ABSTRACT
We reviewed intervention studies designed to increase human papillomavirus (HPV) vaccination coverage to further understand the impact interventions can have on HPV vaccination coverage. We searched 5 databases for intervention studies published from June 2006 to May 2015. Studies were included if they quantitatively measured HPV vaccination coverage as an outcome and were conducted in the United States. We abstracted outcomes, methods, and results from each study and classified by type of intervention conducted. Findings from 34 studies suggest many types of intervention strategies can increase HPV vaccination coverage in different settings, and with modest cost. Interventions were effective especially when implemented in combination at both provider and community levels. However, not all interventions showed significant effects on coverage. More research is needed to identify the best methods for widespread implementation of effective strategies.
Introduction
Human papillomavirus (HPV) is the most common sexually transmitted infection among men and women in the United States.Citation1 Approximately 79 million Americans are infected with HPV, and every year, an estimated 14 million people become newly infected.Citation1 There are more than 100 types of HPV, and though not all lead to disease, some types of HPV can cause genital warts and others cause cancer, including cervical, vulvar, vaginal, penile, anal, and oropharyngeal cancers.Citation1,2 In the United States, approximately 27,000 men and women develop new HPV associated cancers each year.Citation3 Approximately 360,000 people develop genital warts each year.Citation1
HPV vaccination is a means of primary prevention against HPV associated cancers and diseases. There are 3 HPV vaccines licensed for use in the United States. The quadrivalent vaccine (4vHPV) was licensed by the Food and Drug Administration (FDA) in 2006,Citation4 the bivalent vaccine (2vHPV) was licensed in 2009,Citation5 and the 9-valent vaccine (9vHPV) was licensed in 2014.Citation6 The 4vHPV and 2vHPV vaccines protect against virus types 16 and 18, which cause an estimated 66% of cervical cancers, and the 4vHPV vaccine also protects against types 6 and 11, which cause about 90% of genital warts and recurrent respiratory papillomatosis.Citation4-7 The 9vHPV vaccine protects against types 6, 11, 16, 18, 31, 33, 45, 52, and 58 and has the potential to prevent approximately 81% of cervical cancers.Citation2 Clinical trial data have shown that the vaccines are safe and effective, and post-licensure studies indicate that vaccination dramatically reduces the incidence and prevalence of HPV, genital warts, and cervical and anal dysplasias.Citation8-13 All three HPV vaccines that are licensed for use in the United States are recommended by the Advisory Committee on Immunization Practices (ACIP).Citation6
Though safe and effective HPV vaccines have been available since 2006, HPV vaccination coverage in the United States remains low.Citation14 The Healthy People 2020 target for completion of the three-dose HPV vaccination series is 80% for adolescent boys and girls aged 13 to 15 years;Citation15 however, current coverage estimates fall considerably short of this goal. In 2014, only 60.0% of girls aged 13 to 17 years had initiated the series with at least one HPV vaccine dose, and 39.7% had received three doses.Citation14 Coverage for adolescent boys in 2014 was even lower, with only 41.7% of boys aged 13 to 17 years receiving at least one dose and 21.6% receiving three doses.Citation14 Though 2014 estimates demonstrate significant increases from previous years' assessments of HPV vaccination coverage, given comparatively low coverage and slow uptake, it is critical for healthcare providers and public health organizations to increase efforts to improve HPV vaccination coverage and reduce the burden of HPV associated cancers and diseases.Citation14,16
Previously published systematic reviews have compiled some of the available evidence for intervention strategies to increase HPV vaccination coverage, but no comprehensive review has been published to date. Many HPV vaccination systematic reviews have only addressed factors associated with HPV vaccination, such as demographics or perceived barriers, or have reviewed interventions that target intermediate outcomes, such as vaccination knowledge or intention to vaccinate.Citation17-21 One recently published review summarized 14 interventions with HPV vaccination coverage as a study outcome and concluded that most of the interventions reviewed significantly increased HPV vaccination coverage, in contrast to findings from a previous review of educational interventions.Citation21,22 This recent review was an important contribution to the literature on HPV vaccination interventions; however, it only examined non-individual level system or community-based interventions.Citation22
To help healthcare professionals continue to evaluate the entire spectrum of published interventions to improve HPV vaccination coverage, we conducted a comprehensive review of the literature that falls within the Community Guide's categories of interventions to increase appropriate vaccination ().Citation23 The Community Guide is a centralized resource developed by an independent Task Force, the Centers for Disease Control and Prevention (CDC), and other partner organizations.Citation24 Collaborators aggregate and systematically review literature about public health interventions.Citation24 As it relates to vaccination, the Community Guide broadly reviews and evaluates interventions that can increase general vaccination coverage, but the Guide does not present information for specific vaccines or account for the unique challenges that adolescent vaccines such as the HPV vaccine face.Citation23 The present review offers a look at the intervention literature related to HPV vaccination in this framework to understand where strategies categorized by the Community Guide may or may not be promising in the context of HPV vaccination.
Results
Intervention study characteristics
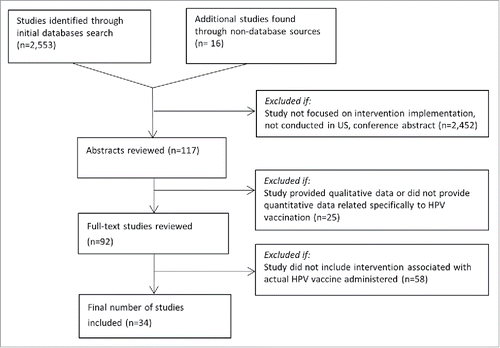
Two thousand five hundred and sixty nine studies were identified in the primary search, resulting in 34 studies eligible for inclusion in the review (). illustrates the characteristics of the studies included for review. A majority of the studies focused exclusively on girls (n = 24, 70.6%) Citation25-32,36-40,42,44-48,50,55-58 and were implemented prior to the 2011 ACIP recommendation that boys receive HPV vaccination (n = 22, 64.7%).Citation25-27,29-32,36-40,42,44,46-48,50,55-58 Studies addressed vaccination for many different age groups, including adolescents and young adults. Sixteen studies (47.1%) assessed intervention effects on more than one vaccine in the adolescent platform, including HPV vaccine,Citation25-27,31-34,38,42,45,46,50,52,53,55,56 while eighteen (52.9%) assessed intervention impact only on HPV vaccination coverage.Citation28-30,35-37,39-41,43,44,47-49,51,54,57,58
Table 1. Intervention categories (n = 34).
Table 2. Study characteristics (n = 34).
