ABSTRACT
Objective: Compare immune persistence from one dose of each of 3 different hepatitis A vaccines when given to school-age children: a domestic, live attenuated hepatitis A vaccine (H2 vaccine); a domestic inactivated hepatitis A vaccine (Healive®); and an imported, inactivated hepatitis A vaccine (Havrix®),.Methods: School-age children were randomized into 1 of 4 groups to receive a single dose of a vaccine: H2 vaccine, Healive®, Havrix®, or hepatitis B vaccine [control]. Serum samples were collected 12 and 24 months after vaccination for measurement of anti-HAV IgG using microparticle enzyme immunoassay. Seropositivity was defined as ≥ 20 mUI/ml. We compared groups on seropositivity and geometric mean concentration (GMC). Results: Seropositive rates for the H2, Healive®, Havrix®, and control groups were 64%, 94.4%, 73%, and 1.0%, respectively, 12-months post-vaccination; and 63%, 95.6%, 72%, and 1.0%, respectively 24-months post-vaccination. Seropositivity was greater for Healive® than for H2 and Havrix® at 12 months (p-values < 0.001) and 24 months (p-values < 0.0001). Average GMCs for the H2, Healive®, Havrix®, and control groups, in mIU/ml, were 29.7, 81.0, 36.4, and 2.9, respectively at 12 months, and 30.9, 112.2, 44.3, and 2.9, respectively, at 24 months. GMCs were greater for Healive® than for H2 and Havrix® at 12 months (p-values < 0.0001 and < 0.001, respectively) and 24 months (p-values < 0.001). No statistically significant differences in seropositivity or GMC were found within groups between 12 and 24 months. Conclusion: Immunity persisted 24 months after a single dose of inactivated hepatitis A vaccine and live attenuated hepatitis A vaccine.
Introduction
Hepatitis A is an acute inflammatory liver disease caused by hepatitis A virus (HAV). Globally, HAV infection causes approximately 1.5 million clinically-apparent hepatitis A cases every year.Citation1 In most developing countries of Asia and Africa, Hepatitis A is highly endemic, with population immunity being acquired through asymptomatic infection in early life.Citation2
HAV has been endemic in China. Since the 1990s, improvements in hygiene, sanitation, safe water, socioeconomic status, and the use of hepatitis A vaccine have led to a decline of hepatitis A-related cases and deaths in China.Citation3 Despite progress, in 2010, many outbreaks and over 30,000 cases were reported to the Chinese Center for Disease Control and Prevention. Hepatitis A is therefore a significant disease that deserves additional attention, especially in Western China.Citation4
In 2008, the China Ministry of Health integrated hepatitis A vaccine into the National Immunization Program (NIP), which provides routinely-recommended vaccines at no charge, regardless of socioeconomic status of the vaccine recipient. Two types of hepatitis A vaccine are currently used in China: inactivated vaccines, available globally, and a live, attenuated vaccine (H2 vaccine), which is manufactured only in China and available in several developing countries, including India.Citation5 NIP currently recommends 1 dose of H2 vaccine or 2 doses of inactivated vaccine in the routine immunization schedule. Currently, Beijing, Shanghai, Tianjin, and Jiangsu use the 2-dose inactivated hepatitis A vaccine option, while other provinces use the 1-dose H2 vaccine option, due mainly to cost considerations. In addition to routine immunization, a large amount of H2 vaccine is used in catch-up programs among school-age children, generally at the expense of the families.
Based on available scientific evidence, inactivated and live attenuated hepatitis A vaccines are highly immunogenic and generate long-lasting protection.Citation5 A single dose of inactivated vaccine is believed to control successfully the morbidity associated with HAV infection. However, there is little evidence comparing directly 1-dose schedules of live versus inactivated hepatitis A vaccines.
NIP does not recommend routine hepatitis A vaccination of children over 3 y of age, and there has been no hepatitis A immunization strategy for school children at high risk of HAV infection. To provide relevant data for policy makers, we conducted a double-blind, randomized clinical trial that compared immune persistence among school-age children of 1-dose vaccine schedules using either live or inactivated hepatitis A vaccines. We report results from this study, which is an extension of a previously published study that compared seroconversion rates at 7, 14, and 28 d after vaccination.Citation6,7 This study extends follow-up time to 12 and 24 months after vaccination.
