ABSTRACT
We conducted a multicentre test negative case control study to estimate the 2013–14 influenza vaccine effectiveness (IVE) against hospitalised laboratory confirmed influenza in 12 hospitals in France, Italy and Spain. We included all ≥18 years hospitalised patients targeted by local influenza vaccination campaign reporting an influenza-like illness within 7 days before admission. We defined as cases patients RT-PCR positive for influenza and as controls those negative for all influenza virus. We used a logistic regression to calculate IVE adjusted for country, month of onset, chronic diseases and age. We included 104 A(H1N1)pdm09, 157 A(H3N2) cases and 585 controls. The adjusted IVE was 42.8% (95%CI: 6.3;65;0) against A(H1N1)pdm09. It was respectively 61.4% (95%CI: −1.9;85.4), 39.4% (95%CI: −32.2;72.2) and 19.7% (95%CI:-148.1;74.0) among patients aged 18–64, 65–79 and ≥80 years. The adjusted IVE against A(H3N2) was 38.1% (95%CI: 8.3;58.2) overall. It was respectively 7.8% (95%CI: −145.3;65.4), 25.6% (95%CI: −36.0;59.2) and 55.2% (95%CI: 15.4;76.3) among patients aged 18–64, 65–79 and ≥80 years. These results suggest a moderate and age varying effectiveness of the 2013–14 influenza vaccine to prevent hospitalised laboratory-confirmed influenza. While vaccination remains the most effective prevention measure, developing more immunogenic influenza vaccines is needed to prevent severe outcomes among target groups.
Introduction
European immunisation strategies recommend yearly influenza vaccination of the population at risk for severe outcome.Citation1 Measuring and reporting influenza vaccine effectiveness (IVE) against hospitalised outcome within the targeted population is critical to evaluate and adapt these strategies.
In a review published in 2011,Citation2 Michiels et al. qualified as “moderate to poor” the evidences regarding IVE in these target groups. Among elderly, for whom a reduced capacity to produce antibodies leads to an impaired immune response (also known as immune-senescence),Citation3,4 the evidence gathered in a 2010 Cochrane review suggested a modest IVE of the trivalent inactivated vaccines (TIV).Citation5
Reporting of IVE among subgroups for which the vaccine may have suboptimal effectiveness may help promoting the development of more immunogenic vaccine products or alternative approaches (e.g. prophylactic antiviral use) for these patients.
In 2011, we set up InNHOVE, a European Network of Hospitals for measuring IVE. The use of a common generic protocol, study site visits and meetings of network partners ensured common practices across hospitals and the possibility to pool data. A pilot phase conducted in 2011–12 reinforced the homogeneity in implementing protocols across study sites.Citation6
In the European Union, the 2013–14 season was marked by a dominant circulation of A(H1)pdm09 A/California/7/2009 (H1N1)-like and A/Texas/50/2012 (H3N2)-like viruses and a sporadic circulation of influenza B viruses.Citation7 The objective of this study was to measure the 2013/14 seasonal IVE against hospitalisation with laboratory-confirmed A(H1N1)pdm09 and A(H3N2) influenza among the adult population targeted for vaccination in 3 EU countries.
Results
Of the 962 eligible patients, we excluded 8 patients due to missing laboratory results and 11 due to missing vaccination status (). We included a total of 157 A(H3N2) and 104 A(H1N1)pdm09 cases. For the A(H3N2) analysis we excluded 220 controls with dates of onset outside the study period and included 585 controls. Similarly, after excluding 357 controls for the A(H1N1)pdm09 analysis, we ended up with 426 controls.
Figure 1. Flowchart of data exclusion for pooled analysis, InNHOVE multicentre study, France, Italy, Spain, 2013-14
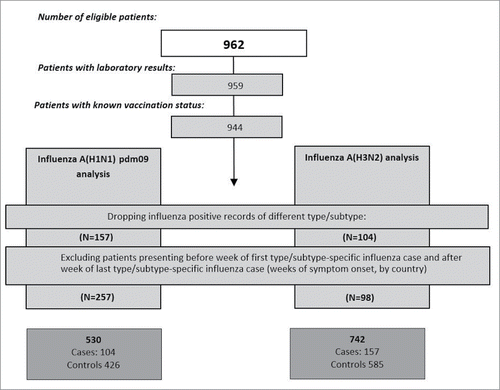
Patients' inclusion started on week 50, 2013 in Spain, on week 1, 2014 in France and on week 3, 2014 in Italy. A(H1N1)pdm09 and A(H3N2) cases were reported throughout the season in France and Spain (; ). We excluded Italy from the A(H1N1)pdm09 analysis as only 2 cases were reported.