Intervention study results
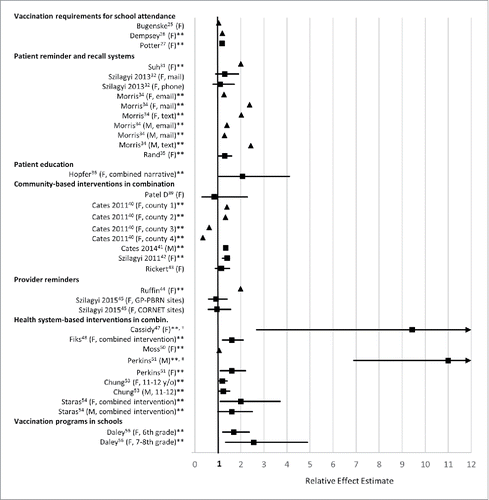
A forest plot of selected results of intervention studies measuring series initiation (≥1 dose of HPV vaccine) is shown in . A forest plot with additional results can be seen in Figure S1. presents the study design, methods, outcomes, and selected results for all of the studies included in the systematic review.
Figure 2. Forest plot of selected HPV vaccination intervention results: series initiation. ▪ : Effect estimates from original source. ▴ : Effect estimates calculated by review authors based on given data in source. *Results presented if study included a measure of series initiation (one or more doses of HPV vaccine). Results were excluded if study did not include a measure of series initiation or if results were not presented in the original paper such that an effect size could be calculated. If studies measured series initiation in more than one population or using different methods, the specifics are indicated in parentheses following the study author's name. F denotes studies that focused on females and M denotes studies that focused on males. Full study characteristics and results can be found in Table 3. **Results were determined to be significant by the original paper. †Effect estimate's confidence intervals could not fit in the plot scale. Cassidy et al. found an OR of 9.43 (2.69–33.10) for series initiation. ‡Effect estimate's confidence intervals could not fit in the plot scale. Perkins et al. found an OR of 11 (6.9–17) for series initiation among males.
Interventions to increase community demand for HPV vaccination
Nineteen studies (55.9%) utilized interventions to increase community demand for HPV vaccination.Citation25-43 Three studies examined the effect of vaccination requirements for school attendance on HPV vaccination coverage.Citation25-27 All three studies were ecological studies examining how vaccination policies for school attendance affected series initiation as a primary outcome.Citation25-27 Vaccination requirements consisted of educational requirements for parents, requirements for HPV vaccination for school attendance, or requirements for another adolescent vaccination for school attendance; however, requirements for school attendance typically had broad opt-out provisions, with waivers available for religious, medical, and/or philosophical objections.Citation25-27 Findings from the school vaccination policy studies consistently showed that while school requirements increased other adolescent vaccinations' coverage, HPV vaccination coverage only increased by a small amount or not at all.Citation25-27 One of the studies demonstrated that in the five states and the District of Columbia that had school entry or educational requirements for HPV vaccination in the 2008–2009 school year, no significant difference was found in 2008–2009 NIS-Teen HPV vaccination coverage for jurisdictions that had requirements vs. those that did not, though requirements for other adolescent vaccinations resulted in significant increases in coverage for those vaccines.Citation25 The two other school policy studies examined whether other adolescent vaccination requirements had any spillover effects on HPV vaccine series initiation.Citation26,27 One study looked at tetanus-diphtheria-acellular pertussis (Tdap) vaccination requirements enacted during or before the 2009–2010 school year and found that a greater, though still small, percentage of females had initiated the HPV vaccination series in states that had Tdap vaccination mandates (percentage point difference: 4.4, p = .004).Citation26 The other study observed the effect that mandates enacted in Michigan in 2010 for Tdap, meningococcal, and varicella vaccination had on HPV vaccination coverage in two cohorts of 6th graders; one cohort of 6th graders in 2009, the other 6th graders in 2010.Citation27 The study found small significant increases in HPV series initiation by age 13 for girls (hazard ratio [HR] 1.18, p < .001), especially if the HPV vaccine was co-administered at the first adolescent visit (HR 1.22, p < .001).Citation27 However, proportionally the increases were much smaller compared to the coverage increases for the other adolescent vaccines; HPV coverage increased approximately 5 percentage points, while Tdap, meningococcal, and varicella coverage increased approximately 20 percentage points.Citation27
Table 3. HPV vaccination intervention study summaries (n = 34). OR: odds ratio, AOR: adjusted odds ratio, RR: risk ratio, ARR: adjusted risk ratio, HR: hazard ratio. Confidence intervals are presented for ratios only if a p value was not presented in the original paper.
Eight studies examined patient reminder and recall systems to increase community demand for HPV vaccination.Citation28-35 Patient reminder and recall systems utilized many methods of reminding or recalling patients; one study used letters,Citation28 one used letters or phone calls,Citation32 two used text messages,Citation29,35 two provided multiple types of reminders,Citation31,33 and two allowed a choice (such as telephone call, letter, text message, Facebook message, or email).Citation30,34 The eight studies addressed a variety of measures, including series initiation, series completion, and receipt of next vaccine dose. The mailed letter intervention found significantly greater HPV series completion in the entire study age range intervention group (percentage point difference: 9.8, p < .01).Citation28 Another study comparing a mailed letter intervention to a telephone reminder intervention found significantly greater dose 2 and series completion in both intervention groups; the mailed letter intervention resulted in an 8 percentage point increase in dose 2 coverage (HR 1.5, p < .05), and the telephone reminder intervention resulted in an 8 percentage point increase in dose 2 (HR 1.6, p < .01) and a 5 percentage point increase in series completion (HR 1.5, p < .05).Citation32 Not all primary measures were found to be significant in this study, however. Series initiation did not differ significantly between intervention and control groups.Citation32
The studies assessing text message reminders showed increases in coverage, with significantly greater on-time receipt of next HPV vaccine dose in an intervention group against two different control groups (adjusted odds ratio [AOR] 2.03 and AOR 1.83, p = .002 and .003) in one studyCitation29 and small differences in series initiation between an intervention and control group (percentage point difference: 3.0; HR 1.3, p = .04) in another study.Citation35 Studies including several types of reminder methods also demonstrated increases in coverage. One used a messaging cascade of multiple types of reminders and found that 22.9% of due or overdue patients received their next dose of HPV, and also showed that this cascade method was most effective at encouraging series completion (p < .0001).Citation33 Another intervention implemented more than one reminder method by using mailed letters and telephone reminders, and found significantly greater HPV series initiation and completion from zero baseline doses in the intervention group (percentage point difference in series initiation: 11.2, p < .05; percentage point difference in completion from zero baseline doses: 7.3, p < .05).Citation31 However, up-to-date series completion from ≥1 baseline doses did not differ significantly between the intervention and control group.Citation31 Studies allowing patient choice of reminder method had mixed results, with one intervention not showing any significant difference in series completion,Citation30 while another demonstrated a range of significant percent increases in initiation and completion (e-mail, mail, text, and telephone call, all p < .05).Citation34
Two randomized controlled trials in females aged 18–26 years utilized education using video technology to deliver messages about HPV vaccination.Citation36,37 One study assessed the effectiveness of a variety of video narrators on series initiation, and found significant greater series initiation in the intervention group that had both a provider expert and peer narrator in the video (percentage point difference: 11, p = .035; odds ratio [OR] 2.07, p = .036).Citation36 The other study looked at how a video-based intervention impacted series completion, and found significantly greater series completion for the group that viewed a short theory-driven video presentation (percentage point difference: 11.5, p = .03; AOR 2.44, p = .001).Citation37