Results
Recruitment and retention
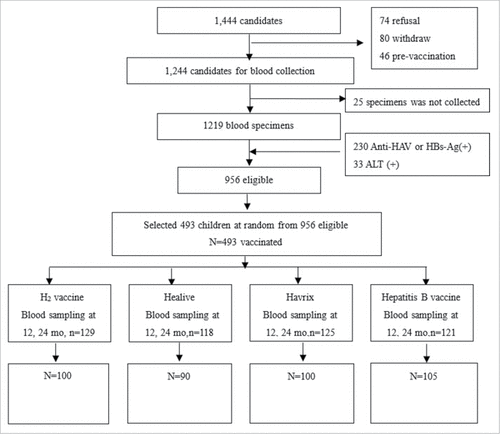
We evaluated 1,444 children for study eligibility. We excluded 488 potential participants after serological testing, but before vaccination, for the following reasons: refusal (N = 74), voluntary withdrawal (N = 80), previous vaccination against HAV (N = 46), lack of serum specimens (N = 25), being anti-HAV IgG positive or HBsAg positive (N = 230), or being ALT positive (N = 33). Of 956 eligible children in 15 schools, 493 were from 8 schools, and of these, 129, 118, 125, and 121 students were assigned at random into 1 of 4 groups, respectively: H2 vaccine, (Chinese live attenuated hepatitis A vaccine), Healive® (Chinese inactivated hepatitis A vaccine), Havrix®(imported inactivated hepatitis A vaccine), and hepatitis B control vaccine (). Characteristics of the subjects are summarized in . ANOVA and Chi-Square analysis showed no differences by age (p = 0.239), sex (p = 0.204), weight (p = 0.748) or height (p = 0.505).
Table 1. Demographic characteristics of subjects (mean ± standard deviation).
Twelve and 24 months after vaccination, 100, 90, 100 and 105 serial serum specimens were collected from children in the 4 groups. Laboratory results from some students showed evidence of HAV infection during the study period (4-fold increase in anti-HAV IgG from 12 to 24 months), and these children were excluded. There were, respectively, 2, 12, 5, and 1 children with 4-fold increases, and 1, 6, 8, and 0 children with more than 4-fold increases excluded.
Immunogenicity
shows seropositivity and GMC results at 12-mo and 24-mo post-vaccination from the 4 groups. At 12 months, seropositivity in the Healive® group was higher than the H2 vaccine group, but there was no statistically difference between the H2 vaccine group and the Havrix® group (P = 0.171). The Healive®group had higher GMC than the H2 vaccine group and the Havrix® group, but the GMCs were not statistically different between the H2 vaccine and Havrix® groups (P = 0.154). The results at 24 months were the same in direction, with similar statistical significance ().
Table 2. Seropositive rates and Geometric mean concentrations of anti-HAV IgG at 12 and 24 months post-vaccination.
Within groups, there were no statistically significant differences between seropositive rates and GMCs between 12 and 24 months post-vaccination. The GMC in the Healive® group at 24 months was higher than at 12 months, but the difference was not statistical significant (P = 0.158).
Discussion
We have shown that over 70% of school-age children, previously seronegative to hepatitis A virus, who were vaccinated with a 1-dose schedule of inactivated hepatitis A vaccine became and remained seropositive 24 months following vaccination with either Healive® or Havrix® vaccines. In contrast, 63% of school children vaccinated with 1 dose of a live, attenuated hepatitis A vaccine, H2 vaccine, became and remained seropositive 24 months later. The 24-month GMC and seropositive rate were greatest for Healive®, at 94% seropositive, than for either H2 or Havrix®. There was no evidence of decline in seropositivity or GMC between 12 and 24 months following vaccination in any of the vaccinated groups.
Study in context of the scientific literature
Our 1-dose schedule study results are consistent with findings from an evaluation conducted in Argentina, following their 2005 implementation of a 12-month, universal, 1-dose, inactivated hepatitis A vaccination policy. Disease surveillance data showed a large decline in the incidence of hepatitis A between 2005 and 2007, attaining a 12-year nadir.Citation11 Our findings are also consistent with a study in middle-age travelers in which a single dose of Havrix® resulted 2 y later in a seropositivity rate of 68% and an average GMC of 32 mIU/m.Citation13
A study conducted in Changzhou, China, showed that 2 doses of Healive® resulted in higher GMCs than Havrix® 3 y following vaccination, with the second dose 6 months after the first dose.Citation6 A single-dose study conducted in Tianjin, China, also showed greater GMCs with Healive® than with Havrix® 6 months following vaccination - 126.1 and 40.9 mIU/ml, respectively.Citation4 These results consistently show greater immunogenicity with Healive®, and we speculate that this may be because Healive® contains more virus antigen than Havrix®.
