ABSTRACT
The goal of this study was to explore the effects of trans-placental tetanus toxoid (TT) and pertussis (PT) antibodies on an infant's response to vaccination in the context of antenatal immunization with tetanus but not with pertussis. 38 mothers received a single dose of TT vaccine during pregnancy. Infants received tetanus and pertussis vaccines at 6, 10 and 14 wk of age. TT and PT anti-IgG secretion by infant lymphocytes was measured at 15 wk. Plasma antibodies were measured at 6 wk (pre-vaccination), 15 wk and 1 y of age. Prior to vaccination, TT and PT antibody were detected in 94.6% and 15.2% of infants. At 15 wk anti-TT-IgG and anti-PT-IgG in plasma was increased by 7–9 fold over pre-vaccination levels, while at 1 y plasma anti-TT-IgG was decreased by approximately 5-fold from the peak and had returned to near the pre-vaccination level. At 1 y plasma anti-PT-IgG was decreased by 2-fold 1 yfrom the 15 wk level. However, 89.5% and 82.3% of infants at 1 y had protective levels of anti-TT and anti-PT IgG, respectively. Pre-vaccination plasma IgG levels were associated with lower vaccine-specific IgG secretion by infant lymphocytes at 15 wk (p < 0.10). This apparent inhibition was seen for anti-TT-IgG at both 15 wk (p < 0.05) and t 1 y (p < 0.10) of age. In summary, we report an apparent inhibitory effect of passively derived maternal antibody on an infants' own antibody response to the same vaccine. However, since the cut-off values for protective titers are low, infants had protective antibody levels throughout infancy.
Introduction
In the first few months of life, trans-placental antibodies help protect infants from infection but this can also influence the development of the infant's own immune response to immunizations. A more general principle of the effect of maternal antibody on infant immune response remains difficult to establish because of contradictory observations. For example, low maternal antibody levels reduce infant vaccine responses to live attenuated measlesCitation1 but not to mumpsCitation2 or rubellaCitation3 vaccinations, while high maternal anti-hepatitis B antibody levels have no effect on the immunogenicity of the hepatitis B vaccine in infants.Citation4 Inhibitory effects of maternal antibodies have also been shown for whole cell but not for acellular pertussis vaccine responses.Citation5,6 In addition, the nature and extent of the inhibitory effect of maternal antibody varies across different studies assessing post-vaccination immune responses in children. Since serum/plasma antibodies only represent the accumulated concentration of soluble antibodies and its level in plasma is also confounded by antibodies generated from the subclinical exposure of antigens in endemic region. It is not possible to discriminate between recently produced antibodies as result of vaccination and preexisting maternal antibodies. Thus assessment of plasma antibody does not provide conclusive evidence of the impact of maternal antibody on the infant immune responses to vaccination.
A more sensitive assessment method is necessary to understand the effect of maternal antibodies derived either from antenatal vaccination or from natural infection on infant response to vaccinations. This issue is particularly important at a time when new maternal immunization programs are being considered as the major public health strategy by international agencies.
At the site of injection, vaccine antigen is taken up by dendritic cells which are activated and then migrate to the draining lymph node where T and B cells are stimulated by vaccine antigens and differentiate into vaccine-specific, antibody-secreting plasma cells. These plasma cells can be found circulating in the peripheral blood 1–2 week after vaccination, on their way from lymph nodes to the bone marrow where they will reside and produce antibody for a long period. The enzyme-linked immunospot (ELISPOT) assay has been used to identify these plasma cells in blood circulation. The assay enumerates “spots” formed on a nitrocellulose plate coated with cognate antigen after incubation of peripheral blood mononuclear cells (PBMC). One spot represents one antibody-secreting cell (ASC). A similar but simpler assay has also been developed called the antibody in lymphocyte supernatant (ALS) assay. It directly measures antibody secreted into the supernatant of the PBMC culture, rather than counting the spots formed on the nitrocellulose membrane in the ELISPOT method. The ALS assay has been validated after oral cholera,Citation7 oral typhoid Citation8,9 as well as systemic tetanus Citation10 vaccinations. The ALS assay has greater flexibility than the ASC assay, as antibody measurements can be performed with frozen lymphocyte supernatants. The ALS and ASC results show 100% concordance in the specificity of vaccine exposure assessment.Citation8
In this observational study, we sought to determine the association between vaccine-induced antibody secreting plasma cell responses (by ALS assay) in infants and maternally derived antibodies obtained from antenatal vaccination (tetanus) and naturally acquired antibodies (pertussis) in a mother–infant cohort in Bangladesh.