The final six “increasing community demand” interventions utilized multiple intervention strategies in combination with each other.Citation38-43 One prospective cohort intervention implemented reminder and recall in a school-located vaccination program.Citation38 The results demonstrated 59% of participants getting any dose of HPV vaccine, but only 25% received dose 2 and 0% completed the full series.Citation38 Two other interventions aimed to increase HPV vaccination among adolescent females and males respectively, utilizing broad, multi-faceted public awareness campaigns targeted to each audience in conjunction with education for healthcare providers.Citation40,41 One of these interventions targeted females using a social marketing campaign, and though significant differences were seen between all intervention counties and each control group (all, p < .01), the 2 counties with higher initiation were only between 1.6 and 2.0 percentage points greater than the controls, and 2 counties were found to have significantly lower initiation rates.Citation40 The other social marketing campaign targeted adolescent males, and while those in the intervention group were more likely to initiate the HPV vaccination series (percentage point difference: 2.1; HR 1.34, p = .002), this was again a small increase, and results were not sustained at a 6 month follow up assessment.Citation41
Another intervention using multiple methods utilized immunization navigators trained to track immunization records, lead reminder and recall efforts, and conduct home visits.Citation42 This intervention resulted in significant positive changes from baseline in series initiation (adjusted risk ratio [ARR] 1.4, p < .001), dose 2 receipt (ARR 1.4, p < .001), and series completion (ARR 1.5, p < .001).Citation42 The final two studies in this category assessed educational vaccination messaging in a school-located clinicCitation43 and a reminder and recall combined with patient education intervention,Citation39 but found no significant effects on measures of HPV vaccination coverage.Citation39,43
Provider or healthcare system based interventions to increase HPV vaccination
Twelve studies (35.3%) were identified as provider or healthcare system-based interventions.Citation44-55 Three studies assessed single method interventions targeted at providers: provider assessment and feedbackCitation46 and provider prompts.Citation44,45 The intervention assessing provider assessment and feedback utilized CDC's AFIX (Assessment, Feedback, Incentives, and eXchange) program and targeted it specifically to HPV vaccination.Citation46 The randomized controlled trial had two intervention groups—one in-person, one web-based—and both found small but significant increases in coverage among 11–12 year olds at 5 months post-intervention (1.5% increase in initiation with in-person AFIX (p = .02) and 1.9% increase with web-based AFIX (p < .01)), but not for 13–18 year olds at 5 months, and results were not sustained for any group at 1 year post-intervention.Citation46 Another study implementing electronic health record-generated provider prompts found significantly greater series initiation in the intervention group (percentage point difference: 13.7, p < .001) and significantly greater timely series completion (point estimates not presented; p < .001).Citation44 However, another randomized controlled trial assessing the use of provider prompts via electronic health record or nurses/staff found no significant effect on series initiation, dose 2, or series completion, despite strong acceptance of the intervention among providers.Citation45
The majority of provider-targeted interventions utilized multiple intervention methods in combination with each other.Citation47-55 Many assessed interventions in more than one primary care practice,Citation48,53 in federally qualified health centers (FQHC),Citation50,51 or in large hospital clinic settings.Citation52,55 Three were focused primarily at the provider level.Citation50,52,55 One intervention presented webinars for FQHC providers based on the CDC's AFIX program principles, with follow-up weekly educational emails for the providers.Citation50 The results found significant, but small increases of 1 to 1.6 percentage points in HPV series initiation (p = .029), dose 2 (p = .001), and series completion (p = .001).Citation50 Another intervention created individualized vaccine catch-up schedules for hospitalized children not up-to-date for HPV vaccination, and increased series initiation in this group by 20.6% (p = not reported),Citation52 while another using provider prompts, clinical decision support, and individualized provider feedback found no significant effect of the intervention on up-to-date status of the participants or timeliness of vaccine receipt.Citation55
The remaining multiple method provider-based interventions implemented complex interventions targeting both provider behavior and community demand.Citation47-49,51,53,54 The first examined the effects of a family-focused intervention consisting of patient education and reminder and recall, and a provider intervention consisting of provider education, assessment and feedback, and provider reminders.Citation48 Both intervention strategies implemented together found the largest increases in HPV vaccination coverage across all measures, compared to when each strategy was implemented separately (series initiation: HR 1.6, p = .001; dose 2: HR 1.3, p = .008; series completion: HR 1.5, p < .001).Citation48 Two other trials first implemented provider education, followed by supplemental interventions such as school-based telephone reminder and recallCitation53 or targeted interventions for each individual practice based on mutually identified barriers to HPV vaccination.Citation51 The intervention outcomes for both studies showed increases in coverage, indicating that series initiation was significantly more likely for females (adjusted odds ratio [AOR] 1.19, p = significant, but not reported;Citation53 OR 1.6, p < .001Citation51) and males (AOR 1.23, p = significant, but not reported;Citation53 OR 11, p < .001Citation51), and next dose receipt was also significantly more likely for females (OR 1.4, p < .05)Citation51 and males (OR 23, p < .05).Citation51 Many of the increases persisted for outcomes measured 6 months post-intervention, except the series initiation results for females.Citation51 Another study demonstrated that patient reminder and recall combined with an in-clinic education and health information system summarizing patient intention data for providers resulted overall in 5% increases in HPV vaccination series initiation, with slightly greater increases for boys (p = significant, but not reported).Citation54
The two studies that observed the largest percentage point increases in HPV vaccination coverage in any of the intervention studies reviewed also had the smallest sample sizes, which is important to note.Citation47,49 These small observational studies took place in single pediatric practice settings and utilized a combination of patient reminder and recall, patient educational materials, provider education, and provider protocols. Large, significant differences were seen in outcomes between intervention and control groups in both studies.Citation47,49 One study demonstrated that series initiation was greater by 50.9 percentage points (p = .001; OR 9.43, p = significant, but not reported) and series completion was greater by 55.6 percentage points (p < .001; OR 22.5, p = significant, but not reported).Citation47 The other study showed that dose 2 completion was greater by 40 percentage points compared to a non-enrolled control group (p < .001) and 46 percentage points compared to a non-intervention group (p < .001), and series completion was greater by 16 percentage points compared to a non-enrolled group (p = .008) and 11 percentage points compared a non-intervention group (p = .018).Citation49