Our finding that seropositivity was low following 1 dose of H2 vaccine, the live, attenuated hepatitis A vaccine, was different than results from a study conducted by Wang and colleagues in Hebei province, China. These investigators showed higher GMCs and seropositivity rates 12 and 24 months following a single dose of H2 vaccine than we found: 81.0 mIU/ml and 80.2% seropositive at 12 months post-vaccination, and 82.1 mIU/ml and 75.7% seropositive after 24 months.Citation15 Similar findings were seen in a study conducted in Xinjiang Uighur Autonomous Region of China, which showed that 12 months following 1 dose of H2 vaccine, the average GMC was 135.8 mIU/ml, and the seropositivity rate was 92.9%.Citation8
Experimental studies have shown that both inactivated and live hepatitis A vaccines are able to induce a T-cell response.Citation16 One study showed a memory response up to 11 y with a booster dose of virosome-formulated inactivated hepatitis A vaccine.Citation17 A memory response was also seen in children who had waning immunity following H2 vaccination and had become seronegative. These children showed a robust antibody response when vaccinated 6 y following their initial H2 vaccine immunization. Although anti-hepatitis A IgG is used to determine effective protection from hepatitis A virus,Citation19 these memory responses may indicated less of a need for a booster dose of vaccine.Citation18 In our study, 17 subjects who received hepatitis A vaccine had rising GMCs between 12 and 24 months, possibly indicating boosting from wild virus. However, no controls had rising antibody levels between 12 and 24 months, and surveillance showed no cases of hepatitis A, casting doubt on speculation that there was boosting from wild virus. The interpretation of the rising antibodies, therefore, is not clear in our study.
Program implications
Implementation in 2008 of universal hepatitis A vaccination in China has had a tremendous impact on the incidence of hepatitis A.Citation8 Although national-level hepatitis A mortality has declined to 1.67 per 100,000 population by 2013,Citation11 there remain high-risk areas in China. Furthermore, none of the available hepatitis A vaccines are made available free to children over 3 y of age, including school-age children.Citation9, 10 Parents or guardians of children over 3 y old must pay out-of-pocket for hepatitis A vaccine, while younger children receive the vaccine at no charge.
The motivation for this study was to determine whether a more economical 1-dose strategy of inactivated hepatitis A vaccine would be sufficiently immunogenic for strategic use by the immunization program to protect school children from HAV infection. A 1-dose schedule is easier to implement and may result in higher uptake than a 2-dose schedule.Citation12
Results from this study are encouraging for implementation of a 1-dose hepatitis A schedule, especially with Healive® vaccine, the inactivated, domestically-produced vaccine. Thus far, our study has 2 y of follow-up data; an additional 3 y of follow-up is planned with these cohorts. Our encouraging results may be able to be extended into a program recommendation for catch-up vaccination efforts, depending on the results of additional follow up.
Limitations
The 2 main limitations to our study are the relatively small sample size and the lost-to-follow-up rate of 19%. The sample size was sufficient to show meaningful differences in immunogenicity, but demonstrating non-inferiority requires more subjects. Subject dropout is a result of migration out of the study area. China is undergoing extensive urbanization and migration, which will challenge the 5-year follow-up that is planned for these cohorts.
Conclusions and next steps
We have shown that a single dose of either Healive® or Havrix® results in seroprotection of at least 70%, and that Healive® remains more immunogenic 24 months following vaccination than H2 vaccine and Havrix®. The cohorts in this study will be followed for an additional 3 y to obtain estimates of longer-duration persistence of humoral immunity. Ultimately, this study, in the context of other hepatitis A vaccination studies, may lead to a program recommendation for efficient use of hepatitis A vaccination among school children in China.
Materials and methods
Setting
The study was conducted in Jingyuan County of Gansu Province, which is considered to be a medium-high HAV endemic area in China. In 2011, data from China's National Notifiable Disease Reporting System (NNDRS) showed that hepatitis A reported morbidity in Gansu Province was 4.9 times greater than the national average (14.0 vs. 2.7 per 100,000). The prevalence of HAV and coverage of hepatitis A vaccine in Jingyuan County were both low. Sixteen cases of hepatitis A were reported in 2007; 31 cases were reported in 2008, for incidences of 3.287 per 100,000 in 2007 and 6.318 per 100,000 in 2008.
Subjects and study design
The design was a double-blind, randomized clinical trial with 4 groups; children in each group received 1 dose of a study vaccine (3 groups) or a control vaccine (1 group; hepatitis B vaccine).