Results
Study population
Cohort characteristics of the parent study including the recruitment strategy and reasons for non-participation have been described previously.Citation11 In this sub-study, infant characteristics () are comparable to the larger study,Citation11 expect that mean birth weight was 4.8% higher in this sub-study. Six infants in this cohort had low birth weight (<2,500g) and three had preterm delivery (gestational age <37 wk). Most of the mothers had normal BMI (18.5 – 25.0). One infant was anemic (hemoglobin <9.5 g/dL) at 6 wk. Infants were mostly normal to marginally underweight at 6 wk of age (Weight-for-Age Z-score (WAZ): -2SD < WAZ < −1 SD) though 15.8% of infants were moderately underweight (WAZ <-2SD). All infants received vaccines on the schedule set out by the Bangladesh national immunization policy for infants ().
Table 1. Characteristics of infants.Footnote1
Infant response to tetanus immunization
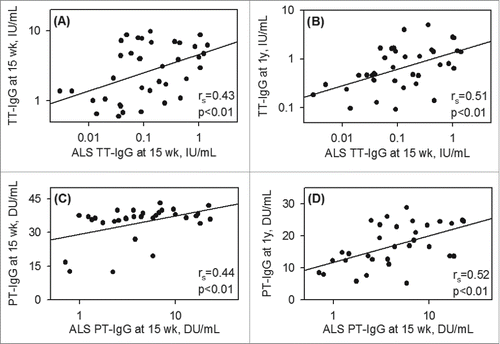
Pre- and post-vaccination concentrations of tetanus specific IgG (TT-IgG) in infants were compared (). As expected, before vaccination, 94.6% (35/37) infants at 6 wk of age had protective, maternally derived TT-IgG, since mothers received tetanus vaccine during pregnancy. At 15 wk of age (7–10d after the third TT and PT vaccines) plasma anti-TT-IgG was increased by 9.23 fold (95% CI: 13.9–4.55) while at 1 y, it was decreased by 4.92 fold (95% CI: 6.00–3.85) and returned to the pre-vaccination level at 6 wk (). In spite of receiving three doses of vaccine, 10.5% (4/38) of infants had plasma antibody below the target cutoff and 56.8% (21/37) infants showed decreased levels at 1 y compared to pre-vaccination level at 6 wk. As anticipated, vaccine-antigen mediated TT-IgG secretion from infant lymphocytes (ALS) at 15 wk was positively correlated with infant plasma TT-IgG level both at 15 wk (rs = 0.430 p < 0.01) and 1 y (rs = 0.505 p < 0.01) of age ( and ). Thus higher antibody production from lymphocytes correlated positively with higher levels in the plasma.
Figure 1. Tetanus vaccine-specific antibody responses of infants (geometric mean with 95% CI). (A) Plasma anti-TT IgG levels of infants at 6, 15 and 1 y of age, received three doses of DTwP-HepB-Hib combination vaccines at 1 mo interval beginning at 6 wk of age. (B) Antibody secreting lymphocyte (ALS) anti-TT IgG responses at 15 wk of age. (C) Plasma anti-TT IgG levels of infants at 15 wk of age and (D) Plasma anti-TT IgG levels of infants at 1 y of age by pre-vaccination (6 wk) antibody levels. Centiles are based on data from this study population. Specific anti-TT IgG (IU/mL) ranges for centile group at 6 wk were: <25th centile 0.08 – 0.51; 25–75th centile 0.58 – 1.41; >75th centile 1.82–12.2. Dotted horizontal line represents protective plasma anti-TT-IgG level. **p < 0.01, *p < 0.05, #p < 0.10.