Figure 2. Number of ILI patients positive for influenza A(H3N2), positive for influenza A(H1N1)pdm09 and negative for any influenza by week of symptom onset, InNHOVE multicentre study, France, Italy, Spain. By study site, 2013-14
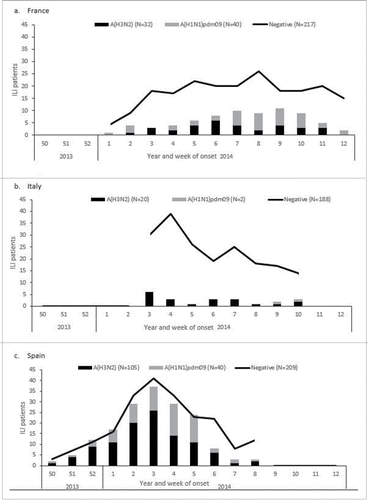
Table 1. Study period, inclusions of laboratory confirmed influenza and test negative controls by vaccination status and study site, InNHOVE multicentre study, France, Italy, Spain, 2013–14.
All recorded vaccines were TIV. In Navarra, Spain, one brand of unadjuvanted vaccine was exclusively distributed. In France, 5 brands of unadjuvanted vaccines were recorded. In Italy, 3 brands, including one adjuvanted vaccine, were reported.
A(H1N1)pmd09 cases were younger than controls (median age 65.2 vs 75.6 years). A lower proportion of cases than controls had chronic diseases (81.7 vs 91.8%), had been hospitalised in the past 12 months (31.7 vs 41.7%) and had never smoked (39.0 vs 53.5%). The 2013–14 vaccine coverage was 34.6% among A(H1N1)pdm09 cases and 56.3% among controls ().
Table 2. Characteristics of Influenza A(H1N1)pdm09 (n=104), Influenza A (H3N2) (n=157) and test-negative controls, InNHOVE multicentre study, France, Italy, Spain, 2013–14.
A(H3N2) cases were of similar age as controls and had comparable chronic diseases. They were less likely than controls to be obese (8.4 vs 19.1%) and to have been hospitalised in the past 12 months (29.9 vs 42.2%). The 2013–14 vaccine coverage was 48.4% among A(H3N2) cases and 54.0% among controls ().
Adjusted for study site, month of onset, age and presence of chronic conditions, the IVE against A(H1N1)pdm09 were respectively 42.0% (95%CI: −25.8;73.3) and 43.8% (95%CI: −6.3;70.3) in France and Spain. The p-value and I2 index testing for heterogeneity between these IVE estimates were respectively 0.999 and 0.0%. The adjusted IVE against A(H3N2) were respectively 31.8% (95%CI: −52.5;69.5), −30.9% (95%CI: −282.3;55.2) and 49.5% (95%CI: 16.6;69.8) in France, Italy and Spain; the p-value associated with the Q-test and the I2 index were respectively 0.216 and 34.8% ().
Table 3. Crude and age adjusted Influenza vaccine effectiveness against influenza A(H1N1)pdm09 and influenza A(H3N2) by study site, InNHOVE multicentre study, France, Italy, Spain, 2013–14.
The pooled adjusted IVE against A(H1N1)pdm09 was 42.8% (95%CI: 6.3;65.0). The adjusted age-group specific IVE was respectively 61.4% (95%CI: −1.9;85.4), 39.4% (95%CI: −32.2;72.2) and 19.7% (95%CI:-148.1;74.0) among patients aged 18–64, 65–79 and 80 years and above ().
Table 4. Crude and age adjusted Influenza vaccine effectiveness against influenza A(H1N1)pdm09 and influenza A(H3N2), overall and by age group, InNHOVE multicentre study, France, Italy, Spain, 2013–14.
The pooled adjusted IVE against A(H3N2) was 38.1% (95%CI: 8.3;58.2). The adjusted age-group specific IVE was respectively 7.8% (95%CI: −145.3;65.4), 25.6% (95%CI: −36.0;59.2) and 55.2% (95%CI: 15.4;76.3) among patients aged 18–64, 65–79 and 80 years and above ().
