smith LA. Safety and immunogenicity of ricin vaccine, RVEcTM, in a Phase 1 clinical trial. Vaccine 2015; 33:7299-7306; PMID:26546259; http://dx.doi.org/10.1016/j.vaccine.2015.10.094