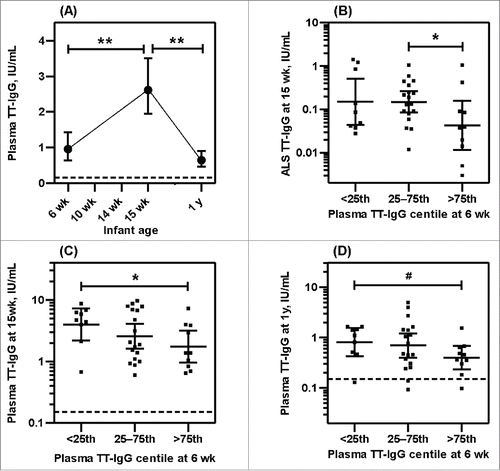
Figure 2. Positive association between vaccine specific ALS and plasma antibody responses Correlation between vaccine specific ALS anti-IgG at 15 wk of age and vaccine specific plasma anti-IgG at 15 wk and at 1 y of age for tetanus (A-B) and pertussis (C-D) vaccines. Spearman correlation coefficients (rs) and p-values are indicated.
There was a trend toward a negative association between pre-vaccination plasma TT-IgG at 6 wk and ALS TT-IgG at 15 wk (rs = −0.215 p = 0.2). Categorizing infants according to the concentration of TT-IgG at 6 wk pre-vaccination: <25th centile, 25–75th centile or >75th centile for this study population, showed significant lower ALS TT-IgG in the >75th centile group than 25–75th centile group of pre-vaccination level (). The lack of a significant difference between the lowest and highest quartiles could be due to our small sample size. Pre-vaccination plasma TT-IgG inversely predicted ALS TT-IgG in the multivariate regression analysis controlling for different covariates (). As a possible consequence of such apparent inhibition of plasma cell development, there were significant inverse association between pre-vaccination TT-IgG at 6 wk and post- vaccination plasma TT-IgG levels both at 15 wk (rs = −0.386 p = 0.02) and at 1 y (rs = −0.314 p = 0.05) of age. Categorizing infants in centiles according to the antibody concentration at 6 wk pre-vaccination showed higher plasma TT-IgG in the <25th centile than >75th centile group at 15 wk (p < 0.05) () and at 1 y (p < 0.10) () of age. Multivariate regression analysis controlling for covariates also showed that pre-vaccination plasma TT-IgG inversely predicted plasma TT-IgG at 15 wk (p < 0.05) and at 1 y of age (p < 0.10) (), In summary, maternal antibody in infant that derived from maternal immunization during pregnancy appears to have an inhibitory effect on infant TT-specific plasma cell development, resulting in reduced plasma antibody accumulation in infant after immunization.
Table 2. Multivariate linear regression analysis of Tetanus (TT) and Pertussis (PT) specific IgG responses in antibody secreting lymphocyte responses (ALS) at 15 wk and plasma antibody level at 1 y of age, received three doses of pentavalent vaccines (DPT/Hib/HBV) at 1 mointerval beginning at 6 wk of age.