Interventions to enhance access to HPV vaccination services
Three intervention studies (8.8%) were designed to primarily enhance access to HPV vaccination services.Citation56-58 Two of these studies aimed to increase access by implementing vaccination programs located in schools.Citation56,57 The first study found significant increases in initiation in both a 6th grade (percentage point difference: 16; ARR 1.69, p = significant, but not reported) and 7–8th grade population (percentage point difference: 13; ARR 2.56, p = significant, but not reported).Citation56 The results of the second study found minimal overall participation in the intervention, with only 2% of the population receiving any dose, though there was high series completion (80%) among those who initiated the series.Citation57 Students at host-school sites compared to satellite sites were also significantly more likely to have received any dose of the HPV vaccine (OR 6.56, p < .05).Citation57
The final study provided grants to reduce out-of-pocket costs to patients associated with receiving the HPV vaccine, but results did not indicate significant differences in number of doses received or on-time completion between those who received grant sponsored doses and those who did not.Citation58
Results from economic analyses
Ten studies assessed the economic effects of implementing HPV interventions.Citation31-34,38,42,46,48,56,57 presents summaries of the economic findings of these studies. Many of these studies implemented reminder and recall interventions.Citation31-34,38,42 One study found that the overall operating costs for implementing mail and telephone reminder and recall ranged from $1,087 to $1,349, with three of the practices demonstrating positive net additional revenues after the study period and one practice demonstrating a net revenue loss, calculated by subtracting total operating costs from total additional revenues for each practice.Citation31 Another mail or telephone reminder and recall intervention demonstrated average costs of $18.78 for mail and/or $16.68 for telephone calls per child per year, with the cost per additional child fully vaccinated at $324.75 for the mail group and $487.03 for the telephone group.Citation32 Additional reminder and recall interventions ranged from between $1.25 and $1.50 for each mailed postcard and < $0.10–$0.80 for each text message/e-mail/phone message.Citation33,34 Reminder and recall costs in a school-based health center setting ranged from $1.12 to $6.87 per child immunized.Citation38 The cost of a full “immunization navigator” program including reminder and recall was found to be $45.74 per child per year, with the cost per additional child fully vaccinated at $465.Citation42
Table 4. Selected results from economic analyses (n = 10).
Costs of other interventions implemented in school-located clinics and with providers/healthcare systems were also evaluated.Citation46,48,56,57 One school-located vaccination clinic found a cost of $23.98 per vaccine administered, not counting the cost to purchase the vaccine.Citation56 The other school-located vaccination clinic intervention used only 36% of their original budget, for a total operating cost of $135,397.44.Citation57 The largest percentage of the expenses went to personnel costs (67%).Citation57 In a study focusing on provider and family interventions, the cost per additional dose delivered for the most effective intervention for increasing series initiation (provider-focused) was $6; for increasing dose 2 and series completion, the most effective intervention (family-focused) was $10 for dose 2 and $6 for dose 3 per additional dose delivered; and for the most effective intervention overall (combined provider and family-focused), the cost was $24 for dose 1, $42 for dose 2, and $189 for dose 3.Citation48 Finally, AFIX webinars for providers showed promise in reducing travel costs; the cost of delivering the intervention was $152 per clinic for in-person consultations, and $100 per clinic for webinar consultations.Citation46
Discussion
Of the 34 HPV vaccination intervention studies identified, the majority of the studies were designed to increase community demand for HPV vaccination, but a substantial number of studies also targeted providers and healthcare systems. Only a few studies reviewed addressed access to vaccination services. Overall, while most of the intervention methods identified in the literature were based on evidence-based vaccination intervention categories recommended by the Community Guide,Citation23 there was considerable variation in the efficacy of these interventions in the context of HPV vaccination. A substantial number of studies did show significant increases in HPV vaccination coverage following the intervention. The only Community Guide category of intervention that we reviewed that is not currently recommended by the Community Guide is patient education when used alone, due to insufficient evidence.Citation23 The studies reviewed here should be examined both in context with their methods and with overall Healthy People 2020 targets, which will require large increases in coverage, to understand the potential impact that these reviewed interventions may have on HPV vaccination coverage.
The single-method intervention strategies that frequently produced statistically significant increases in HPV vaccination coverage were reminder and recallCitation28-35 and patient education.Citation36,37 Reminder and recall interventions were the most common type of intervention reviewed. Reminder and recall intervention methods ranged from improving series initiation with a reminder message that a child is due to start the HPV vaccine series, to recall efforts to get children overdue for dose 2 or 3 to get caught up and complete the series. Regardless of type of reminder or recall utilized (text, mail, phone, e-mail, etc.), many interventions resulted in a range of increases in a variety of measures of HPV vaccination coverage,Citation28,29,31-35 though not all resulted in increases.Citation30 The Community Guide does not recommend patient education when used alone as a strategy to increase vaccination coverage due to insufficient evidence;Citation23 however, the two patient education studies reviewed here demonstrate some potential promise of this type of intervention as it relates to HPV vaccination.Citation36,37 The interventions solely implementing patient education both used video as a means of delivering information about the HPV vaccine, but these interventions were directed at young adults aged 18–26 years, not the target age group for routine vaccination in the United States. Both trials demonstrated significant differences in coverage compared to non-intervention groups, though their sample sizes were moderate.Citation36,37
Provider assessment and feedback, when used alone, produced significant but small increases in HPV vaccination initiation, though these changes were not sustained one year after the intervention period.Citation46 Again, in considering the findings of these studies, it is important to keep in context that large increases in coverage are needed to reach the Healthy People 2020 targets.
Numerous effective multi-component interventions were implemented in the communityCitation38-43 or in healthcare systems.Citation47-55 Methods varied across studies, though the most common strategy included in the multi-component interventions was some type of reminder and recall system. These efforts were frequently accompanied by education of parents and/or providers, enhanced practice-based IT systems, and/or provider incentives. The studies took place in a variety of settings and there was a wide range in the number of participants. Many of the largest increases in coverage were seen as a result of these multiple strategy interventions, particularly those that involved a provider-targeted strategy. While not all of the multi-component interventions found large or significant increases, many of the studies reviewed demonstrated that implementing multiple strategies to address barriers to vaccination can be effective in increasing HPV vaccination coverage.