Discussion
Our results suggest that the 2013–14 influenza vaccines provided a moderate protection against hospitalisation with laboratory confirmed A(H1N1)pdm09 (42.8%) and A(H3N2) (38.1%) influenza among adults targeted by the vaccination program. Our data also suggest different IVE by age-group. The highest IVE point estimate was among those aged < 65 years for A(H1N1)pdm09 and those aged > 80 for A(H3N2).
We used a test-negative case control design to recruit our patients. This approach, widely used and validated among GP based IVE studies, assumes that, by recruiting patients presenting with the same syndrome, we are likely to minimise selection biases[16–18]. In our study, A(H3N2) cases were of similar age as controls but tended to have less chronic diseases (non-significant differences). A(H1N1)pdm09 cases were younger and had significantly less chronic diseases than controls. A higher proportion of controls with comorbidities could lead to an overestimation of the VE if the presence of comorbidities was associated with higher vaccine uptake. Our estimates were adjusted for presence of chronic diseases, age, time and study sites and stratified by age-group. Adjusting for chronic diseases had little effect on the IVE estimates, even within each age group. Adjusting on specific categories of comorbidities or hospitalisation for chronic conditions in the previous year had a marginal effect on the IVE estimates. However, some stratified analyses led to small sample size, increasing variability and limiting interpretation of positive and negative confounding. Furthermore, we cannot exclude that residual confounding biases our results.
Vaccination status was missing in 1.6% of eligible patients. While its ascertainment was based on a registry in Navarra, we relied on patients, pharmacist and/or GP memory in France and Italy. We cannot exclude some misclassification. However, as all patients presenting at the hospitals had similar signs and as the vaccination was ascertained prior to the laboratory results, there is no reason to expect a differential misclassification bias between cases and controls.
European virological surveillance data suggested that A(H1N1)pm09 and A(H3N2) circulating and vaccine viruses were antigenically similar in the 2013–14 season.Citation11 For A(H3N2), while the French national laboratory reported no significant mutation,Citation12 the region of Navarra, Spain, reported some genetic differences between the circulating and vaccine viruses.Citation13 Of particular interest was the L157S mutation, located in the HA1 antigenic site B near the receptor binding site, observed in 16/17 patients. This site is located close to 4 of the 7 positions (positions 145, 155, 156, 158, 159, 189 and 193) associated with all majors H3 antigenic change since 1968.Citation14 The L157 mutation observed as part of the virological surveillance scheme, if present in the patients included in the study and if affecting antigenicity, could partially explain the moderate IVE against A(H3N2) we observed this season. A representative virus characterization from the patients included in the various study sites of our multicenter study would be of great value to better interpret vaccines performances against given subtypes as well as IVE differences between study sites.
The age-group specific IVE results need to be read in the light of the small sample size in the various age groups, particularly among the A(H1N1)pdm09 cases aged ≥80 years and the A(H3N2) cases aged less than 65 years. The confidence intervals surrounding the IVE were very large in these 2 groups. The proportion of patients aged 65 and above was lower among A(H1N1)pdm09 cases compared to controls and A(H3N2) cases. This may reflect the lower incidence of A(H1N1)pdm09 influenza among the older age group compatible with a former exposure to A(H1N1) virus.Citation15,16 Recent past natural infections may play a booster role on the immunological response to seasonal vaccination.Citation17,18 Immune senescenceCitation4 and a lower incidence of A(H1N1)pdm09 among elderly since the 2009 pandemic could partially explain the lower IVE against this subtype observed among patients. The higher IVE against A(H3N2) among elderly would require further investigation as it is in contradiction with the principles of immune senescence. We cannot exclude that variability due to small sample size, or biases due to unmeasured confounding, lead to this unexpectedly high estimate. Larger age group specific sample size would be needed to measure and compare IVE estimates between age groups with more precision. A better documentation of past natural infections and vaccinations would be useful to understand these differences, if any. Furthermore, larger sample size would be required to investigate the effect of repeated vaccination as only few patients have changing vaccination status over time.