Infant response to pertussis immunization
The kinetics of the PT-IgG responses to vaccination was similar to tetanus. As mothers did not receive pertussis vaccines during pregnancy, plasma PT-IgG in infants was found below the cut-off of protection at 6 wk of age before vaccination (). However, 15.2% (5/33) infants had plasma PT-IgG above the cut-off value at 6 wk, indicated natural exposure of pertussis antigen in mothers. At 15 wk of age plasma anti-PT-IgG was increased by 7.14 fold relative to 6 wk (95% CI: 10.2–4.07), similar to the finding with TT-IgG, and by 1 y of age it was decreased by 2.25 fold (95% CI: 2.54–1.96), but this fold-reduction in anti-PT-IgG was significantly lower than was seen with the anti-TT-IgG response (p < 0.01). In addition, and again in contrast to tetanus, plasma PT-IgG remained significantly higher at 1 y of age compared to 6 wk (). However, 17.7% (6/34) infants had PT-IgG below protection level at 1 y. Similar to tetanus, as anticipated, PT-IgG secretion from infant lymphocytes (ALS) at 15 wk was positively correlated with infant plasma PT-IgG level both at 15 wk (rs= 0.438 p < 0.01) and at 1 y (rs = 0.523 p < 0.01) of age ( and ) but there was a negative association between pre-vaccination plasma PT-IgG at 6 wk and ALS PT-IgG at 15 wk (rs = −0.473 p < 0.01). When infants were categorized according to the concentration of PT-IgG at 6 wk pre-vaccination (<25th centile, 25–75th centile or >75th centile), a lower ALS PT-IgG was seen in the >75th centile group compared to <25th centile (p < 0.05) and the 25–75th centile (p < 0.10) groups (). Pre-vaccination plasma PT-IgG also inversely predicted ALS PT-IgG marginally (p<0.10) in multivariate regression analysis controlling for covariates (). In contrast to tetanus, this apparent inhibitory effect on plasma cell developement showed no significant effect in the accumulation of plasma PT-IgG level at 15 wk and 1 y of age and there were no significant correlations between pre-vaccination PT-IgG at 6 wk and post- vaccination plasma levels either at 15 wk (rs = −0.172 p = 0.34) or at 1 y (rs = −0.184 p = 0.30) of age. Categorizing infants in centiles according to the pre-vaccination concentration of PT-IgG also showed no association ( and ) nor was an association seen in multivariate regression analysis controlling for different covariates ().
Figure 3. Pertussis vaccine specific antibody responses of infants (geometric mean with 95% CI). (A) Plasma anti-PT IgG levels of infants at 6, 15 and 1 y of age, received three doses of DTwP-HepB-Hib combination vaccines at 1 mo interval beginning at 6 wk of age. (B) Antibody secreting lymphocyte (ALS) anti-PT IgG responses at 15 wk of age. (C) Plasma anti-PT IgG levels of infants at 15 wk of age and (D) Plasma anti-PT IgG levels of infants at 1 y of age by pre-vaccination (6 wk) antibody levels. Centiles are based on data from this study population. Specific anti-PT IgG (DU/mL) ranges for centile group at 6 wk were: <25th centile 0.82 – 4.60; 25–75th centile 5.41 – 9.87; >75th centile 10.1–21.6. Dotted horizontal line represents protective plasma anti-PT-IgG level. **p < 0.01, *p < 0.05, #p < 0.10.
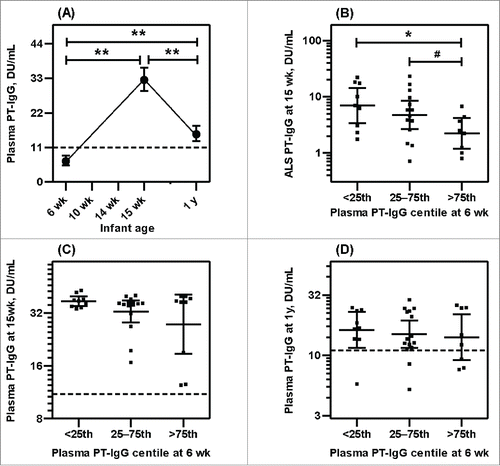
Multivariate regression analysis however, revealed sex differences in the pertussis vaccine response in that girls showed higher anti-PT-IgG secretion by PBMC and higher plasma PT-IgG than boys both at 15 wk and 1 y of age ().