The intervention methods that had inconsistent support for their effectiveness or did not find significant findings were school-located vaccination services, provider reminders used alone, vaccination requirements for school attendance, and programs to reduce out of pocket costs. Vaccination programs in schools and provider reminders used alone are both evidence-based strategies cited by the Community Guide's recommendations,Citation23 but results presented in the studies focused on HPV vaccination were inconsistent.Citation44,45,56,57 Both categories had one study that had significant resultsCitation44,56 and one that did not have significant results.Citation45,57 Vaccination requirements for school attendance have been shown to be effective in increasing vaccination coverage for other vaccines,Citation23 but no difference was seen in series initiation with a middle school entry requirement for HPV vaccination.Citation25 It is important to note that requirements for HPV vaccination for school attendance have broad opt-out provisions, with waivers available for religious, medical, and/or philosophical objections. There did appear to be small spill-over effects to HPV vaccination from other adolescent vaccination requirements,Citation26,27 though small spill-over effects may not help HPV vaccination coverage increase by the amount needed to reach the Healthy People 2020 target of 80% coverage. Programs to reduce out of pocket costs similarly are cited to increase vaccination per the Community Guide's recommendations,Citation23 but for the study examining HPV vaccination specifically, the strategy did not significantly influence coverage.Citation58 For those strategies with inconsistent evidence, more research may be needed to better understand the influence of those strategies on HPV vaccination coverage. For those strategies that did not demonstrate changes in coverage, many potential barriers to HPV vaccination have been identified in the literature,Citation20,21 and it may be that these particular intervention strategies do not address an adequate number of barriers to see a consistent change in vaccination delivery. Beyond addressing an adequate number of barriers, additional baseline differences in study conditions such as study design or community context could have affected study outcomes.
Some interventions by design may be more resource-intensive than others, but many studies' economic analyses demonstrated that interventions could be implemented with modest cost.Citation31-34,38,42,46,48,56,57,59 Due to variations in measures of cost, it was difficult to directly compare economic measures across the studies. However, the data from the studies reviewed generally found that implementing an HPV vaccination intervention is not cost-prohibitive.
Though HPV vaccines have been available for more than 9 years, HPV vaccination intervention research specifically focused on increasing coverage is just emerging. The studies reviewed here indicate that many types of interventions may be promising. With this knowledge in mind, an important next step for researchers and practitioners to consider is how to translate evidence into practice. Many of the interventions reviewed here are targeted to specific populations, so additional efforts are needed to study how intervention models can be implemented on a wide scale and adapted to different settings. Additional research is needed to examine how strategies can be customized and commonly integrated into practice.
In addition to this, there is also a need to examine more closely the effects of interventions to improve provider communication and recommendations for vaccination. The literature shows that though provider recommendations for HPV vaccination are highly correlated with higher coverage, many providers do not routinely recommend HPV vaccination.Citation60-62 Though this fact is demonstrated strongly in the literature, few of the studies reviewed in this paper aimed to strengthen the provider recommendation through interventions. As this is one of the most highly correlated factors in the literature with higher HPV vaccination coverage, this suggests that there is a need for more intervention research in this area.
Another important takeaway from this review is the need for continued aggregation of evidence resulting from vaccination intervention research, especially as it relates to specific vaccinations. Given that barriers to HPV vaccination are often unique and increasing coverage may require different approaches, intervention research specific to HPV vaccination must be reviewed in context of other vaccination intervention research. Having more specificity in recommendations from the Community Guide regarding individual vaccinations and populations would be helpful. As more HPV vaccination intervention research continues to be published, reviews such as these should continue to be updated to highlight trends of what is working in the field.
Of the studies identified for inclusion in this review, there was a high amount of variability between intervention strategies, populations, and the measures used. This review demonstrates a clear need for more studies with population level samples, comparison groups, and randomized designs to help indicate which interventions may be most effective in increasing coverage. There is also a strong need to implement and evaluate HPV vaccination interventions that include coverage of males as an outcome. Finally, as several of the studies reviewed indicated that strategies implemented in combination show promise in their ability to increase vaccination coverage, particularly when the intervention includes a provider-focused element, more research should be conducted to assess these types of multi-component interventions.
One major limitation of this systematic review was that many studies had limited generalizability in their results, as many had small sample sizes or were observational studies. Without a comparison group in several of the studies, it is difficult to state conclusively whether the intervention was truly responsible for changes seen. Additionally, each study measured HPV vaccine uptake differently. This made it difficult to evaluate and compare effectiveness across studies, and precluded meta-analysis. It is important, when evaluating the impact of each intervention, to consult the original studies for a deeper understanding of study design and measures. Another limitation is that the ACIP recommendations only extended to include boys in 2011, which is a likely factor in the limited number of interventions found in the literature that targeted boys. This dearth in the literature impacts the knowledge about interventions to increase HPV vaccination coverage in boys. A final limitation is that interventions conducted outside of the United States were not included in this review. The decision to exclude studies conducted in international settings was based on differences in healthcare systems and school-based vaccination policies compared to the United States, but successful interventions could have been evaluated and then assessed for feasibility or adaption to United States settings. This decision could also be seen as a strength of this review, however, as differences in context may render comparisons invalid.
Conclusion
This review presented a thorough examination of the literature from licensure of the first HPV vaccine to the present, making information on HPV vaccination interventions easily accessible for practitioners working on this priority public health issue. Variable study populations and outcome measures precluded meta-analysis, but the literature overall indicates that there are several strategies that may be promising. While single strategies can be effective, interventions may be most successful when implemented in combination with each other to address multiple barriers at community and provider levels.
Most importantly, because the evidence suggests that several of the interventions reviewed here may be effective, more research is needed to translate the evidence into comprehensive strategies that can be implemented in a variety of settings. Additional efforts are needed to study how strategies can be adapted and integrated into practice on a wide scale.
Methods
Search strategy and selection criteria
Two authors searched PubMed, Web of Science, Wiley Online Library, Cumulative Index to Nursing and Allied Health Literature, and Google Scholar databases to capture a comprehensive list of peer-reviewed studies published between June 2006 and May 2015. Relevant MeSH (Medical Subject Headings) search term keywords were entered in each database (HPV, human papillomavirus, adolescent, vaccine or vaccination, immunization, intervention, coverage), and the search was limited to English language and peer-reviewed literature.