The sample size calculation was described previously,Citation6 and showed that each group required 80 subjects to find significant differences in seropositive rates and geometric mean concentrations of anti-HAV IgG antibody. To account for a potential loss of 10% of subjects, the desired sample size for each group was adjusted to 90 to 100 children.
In total, 1,444 first to third grade children from Jingyuan County were enrolled in our 2009 study. Subjects were excluded for any of the following: previous vaccination with any hepatitis A vaccine, a contraindication to hepatitis A or hepatitis B vaccine, a history of hepatitis A, liver or spleen enlargement, being anti-HAV positive, being HBsAg positive, or having an abnormal alanine aminotransferase level.
After applying exclusion criteria, 956 children remained eligible for the study. Among eligible children, 493 were selected randomly and then assigned at random to 1 of 4 groups using a spreadsheet-based algorithm. Children in each group received one dose of a vaccine. Group A children received freeze dried live attenuated hepatitis A vaccine (106.5TCID50, Zhejiang Pukang Biotech Limited, batch number 20090402,H2 strain); Group B children received domestic inactivated hepatitis A vaccine (Healive®, 250 UI, Sinovac Biotech Limited, batch number 20090305); and Group C children received imported inactivated hepatitis A vaccine (Havrix®, 720 EU, GlaxoSmithKline, batch number XHAVB288A1). Group D was used as a control group, and children in this group received hepatitis B vaccine (5ηg/ml, Shenzhen Kangtai Biological Products Company Limited, batch number 20080920).
Subjects were identified by an assigned random number. Participants and vaccinators were blind to which vaccine was being administered. All vaccines were administrated in the deltoid muscle, and all vaccines were, and are, licensed by the China Food and Drug Administration for routine use.
Ethical review
The study was approved by the Committee of Ethics and Research of Gansu Provincial Center for Disease Control and Prevention. Written informed consent was obtained from all participants before any study procedure was performed.
Specimen collection and testing
Serum specimens were collected from all available subjects 12 and 24 months following vaccination. At 12 months following vaccination, we obtained serum from 440 subjects (a loss to follow-up rate of 10.75%); at 24 months we obtained serum from 410 subjects (loss rate of 16.83%). Serum from 395 children was available at both 12 and 24 months following vaccination for testing and analysis.
Anti-HAV IgG quantitative testing was performed using a micro-particle enzyme immunoassay (HAVAB 2.0, Abbott Laboratories, Chicago, USA) at the Beijing IPE Center Clinical Laboratory. Anti-HAV IgG values ≥ 20 mIU/ml were considered seropositive. Infection with wild virus was inferred if the Anti-HAV level at 24 month was 4 times higher than at 12 month.
Statistical analyses
We analyzed laboratory results from subjects with 12- and 24-months serum samples and calculated proportions of seropositive subjects per group and the average anti-HAV IgG GMCs and 95% confidence intervals (CI). Pearson's Chi-Square was used to compare seropositive rates, and analysis of variance (ANOVA) was used to compare GMCs. P-values < 0.05 (2-tailed) were considered statistically significant. We used SPSS 16.0 (SPSS Inc., Chicago, IL USA) statistical software to perform analyses.
Disclosure of potential conflicts of interest
The authors declare that they have no potential conflicts of interest.
Acknowledgments
The authors would like to extend their gratitude to the project County Center for Disease Control and Prevention. Special thanks to Dr. Lance Rodewald in WHO China for polishing the English
Funding
The trial was funded by Health Bureau of Gansu Province and project of Study on Immune Effects of different Hepatitis A Vaccines and Appropriate Technology on Emergent vaccination (GWGL2011-30).