No significant correlations were detected between pre-vaccination TT-IgG and ALS PT-IgG at 15 wk (rs = −0.222 p = 0.23) or plasma PT-IgG at 1 y of age (rs = −0.244 p = 0.17). Although a trend of negative association appeared between pre-vaccination TT-IgG and plasma PT-IgG at 15 wk (rs = −0.331 p = 0.06), multivariate regression analysis controlling for mode of delivery, infant sex, birth weight, gestational age, and pre-vaccination antibody level revealed no significant association between these variables (data not shown). Thus, maternal antibody that inhibits infant vaccine response appears to be antigen-specific.
In summary, our study results indicated that maternal antibody derived from natural exposure of pertussis antigen although small in concentration was nonetheless able to inhibit plasma cell development, as was seen for tetanus antibody obtained from maternal immunization. However, maternal pertussis antibodies from natural exposure showed no effect on infant plasma antibody accumulation after immunization.
Discussion
Our study is the first to investigate the effect of maternally derived antibody on the infant's antibody secretion abilities of lymphocytes in response to infant immunization. Our data suggests that maternal antibodies regardless of antenatal vaccination or natural exposure showed similar inhibitory effects on antigen-specific plasma cell development.
Pre-vaccination anti-PT-IgG in infants was presumably derived from maternal subclinical natural exposure to pertussis in their childbearing age as pertussis is endemic worldwide, even in areas with high vaccination rates. Low level of pre-vaccination anti-PT-IgG () could be as a result of rapid degradation of maternal antibodies despite efficient active placental transfer.Citation12,13 The half-life of maternal PT-IgG is estimated to be ˜40 days Citation14 and our study infants started their vaccination at ˜45 days (). However, such a low titer of maternal antibody might have higher ability to bind and neutralize vaccine Ag and prevent processing by the infant immune system to produce vaccine-specific antibodies, thus decreasing plasma cell development after immunizations as we observed in this study. Both quantity and quality of antibodies after infection or vaccination increase over time, as is expected during maturation of the antibody response. In antigen-antibody interactions, avidity is commonly referred to as “functional affinity” in which it indicates the strength of antibody to interact with multiple antigen-binding sites simultaneously with the target antigenic epitopes. In healthy adult individuals with previous, possibly silent pertussis infections were found to have higher antibody avidity but a lower concentration than those who received the booster pertussis vaccination.Citation15 Several other vaccine studies also indicate that maternal antibodies in the offspring of naturally immune are more potent than vaccinated women e.g. vaccinating women with live attenuated measles vaccine offered shorter term protection against measles to their children than naturally infected mothers,Citation16-18 suggest generation of higher avidity antibodies after infection than after vaccination. Such antibody, although low in concentration, can bind pertussis vaccine antigen more effectively to promote opsono-phagocytosis and enhance clearance by macrophages and neutrophils.
In addition to quality, quantity of passively derived maternal antibodies is important to neutralize vaccine antigen. Mothers in our study received tetanus vaccine during pregnancy and as a consequence a high level of pre-vaccination anti-TT-IgG develops and likely persists longer in infants and thus neutralizes vaccine antigens during the course of infant immunizations to produce a long-lasting inhibitory effect on the development of the anti-TT-IgG plasma cell response, which would diminish plasma anti-TT-IgG accumulation and protection in infants ().