Studies were eligible for inclusion if they fulfilled the following criteria: interventions were designed to measure increases in HPV vaccination coverage as an outcome, provided quantitative data for HPV vaccination coverage specifically, and were conducted in the United States. Both randomized controlled trials and observational studies were eligible. Intervention studies that only provided qualitative results, were only published as conference abstracts, or only assessed HPV knowledge, attitudes, or intention to get vaccinated, were excluded. After review of article titles and abstracts, relevant full-text articles were obtained and independently reviewed by two authors to verify that they met the criteria. Finally, references from systematic reviewsCitation17-22 as well as references from the studies identified were also assessed for inclusion in the review. Discrepancies related to inclusion criteria were resolved through discussions among all three authors.
Data extraction
Following the final round of selection of studies, two authors independently extracted information about intervention methods, study design, outcomes, participants, and results. Though outcome measures varied largely across studies, the principal summary measures extracted were percentage changes in coverage, risk ratios, hazard ratios, and/or odds ratios pertaining to receipt of dose 1, 2, and/or 3 of the series, up-to-date status, and timeliness of receipt. If study results were presented as percentage differences between intervention and control groups, one author calculated percentage point changes between groups to provide a common metric for comparison. If studies examined any economic dimensions of intervention implementation, this information was also extracted. The independent abstractions were compared and discrepancies were resolved through discussion.
Based on a review of each study's intervention methods, each study was classified in an intervention category to group similar types of interventions together for in-depth review. Intervention categories were adapted from categories used by the Community Guide's section on Increasing Appropriate Vaccination and were adapted to specifically include only HPV vaccination.Citation23 The broad categories of interventions identified in the literature were “increasing community demand for HPV vaccination,” “provider- or system-based interventions to increase HPV vaccination,” and “enhancing access to HPV vaccination services.” Interventions were further classified within each category by intervention method implemented. Definitions for methodological categories can be found in . It is important to note that though the classification of interventions in this review is based on the Community Guide's categorizations, this review is not the same as the reviews conducted by the Community Guide. The review design did not allow for abstracting data in the same detail as the Community Guide, there was no quality of evidence analysis to put forth a recommendation or non-recommendation, and no separate economic evaluation of interventions beyond what intervention study authors put forth themselves was conducted. Additionally, a risk of bias assessment was not performed, as that was beyond the scope of this review.
Abbreviations
| CDC | = | Centers for Disease Control and Prevention |
| HPV | = | human papillomavirus |
| 4vHPV | = | quadrivalent HPV vaccine |
| 2vHPV | = | bivalent HPV vaccine |
| 9vHPV | = | 9-valent HPV vaccine |
| FDA | = | Food and Drug Administration |
| ACIP | = | Advisory Committee on Immunization Practices |
| MeSH | = | Medical Subject Headings |
| Tdap | = | tetanus-diphtheria-acellular-pertussis |
| HR | = | hazard ratio |
| OR | = | odds ratio |
| AOR | = | adjusted odds ratio |
| RR | = | risk ratio |
| ARR | = | adjusted risk ratio |
| AFIX | = | assessment, feedback, incentives, and eXchange |
| FQHC | = | federally qualified health centers |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental_Material.pdf
Download PDF (198.7 KB)Acknowledgments
This publication was supported by Cooperative Agreement Number 3U36OE000002 from the Centers for Disease Control and Prevention and the Association of Schools and Programs of Public Health. The findings and conclusions of this publication do not necessarily represent the official views of CDC or ASPPH. This publication was also supported by 2013 Prevention and Public Health Funds, Immunization Program Technical and Analytical Assistance in Support of HPV Vaccination, Contract #200-2009-28537 Task Order-091.
References
- Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MC, Su J, Xu F, Winstock H. Sexually transmitted infections among US women and men: prevalence and incidence estimates 2008. Sex Transm Dis 2013; 40(3):187-93; PMID:23403598
- Saraiya M, Unger ER, Thompson TD, Lynch CF, Hernandez BY, Lyu CW, Steinau M, Watson M, Wilkinson EJ, Hopenhayn C, et al. US assessment of HPV types in cancer: implications for current and 9-valent HPV vaccines. J Natl Cancer I 2015; 107(6):djv086
- Markowitz LE, Dunne EF, Saraiya M, Chesson H, Curtis CR, Gee J, Bocchini JA, Unger ER. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report (MMWR) 2014; 63(RR05):1-30
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report (MMWR) 2007; 56(RR02):1-24
- Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report (MMWR) 2010; 59(20):626-9
- Petrosky E, Bocchini JA, Hariri S, Chesson H, Curtis CR, Saraiya M, Unger ER, Markowitz LE. Use of 9-Valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report (MMWR) 2015; 64(11):300-4
- Lacey CJN, Lowndes CM, Shah KV. Chapter 4: burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 2006; 24(Suppl 3):S3/34-41
- Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine 2012; 30(Suppl 5):F123-38; PMID:23199956
- Markowitz LE, Hariri S, Lin C, Dunne EF, Steinau M, McQuillan G, Unger ER. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis 2013; 208:385-93; PMID:23785124
- Leval A, Herweijer E, Arnheim-Dahlstrom L, Walum H, Frans E, Sparén P, Simard JF. Incidence of genital warts in Sweden before and after quadrivalent human papillomavirus vaccine availability. J Infect Dis 2012; 206(6):860-6; PMID:22815381