This season, the InNHOVE network included a total of 12 hospitals. The recruitment approaches ensured a systematic inclusion of patients hospitalised for influenza related conditions and presenting with an ILI onset in the past 7 days. We did not find statistically significant difference between study site specific estimates of IVE against A(H1N1)pdm09 and A(H3N2). However, small study site specific sample size made it difficult to observe a statistically significant heterogeneity. While French and Spanish specific IVE estimates against A(H1N1)pdm09 were close, large differences could be observed between study sites for A(H3N2) analyses. Although access to care is high in the 3 participating regions, different access to vaccination and to hospitalization in case of severe ILI across study sites cannot be excluded. If not taken into account when adjusting on the age and the presence of chronic conditions, residual confounding of a different magnitude across study sites could partially explain the observed differences. If these differences in estimates were to reflect true differences in IVE across study sites, possible explanations could be the use of distinct vaccine types and brands or the circulation of different viruses across study sites. Our sample size was too small to provide product specific IVE estimates with interpretable confidence intervals. In the future, we suggest that each study site select a systematic sample of viruses for genetic and antigenic characterization. This would allow for a better interpretation of differences in IVE between study sites.
While seasonal vaccination remains the most effective prevention measure against influenza,Citation19 our study suggests that developing more immunogenic influenza vaccines is needed to prevent severe outcomes among those targeted by the vaccination. The InNHOVE project represents a good example of a European network of hospitals working according to the same generic protocol, allowing pooling data together. Encouraging this initiative and further increasing this network is the best way to compute strain, age groups and product specific estimates.
Material and methods
We conducted a multicentre case control study using a test-negative design in 12 hospitals located in France (6 hospitals), Italy (2 hospitals), and Spain (4 hospitals in Navarra region). Study sites adapted the generic study protocol to their local settings.Citation20
The competent authorities of each country/region approved the protocol. All study sites complied with “The Ethical Principles for Medical Research Involving Human Participants of the World Medical Association and the Declaration of Helsinki” (World Medical Association, Inc. Available at: www.wma.net/en/30publications/10policies/b3/index.html). According to country specific requirements for ethical approval, all participants (or their legal tutor) provided written consent for recruitment into the study.
The “Ile de France IV” Ethical Committee (“Comité de Protection des Personnes Ile-de-France IV,” Paris, France), the Ethical Committee of the Catholic University of Rome, Italy and the Navarra Ethical Committee for Medical Research, Spain gave their approval to conduct the study.
Study population
The study population included all community-dwelling adults (≥18 years) belonging to the target group for influenza vaccination, with no contra-indication for vaccination, likely to be hospitalized in one of the participating hospitals in case of influenza related illness.
Individuals were considered to belong to target group for vaccination if they had at least one of the medical conditions or the age required to fall into this group according to the country specific recommendations for vaccination.Citation21-23
Study period
In each study site, the study period lasted from the week of the first laboratory confirmed case of influenza to the week of the last laboratory confirmed case of influenza followed by 2 weeks without a case. We defined separately the study period for A(H1N1)pdm09 and A(H3N2).
Patient inclusion
Study teams approached all patients admitted for at least 24 hours in one of the participating hospitals with influenza related conditions. These conditions included acute myocardial infarction or acute coronary syndrome; heart failure; pneumonia and influenza; chronic pulmonary obstructive disease; myalgia; altered consciousness, convulsions, febrile-convulsions; dyspnoea/respiratory abnormality; respiratory abnormality; shortness of breath; respiratory abnormality necrotising enterocolitis; respiratory symptoms/chest symptoms; acute cerebrovascular disease; sepsis; systemic inflammatory response syndrome.
The influenza-like-illness (ILI) case definition included the presence of at least one of the following systemic symptoms: fever or feverishness, malaise, headache, myalgia; and at least one of the following respiratory symptoms: cough, sore throat, shortness of breath. Study teams proposed to those reporting an ILI onset in the past 7 days and no later than 48 hours after admission to undergo a nasopharyngeal swab, to be interviewed and included in the study.
Data collection
Study teams collected data on demographics, chronic diseases and their severity (using as proxy the number of hospital admissions due to chronic diseases in the past 12 months), number of GP consultations in the previous 3 months (to adjust for health seeking behavior), smoking status, vaccination against influenza in the last 2 seasons (including vaccine brand for the 2013–14 season) and functional status before onset using the Barthel indexCitation24 through patient/family interview and from medical records. Patients with a Barthel index below or equal to 60 were considered as having a low functional status.