Few studies have addressed the mechanism by which maternal antibodies inhibit seroconversion after infant immunization. The proposed mechanisms include antibody feedback regulation Citation19 and epitope masking of antigens by passive antibodies to prevent B cell recognition through the B cell receptors. The magnitude of this effect would depend on the concentration of antibody present in the circulation. However, experimentally it has been shown that one antibody at a high concentration is less efficient in inhibiting active vaccination response than several antibodies at lower concentrations.Citation20 Presumably natural infection produced a more diverse array of antibodies than vaccines in the subclinical maternal pertussis infection as observed in our study. Nevertheless, inhibition of B cell responses can also be mediated by binding Fc region of passive antibody-vaccine Ag complex to the B cell Fcγ-receptor IIB, results in a stronger negative signal that inhibits both the proliferation Citation21 and the antibodies secretion of B cells.Citation20
In this study, we observed a 9-fold increase in plasma anti-TT-IgG at 1 wk after three doses of primary immunizations (at 1.5, 2.5 and 3.5 mo of age), while at 1 y of age, it was decreased by about 5-fold from the peak and had returned to the pre-vaccination level (). One study in the US showed a 2.5-fold decrease in infant plasma anti-TT-IgG at 4 wk after three doses of primary immunizations (at 2, 4 and 6 mo of age) compare to pre-vaccination level whose mothers received Tdap vaccine during pregnancy.Citation22 We observed that 84.2% of infants had a non-protective level of PT-IgG in plasma at 6 wk of age. These infants were born to unvaccinated mothers, similar to the studies in US population.Citation13 There was a 7-fold increase in plasma anti-PT-IgG at 15 wk of age (), while another study has shown an 11 to 18-fold increases in antibodies specific for four different pertussis antigens 4 wk after primary immunizations of infants whose mothers did not receive pertussis vaccine during pregnancy.Citation22 However, there was a 2.25-fold decreased at 1 y from the peak level at 15 wk of age (). Thus vaccine-induced antibody levels in young infants appear quite unstable and a fourth dose of vaccine may be required in later infancy as reported.Citation22
Infants have immature immune systems that can result in poor vaccine responses and multiple immunizations are required in order to obtain protective level of antibody.Citation23 In addition, colonization of gut microbiota during early infancy, which depends on regional environment factors,Citation24 can influence memory B-cell maturation,Citation25 thymic development and T-cell responses to vaccines in early infancy.Citation26 Our study, however, also indicates rapid waning of vaccine-induced antibody level in infants, and may indicate immature mechanisms involved in plasma IgG homeostasis. Maintaining a high IgG titer is regarded as evidence of continued protection, but the mechanisms by which this is attained remain unclear. ‘Neonatal’; Fc receptor (FcRn) is considered involved in plasma IgG homeostasis.Citation27 FcRn is ubiquitously found in human endothelial cells,Citation28 and acts as a protective receptor which binds and recycles plasma IgG while unbound IgG accumulates in the lysosomal pathway for degradation.Citation27 The functional maturity of FcRn in neonates and infants has not been described.
One intriguing finding in this study is the sex difference in pertussis vaccine response. Girls showed a significantly higher plasma cell response than boys and this difference was also reflected in their plasma antibody accumulation (). In adults, females show a higher humoral immune responses to several viral vaccines including influenza, yellow-fever and hepatitis B.Citation29
Our study has several limitations. Firstly, the sample size of our follow-up cohort was small due to requiring follow-up within a particular time frame. Although the demographic characteristics of our study infants () are comparable to the parent study,Citation11 the mean birth weight was 4.8% higher in this follow-up group, which could bias our results. Our study population also differs from the general Bangladeshi population, for example, enrolment of mothers only from maternity clinic and high proportion of delivery by caesarean section. Clinic enrollment did not skew our study subjects toward higher socio-economic status, since this public clinic provides maternal and child care at highly subsidized rate or at no cost. According to Bangladesh Demographic and Health Survey 2011, in urban area three in five births in a facility are delivered by c-section.Citation30 These confounding factors were controlled in multivariate analysis. Finally, we assessed “protective” plasma antibody threshold by using internationally agreed correlates of protection exist for tetanus, but there is no agreed correlate for pertussis. We reported a “positive” response to pertussis as defined by the manufacturer of the ELISA kit that estimates anti-PT-IgG to Bordetella pertussis and Bordetella pertussis toxin. Measuring the concentrations of antibodies to pertussis toxin, filamentous hemagglutinin, pertactin, and fimbriae separately could have provided additional information.