- Read TR, Hocking JS, Chen MY, Donovan B, Bradshaw CS, Fairley CK. The near disappearance of genital warts in young women 4 years after commencing a national human papillomavirus (HPV) vaccination programme. Sex Transm Infect 2011; 87:544-7; PMID:21970896
- Brotherton JM, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet 2011; 377(9783):2085-92; PMID:21684381
- Powell SE, Hariri S, Steinau M, Bauer HM, Bennett NM, Bloch KC, Niccolai LM, Schaffer S, Unger ER, Markowitz LE. Impact of human papillomavirus (HPV) vaccination on HPV 16/18-related prevalence in precancerous cervical lesions. Vaccine 2012; 31(1):109-13; PMID:23137842
- Reagan-Steiner S, Yankey D, Jeyarajah J, Elam-Evans-LD, Singleton JA, Curtis CR, MacNeil J, Markowitz LE, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years: United States, 2014. Morbidity and Mortality Weekly Report (MMWR). 2015; 64(29):784-92
- Healthy People 2020 objectives: immunization and infectious diseases. Available at https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed May 30, 2015
- President's Cancer Panel. Accelerating HPV vaccine uptake: urgency for action to prevent cancer. A report to the President of the United States from the President's Cancer Panel. Bethesda, MD: National Cancer Institute. 2014. Available at http://deainfo.nci.nih.gov/advisory/pcp/annualReports/HPV/index.htm. Accessed May 30, 2015
- Fu L, Bonhomme L, Cooper S, Joseph J, Zimet G. Educational interventions to increase HPV vaccination acceptance: a systematic review. Vaccine 2014; 32:1901-20; PMID:24530401
- Brewer N, Fazekas K. Predictors of HPV vaccine acceptability: a theory-informed, systematic review. Prev Med 2007; 45:107-14; PMID:17628649
- Gamble H, Klosky J, Parra G, Randolph M. Factors influencing familial decision-making regarding human papillomavirus vaccination. J Pediatr Psych 2010; 35(7):704-15; http://dx.doi.org/10.1093/jpepsy/jsp108
- Holman D, Bernard V, Roland K, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr 2014; 168(1):76-82; PMID:24276343; http://dx.doi.org/10.1001/jamapediatrics.2013.2752
- Walhart T. Parents, adolescents, children and the human papillomavirus vaccine: a review. Int Nurs Rev 2012; 59:305-11; PMID:22897180; http://dx.doi.org/10.1111/j.1466-7657.2012.00991.x
- Niccolai L, Hansen C. Practice- and community-based interventions to increase human papillomavirus vaccine coverage: a systematic review. JAMA Pediatr 2015; 169(7):686-92; PMID:26010507; http://dx.doi.org/10.1001/jamapediatrics.2015.0310
- Community Preventive Services Task Force. The guide to community preventive services: increasing appropriate vaccination. Available at |www.thecommunityguide.org/vaccines/index.html. Last updated: February 13, 2015. Accessed May 30, 2015
- Community Preventive Services Task Force. What is the Community Guide? Available at www.thecommunityguide.org/about/index.html. Last updated: April 6, 2015. Accessed October 26, 2015
- Bugenske E, Stokley S, Kennedy A, Dorell C. Middle school vaccination requirements and adolescent vaccination coverage. Pediatrics 2012; 129:1056-63; PMID:22566425; http://dx.doi.org/10.1542/peds.2011-2641
- Dempsey A, Schaffer S. Human papillomavirus vaccination rates and state mandates for tetanus-containing vaccines. Prev Med 2011; 52(3–4):268-9; PMID:21195727
- Potter R, DeVita S, Vranesich P, Boulton M. Adolescent immunization coverage and implementation of new school requirements in Michigan, 2010. Am J Public Health 2014; 104(8):1526-33; PMID:24922144; http://dx.doi.org/10.2105/AJPH.2014.301910
- Chao C, Preciado M, Slezak J, Xu L. A randomized intervention of reminder letter for human papillomavirus vaccine series completion. J Adolescent Health 2015; 56:85-90; http://dx.doi.org/10.1016/j.jadohealth.2014.08.014
- Kharbanda E, Stockwell M, Fox H, Andres R, Lara M, Rickert V. Text message reminders to promote human papillomavirus vaccination. Vaccine 2011; 29:2537-41; PMID:21300094; http://dx.doi.org/10.1016/j.vaccine.2011.01.065
- Patel A, Stern L, Unger Z, Debevec E, Roston A, Hanover R, Morfesis J. Staying on track: a cluster randomized controlled trial of automated reminders aimed at increasing human papillomavirus vaccine completion. Vaccine 2014; 32: 2428-33; PMID:24631099; http://dx.doi.org/10.1016/j.vaccine.2014.02.095
- Suh CA, Saville A, Daley MF, Glazner JE, Barrow J, Stokley S, Dong F, Beaty B, Dickinson LM, Kempe A. Effectiveness and net cost of reminder/recall for adolescent immunizations. Pediatrics 2012; 129(6):1437-45; http://dx.doi.org/10.1542/peds.2011-1714
- Szilagyi PG, Albertin C, Humiston SG, Rand CM, Schaffer S, Brill H, Stankaitis J, Yoo, BK, Blumkin AK, Stokley S. A randomized trial of the effect of centralized reminder/recall on immunizations and preventive care visits for adolescents. Acad Pediatr 2013; 13(3):204-13; PMID:23510607; http://dx.doi.org/10.1016/j.acap.2013.01.002
- Bar-Shain D, Stager M, Runkle A, Leon J, Kaelber D. Direct messaging to parents/guardians to improve adolescent immunizations. J Adolescent Health 2015; 56:S21-26; http://dx.doi.org/10.1016/j.jadohealth.2014.11.023
- Morris J, Wang W, Wang L, Peddecord KM, Sawyer M. Comparison of reminder methods in selected adolescents with records in an immunization registry. J Adolescent Health 2015; 56:S27-32; http://dx.doi.org/10.1016/j.jadohealth.2015.01.010
- Rand CM, Brill H, Albertin C, Humiston SG, Schaffer S, Shone LP, Blumkin AK, Szilagyi PG. Effectiveness of centralized text message reminders on human papillomavirus coverage for publicly insured adolescents. J Adolescent Health 2015; 56:S17-20; http://dx.doi.org/10.1016/j.jadohealth.2014.10.273
- Hopfer S. Effects of a narrative HPV vaccine intervention aimed at reaching college women: a randomized controlled trial. Prev Sci 2012; 13:173-82; PMID:21993613; http://dx.doi.org/10.1007/s11121-011-0254-1
- Vanderpool RC, Cohen EL, Crosby RA, Jones MG, Bates, W, Casey BR, Collins T. “1-2-3 Pap” intervention improves HPV vaccine series completion among Appalachian women. J Commun 2013; 63:95-115; PMID:26560123; http://dx.doi.org/10.1111/jcom.12001
- Kempe A, Barrow J, Stokley S, Saville A, Glazner JE, Suh C, Federico S, Abrams L, Seewald L, Beaty B, et al. Effectiveness and cost of immunization recall at school-based health centers. Pediatrics 2012; 129:1446-52; http://dx.doi.org/10.1542/peds.2011-2921