We defined an individual as vaccinated against seasonal influenza if he/she had received at least one dose of influenza vaccine more than 14 days before ILI symptoms onset. Patients not vaccinated or vaccinated less than 15 days prior to ILI onset were considered unvaccinated. The Spanish study team documented patients' vaccination status using vaccination registers. The French and Italian sites collected that information through interview with the patient/family and call to general practitioners (GPs) or pharmacists to check the information.
Reverse transcription polymerase chain reaction (RT-PCR) was used to detect influenza viruses and to classify positive patients as cases of influenza A(H3N2) or A(H1N1)pdm2009. Laboratory tests were centralised within each study site.
Case definition
We defined cases as hospitalised ILI patients belonging to the study population and testing positive for A(H1N1)pdm09 or A(H3N2). Controls were hospitalised ILI patients belonging to the study population and testing negative for all influenza virus.
Data analysis
Study sites sent anonymised datasets to the pooled analysis coordinator through a secured web based platform (Voozanoo™).
To avoid introducing too much variability, for each influenza A subtype, we excluded from the pooled analysis study sites with less than 10 cases (arbitrary set threshold).
We assessed heterogeneity across study sites using the significance of the Cochran Q-test and the I2 index that quantifies the proportion of the variance attributable to differences between study sites.Citation25
We conducted a separate analysis for each type/subtype of influenza. We estimated the pooled IVE as 1 minus the odds ratio (OR) of being vaccinated in cases versus controls, using a one-stage method with study site as a fixed effect in the model.Citation26 We used a multivariable logistic regression model to compute IVE adjusted for month of onset, study site and age modeled as restricted cubic spline with 4 knots. We further calculated IVE adjusted for the presence of any or specific chronic conditions. We measured stratified IVE by age group (< 65, 65–79; 80 years and above) adjusting for all the potential confounding factors.
Disclosure of potential conflicts of interest
No conflict of interest. GlaxoSmithKline and Sanofi Pasteur MSD financially supported the study. They had no role in study design, data collection, pooled analysis, and publication.
Acknowledgments
We would like to thank Vivek Shinde, Hélène Bricout, Bruno Ciancio Germaine Hanquet and Jim McMenamin for their scientific inputs in piloting this study. Many thanks also to EpiConcept colleagues for their contributions: Thomas Seyler for initiating the network, Esther Kissling for her great input on data management, Marta Valenciano for her reviews. We are grateful to all patients, medical and laboratory staff, study nurses and epidemiologists from the 4 study sites who actively participated in the study.
References
- Mereckiene J, Cotter S, Nicoll A, Lopalco P, Noori T, Weber J, D’Ancona F, Levy-Bruhl D, Dematte L, Giambi C, et al. Seasonal influenza immunisation in Europe. Overview of recommendations and vaccination coverage for three seasons: pre-pandemic (2008/09), pandemic (2009/10) and post-pandemic (2010/11). Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull 2014; 19:20780; PMID:24786262
- Michiels B, Govaerts F, Remmen R, Vermeire E, Coenen S. A systematic review of the evidence on the effectiveness and risks of inactivated influenza vaccines in different target groups. Vaccine 2011; 29:9159-70; PMID:21840359; http://dx.doi.org/10.1016/j.vaccine.2011.08.008
- Saurwein-Teissl M, Lung TL, Marx F, Gschösser C, Asch E, Blasko I, Parson W, Böck G, Schönitzer D, Trannoy E, et al. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol Baltim Md 1950 2002; 168:5893-9
- Haq K, McElhaney JE. Immunosenescence: Influenza vaccination and the elderly. Curr Opin Immunol 2014; 29:38-42; PMID:24769424; http://dx.doi.org/10.1016/j.coi.2014.03.008
- Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2010; 17:CD004876; PMID:20166072