References
- Zhuang FC, Du JB, Mao JS. Prevention and comparison on hepatitis A vaccine. Chin J Vacc Immun 2007; 13(1):79–83
- Verma R, Khanna P. Hepatitis A vaccine should receive priority in National Immunization Schedule in India. Hum Vacc Immunother 2012; 8(8):1132-4; PMID:22854671; http://dx.doi.org/10.4161/hv.20475
- Sui HT, Liang XF, Yin DP, Cui FQ, Wang HQ. Epidemic characteristics on hepatitis A in China during 1990∼2006. Chin J Vacc Immun 2007; 13(5):466–9
- Zheng H, Cui FQ. The Immunogenicity and Impact Factors of Hepatitis A Attenuated Live Vaccine and Inactivated Vaccine. Chin J Vacc Immun 2009: 15(4):371-4; PMID: 20077742
- WHO position paper on hepatitis A vaccines: June 2012-recommendations. Vaccine 2013; 31(2):285-6; PMID:23142134
- Zheng H, Chen Y, Wang F, Gong X, Wu Z, Miao N, Zhang X, Zhang X, Li H, Chen C, Hou X, et al. Comparing live attenuated and inactivated hepatitis A vaccines: An immunogenicity study after one single dose. Vaccine 2011; 29(48):9098-103; PMID: 21875638; http://dx.doi.org/10.1016/j.vaccine.2011.08.078
- Li H, Zhang X S, An J. Evaluation on the effect of immunization and safety of live attenuated and inactivated hepatitis A vaccine in China. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi 2013; 34(1):24-7; PMID:23648244
- Liu XE, Wushouer F, Gou A, Kuerban M, Li X, Sun Y, Zhang J, Liu Y, Li J, Zhuang H. Comparison of immunogenicity between inactivated and live attenuated hepatitis A vaccines A single-blind, randomized, parallel-group clinical trial among children in Xinjiang Uighur Autonomous Region, China. Hum Vacc Immunother 2013; 9(7):1460-5; PMID: 23571173; http://dx.doi.org/10.4161/hv.24366
- Ott JJ,Wiersma ST. Single-dose administration of inactivated hepatitis A vaccination in the context of hepatitis A vaccine recommendations. Int J Infect Dis 2013; 17(11):e939-44; PMID:23791857
- Van DP, Banatvala J, Fay O, Iwarson S, McMahon B, Van HK, Shouval D, Bonanni P, Connor B, Cooksley G, et al. Hepatitis A booster vaccination: Is there a need? Lancet 2003; 362(9389):1065-71; PMID:14522539; http://dx.doi.org/10.1016/S0140-6736(03)14418-2
- Vacchino MN. Incidence of Hepatitis A in Argentina after vaccination. J Viral Hepatitis, 2008,15 suppl 2(Supplement s2):47-50; PMID:18837834; PMID:18837834; http://dx.doi.org/10.1111/j.1365-2893.2008.01029.x
- Hollinger FB, Bell B, Levy‐Bruhl D, Shouval D, Wiersma S, Van Damme P. Hepatitis A and B vaccination and public health. J Viral Hepatitis 2007; 14(Supplements1):1-5; PMID:17958635; http://dx.doi.org/10.1111/j.1365-2893.2007.00924.x
- Sten I, Magnus L, Lena WM. Excellent booster response 4–6 y after a single primary dose of an inactivated hepatitis A vaccine. Scand J Infect Dis 2002; 34(2):110–111(112); PMID:11928839
- Zhang ZL, Zhu XJ, Wang X, Liang M, Sun J, Liu Y, Gao ZG, Wu JY, Dong XJ, Liu RK, et al. Interchangeability and tolerability of two inactivated hepatitis A vaccines in Chinese children. Vaccine 2012; 30(27):4028-33; PMID:22537990
- Wang XY, Xu ZY, Ma JC, Lorenz VS, Zhang Y, Hao ZY, Oak PH, Zhang YL, Tian MY, Ouyang P Ying, et al. Long-term immunogenicity after single and booster dose of a live attenuated hepatitis A vaccine: Results from 8-year follow-up. Vaccine 2007; 25(3):446–9; PMID:16949710; http://dx.doi.org/10.1016/j.vaccine.2006.08.004
- Yang ZN, Wang ZJ, Xu J, Xu LF, Jiang DC. Primary research on cellular immune response induced by live attenuated and inactivated hepatitis A vaccines. Chin J Microbiol Immunol 2008; 28(12):1131–6
- Christoph H, Robert V D P, Beck B R, Frösner G, Hunt M, Herzog C. Successful memory response following a booster dose with a virosome-formulated hepatitis a vaccine delayed up to 11 years. Clin Vacc Immunol Cvi 2011,18(5):885-887; PMID: 21411599; http://dx.doi.org/10.1128/CVI.00358-10
- Zhuang FC, Jiang Q, Gong YP, Mo SH, Xin YJ, Qian W, Chen NL, Zhang SY, Chai SA, Mao JS. Epidemiological effects of live attenuated hepatitis A vaccine (H2-strain): Results of a 10-year observation. Chin J Epidemiol 2001; 22(3):188–190; PMID:11860874
- X Zy, Xy W. Live attenuated hepatitis A vaccines developed in China. Hum Vacc Immunother 2014; 10(3):659-66; PMID:24280971