- Patel D, Zochowski M, Peterman S, Dempsey A, Ernst S, Dalton V. Human papillomavirus vaccine intent and uptake among female college students. J Am Coll Health 2012; 60(2):151-61; PMID:22316412; http://dx.doi.org/10.1080/07448481.2011.580028
- Cates J, Shafer A, Diehl S, Deal A. Evaluating a county-sponsored social marketing campaign to increase mothers' initiation of HPV vaccine for their pre-teen daughters in a primary rural area. Soc Mar Q 2011; 17(1):4-26; PMID:21804767; http://dx.doi.org/10.1080/15245004.2010.546943
- Cates J, Diehl S, Crandell J, Coyne-Beasley T. Intervention effects from a social marketing campaign to promote HPV vaccination in preteen boys. Vaccine 2014; 32:4171-8; PMID:24886960; http://dx.doi.org/10.1016/j.vaccine.2014.05.044
- Szilagyi PG, Humiston SG, Gallivan S, Albertin C, Sandler M, Blumkin AK. Effectiveness of a citywide patient immunization navigator program on improving adolescent immunizations and preventive care visit rates. Arch Pediatr Adol Med 2011; 165(6):547-53; http://dx.doi.org/10.1001/archpediatrics.2011.73
- Rickert V, Auslander B, Cox D, Rosenthal S, Rupp R, Zimet G. School-based HPV immunization of young adolescents: effects of two brief health interventions. Hum Vaccin Immunother 2015; 11(2):315-21; PMID:25692717; http://dx.doi.org/10.1080/21645515.2014.1004022
- Ruffin MT, Plegue MA, Rockwell PG, Young AP, Patel DA, Yeazel MW. Impact of an electronic health record (EHR) reminder on human papillomavirus (HPV) vaccine initiation and timely completion. J Am Board Fam Med 2015; 28(3):324-33; PMID:25957365; http://dx.doi.org/10.3122/jabfm.2015.03.140082
- Szilagyi PG, Serwint JR, Humiston SG, Rand CM, Schaffer S, Vincelli P, Dhepyasuwan N, Blumkin A, Albertin C, Curtis CR. Effect of provider prompts on adolescent immunization rates: a randomized trial. Acad Pediatr 2015; 15:149-57; PMID:25748976; http://dx.doi.org/10.1016/j.acap.2014.10.006
- Gilkey MB, Dayton AM, Moss JL, Sparks AC, Grimshaw AH, Bowling JM, Brewer NT. Increasing provision of adolescent vaccines in primary care: a randomized controlled trial. Pediatrics 2014; 134:346-53; http://dx.doi.org/10.1542/peds.2013-4257
- Cassidy B, Braxter B, Charron-Prochownik D, Schlenk E. A quality improvement initiative to increase HPV vaccine rates using an educational and reminder strategy with parents of preteen girls. J Pediatr Health Care 2014; 28(2):155-64; PMID:23522561; http://dx.doi.org/10.1016/j.pedhc.2013.01.002
- Fiks AG, Grundmeier RW, Mayne S, Song L, Feemster K, Karavite D, Hughes CC, Massey J, Keren R, Bell LM, et al. Effectiveness of decision support for families, clinicians, or both on HPV vaccine receipt. Pediatrics 2013; 131:1114-24; PMID:23650297; http://dx.doi.org/10.1542/peds.2012-3122
- Matheson EC, Derouin A, Gagliano M, Thompson JA, Blood-Siegfried J. Increasing HPV vaccination series completion rates via text message reminders. J Pediatr Health Care 2014; 28(4):35-9; PMID:23195652; http://dx.doi.org/10.1016/j.pedhc.2013.09.001
- Moss JM, Reiter P, Dayton, A, Brewer NT. Increasing adolescent immunization by webinar: a brief provider intervention at federally qualified health centers. Vaccine 2012; 30:4960-3; PMID:22652406; http://dx.doi.org/10.1016/j.vaccine.2012.05.042
- Perkins R, Zisblatt L, Legler A, Trucks E, Hanchate A, Sheinfeld Gorin S. Effectiveness of a provider-focused intervention to improve HPV vaccination rates in boys and girls. Vaccine 2015; 33:1223-9; PMID:25448095; http://dx.doi.org/10.1016/j.vaccine.2014.11.021
- Pahud B, Clard S, Herigon JC, Sherman A, Lynch DA, Hoffman A, Jackson MA. A pilot program to improve vaccination status for hospitalized children. Hosp Pediatr 2015; 5(1):35-41; PMID:25554757; http://dx.doi.org/10.1542/hpeds.2014-0027
- Chung R, Walter E, Kemper A, Dayton A. Keen on teen vaccines: improvement of adolescent vaccine coverage in rural North Carolina. J Adolescent Health 2015; 56:S14-16; http://dx.doi.org/10.1016/j.jadohealth.2014.10.272
- Staras S, Vadaparampil S, Livingston M, Thompson L, Sanders A, Shenkmen E. Increasing human papillomavirus vaccine initiation among publicly insured Florida adolescents. J Adolescent Health 2015; 56:S40-46; http://dx.doi.org/10.1016/j.jadohealth.2014.11.024
- Bundy DG, Persing NM, Solomon BS, King TM, Murakami P, Thompson RE, Engineer LD, Lehmann CU, Miller MR. The ImmProve Project: Leveraging electronic health record data to promote immunization delivery. Acad Pediatr 2013; 13(5):458-65; PMID:23726754http://dx.doi.org/10.1016/j.acap.2013.03.004
- Daley MF, Kempe A, Pyrzanowski J, Vogt TM, Dickinson LM, Kile D, Fang H, Rinehart DJ, Shlay JC. School-located vaccination of adolescents with insurance billing: cost, reimbursement, and vaccination outcomes. J Adolescent Health 2014; 54:282-8; http://dx.doi.org/10.1016/j.jadohealth.2013.12.011
- Stubbs B, Panozzo C, Moss J, Reiter P, Whitesell D, Brewer N. Evaluation of an intervention providing HPV vaccine in schools. Am J Health Behav 2014; 38(1):92-102; PMID:24034684; http://dx.doi.org/10.5993/AJHB.38.1.10
- Harper DM, Verdenius I, Harris GD, Barnett AL, Rosemergey BE, Arey AM, Wall J, Malnar GJ. The influence of free quadrivalent human papillomavirus vaccine (HPV4) on the timely completion of the three dose series. Prev Med 2014; 61:20-5; PMID:24440159; http://dx.doi.org/10.1016/j.ypmed.2014.01.007
- Chesson H, Ekwueme D, Saraiya M, Watson M, Lowy D, Markowitz LE. Estimates of the annual direct medical costs of the prevention and treatment of disease associated with human papillomavirus in the United States. Vaccine 2012; 30(42):6016-9; PMID:22867718; http://dx.doi.org/10.1016/j.vaccine.2012.07.056
- Rosenthal S, Weiss T, Zimet G, Ma L, Good M, Vichnin M. Predictors of HPV vaccine uptake among women aged 19–26: importance of a physician's recommendation. Vaccine 2011; 29(5):890-895; PMID:20056186; http://dx.doi.org/10.1016/j.vaccine.2009.12.063
- Hofstetter A, Rosenthal S. Health care professional communication about STI vaccines with adolescents and parents. Vaccine 2014; 32(14):1616-23; PMID:23791695; http://dx.doi.org/10.1016/j.vaccine.2013.06.035
- Hofstetter A, Rosenthal S. Factors impacting HPV vaccination: lessons for health care professionals. Exp Rev Vaccines 2014; 13(8):1013-26; http://dx.doi.org/10.1586/14760584.2014.933076