- Rondy M, Puig-Barbera J, Launay O, Duval X, Castilla J, Guevara M, Costanzo S, de Gaetano Donati K, Moren A. 2011–12 Seasonal Influenza Vaccines Effectiveness against Confirmed A(H3N2) Influenza Hospitalisation: Pooled Analysis from a European Network of Hospitals. A Pilot Study. PloS One 2013; 8:e59681; PMID:23565159; http://dx.doi.org/10.1371/journal.pone.0059681
- European Centre for Disease Prevention and Control. Weekly influenza surveillance overview - 23 May 2014 - Surveillance report[Internet]. Available from: http://www.ecdc.europa.eu/en/publications/Publications/influenza-surveillance-overview-23-may-2014.pdf
- Valenciano M, Kissling E, Ciancio BC, Moren A. Study designs for timely estimation of influenza vaccine effectiveness using European sentinel practitioner networks. Vaccine 2010; 28:7381-8; PMID:20851086; http://dx.doi.org/10.1016/j.vaccine.2010.09.010
- Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013; 31:2165-8; PMID:23499601; http://dx.doi.org/10.1016/j.vaccine.2013.02.053
- Foppa IM, Haber M, Ferdinands JM, Shay DK. The case test-negative design for studies of the effectiveness of influenza vaccine. Vaccine 2013; 31:3104-9; PMID:23624093; http://dx.doi.org/10.1016/j.vaccine.2013.04.026
- European Centre for Disease Prevention and Control. Influenza virus characterisation Summary Europe, July 2014[Internet]. Available from: http://ecdc.europa.eu/en/publications/Publications/influenza-characterisation-report-july-2014.pdf
- Équipes de surveillance de la grippe. Epidemiological and virological influenza activity in mainland France: 2013–14 season (in French). BEH2014;
- Castilla J, Martínez-Baz I, Navascués A, Fernandez-Alonso M, Reina G, Guevara M, Chamorro J, Ortega MT, Albéniz E, Pozo F, et al. Vaccine effectiveness in preventing laboratory-confirmed influenza in Navarre, Spain: 2013/14 mid-season analysis. Euro Surveillance 2014; 19(6).
- Koel BF, Burke DF, Bestebroer TM, van der Vliet S, Zondag GCM, Vervaet G, Skepner E, Lewis NS, Spronken MIJ, Russell CA, et al. Substitutions near the receptor binding site determine major antigenic change during influenza virus evolution. Science 2013; 342:976-9; PMID:24264991; http://dx.doi.org/10.1126/science.1244730
- Rimmelzwaan GF, Fouchier RAM, Osterhaus ADME. Age distribution of cases caused by different influenza viruses. Lancet Infect Dis 2013; 13:646-7; PMID:23886322; http://dx.doi.org/10.1016/S1473-3099(13)70181-6
- Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, Liu F, Dong L, DeVos JR, Gargiullo PM, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009; 361:1945-52; PMID:19745214; http://dx.doi.org/10.1056/NEJMoa0906453
- Davies JR, Grilli EA. Natural or vaccine-induced antibody as a predictor of immunity in the face of natural challenge with influenza viruses. Epidemiol Infect 1989; 102:325-33; PMID:2703026; http://dx.doi.org/10.1017/S0950268800030004
- Smith DJ, Forrest S, Ackley DH, Perelson AS. Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci U S A 1999; 96:14001-6; PMID:10570188; http://dx.doi.org/10.1073/pnas.96.24.14001
- Background Paper on Influenza Vaccines and Immunization. SAGE Working Group. 2012 [Internet]. 2013[cited 2013 Apr 19]; Available from: http://www.who.int/immunization/sage/meetings/2012/april/1_Background_Paper_Mar26_v13_cleaned.pdf
- Seyler T, Rondy M, Valenciano M, Moren A. Protocol for hospital-based case control studies to measure seasonal influenza vaccine effectiveness against laboratory confirmed influenza hospitalisations across the European Union and European Economic Area Member States. 2012.
- Calendrier vaccinal et recommandations vaccinales 2013 du ministère des affaires sociales et de la santé selon l’avis du Haut Conseil de la santé publique.; 2013 vaccination schedule and recommendations from the Haut conseil de la santé publique in France.
- Instituto de Salud Pública y Laboral de Navarra. Protocolo de vacunación antigripal 2013–2014. [Influenza vaccination protocol 2013–2014].
- Prevenzione e controllo dell’influenza: raccomandazioni per la stagione 2013–2014. [Prevention and control of influenza: recommendations for the season 2013–2014].
- MAHONEY FI, BARTHEL DW. FUNCTIONAL EVALUATION: THE BARTHEL INDEX. Md State Med J 1965; 14:61-5; PMID:14258950
- Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 2006; 11:193-206; PMID:16784338; http://dx.doi.org/10.1037/1082-989X.11.2.193
- Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001.