In summary, our data shows an inhibitory association of higher concentrations of maternal antibody with subsequent plasma cell development (using the ALS assay) and plasma antibody concentrations in response to infant vaccination; this effect appears similar in magnitude irrespective of antenatal immunization or natural exposure to infection in women of childbearing age. Our study provides evidence that maternal antibody down-regulates the infant immune response to vaccinations. This conclusion aligns with the results of previous studies examining plasma antibody concentrations after vaccination. However, maternal immunization seems the only way to protect infants from infection during neonatal period. We found 89.5% and 82.3% infants at 1 y had protective level of anti-TT and anti-PT IgG in plasma respectively. Thus maternal TT immunization does not compromise the protection level in the long run but insured 94.6% protection in infants from tetanus during the neonatal period, which is considerable benefit as a result of maternal immunization and public health policy perspective to initiate such programs considering the risk of infection during neonatal period as well as regional epidemiology of infection.
Materials and methods
Study population
The presented data in this manuscript were generated during follow-up of a randomized placebo-controlled clinical trial of 50,000 IU vitamin A supplementation of newborn babies within 48 h of birth, conducted at icddr,b Dhaka Bangladesh between January 2012 and August 2013.Citation11 The study protocol was peer reviewed and approved by the Research Review Committee (RRC) and Ethical Review Committee (ERC) of the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) (FWA-00001468; IRB-00001822). The study protocol, recruitment process and cohort characteristics have been described.Citation11 Briefly the study was a block-randomized, double-masked, placebo-controlled intervention trial in which equal numbers of girls (n = 153) and boys (n = 153) were enrolled.Citation11 Informed written consents were obtained from mothers along with additional written consents of using stored biological samples for future studies. Infants received the following vaccines in primary healthcare according to the immunization schedule for infants in Bangladesh: BCG (Serum Institute of India, Ltd) and trivalent OPV (OPVERO; Sanofi Pasteur) within 48 h of birth, pentavalent DTwP-HepB-Hib vaccine (Diphtheria/Tetanus/whole cell Pertussis-Hepatitis B-Haemophilus influenzae type b vaccine; Quinvaxem; Berna Biotech Korea Corporation) and OPV at 6, 10 and 14 weeks of age and measles-rubella vaccines (Serum Institute of India, Ltd) at 9 months of age. All mothers received only a single dose of tetanus vaccine (Serum Institute of India, Ltd) in their third trimester as part of antenatal care policy. Using structured questionnaires, infant morbidity, dietary and anthropometry data were collected on weekly basis up to 15 wk of age since birth.Citation11 The data of 38 infants in this manuscript were selected who were free from infection in the parent trial, included from the same order of recruited newborns in the parent trial and followed them up to 1 y of age. This data presented in this manuscript did not include group analysis of the parent study as it was of insufficient size to allow meaningful comparison. We utilized the spare plasma and cell culture samples for antibody analysis. Other parameters such as birth history, anthropometry and blood panel of biochemical indicators were obtained from the parent study.Citation11
Sample collection
Peripheral blood samples from infants were obtained at 6 wk of age immediate before vaccination and again at 15 wk and 1 y of age. 15 wk blood sampling was 7–10 d after the third dose of DTwP-HepB-Hib vaccination for the ALS assessment. PBMC were separated from fresh heparinaized blood using a standard density gradient method (Ficoll-Paque-PLUS, Amersham Biosciences) and plasma samples were extracted and stored at −80°C.
Assay for TT and PT specific IgG secretion abilities of infant PBMC by ALS method
In the ALS method, PBMC were washed twice with PBS to remove residual plasma containing antibody. PBMC at 1 × 107cells/mL were cultured in plating medium (RPMI, 10% fetal calf serum, and 1% penicillin–streptomycin) for 2 days at 37°C in 5% CO2 without any stimulants. Cells were then centrifuged at 750 × g for 10 min and supernatant was preserved with protease inhibitor and stored at −80°C. These assays were conducted at 15 wk of age.
TT and PT specific IgG measurement
Bordetella pertussis specific anti-IgG in stored plasma and ALS samples was measured by using Bordetella pertussis IgG ELISA kit (20-BPGHU-E01, ALPCO Diagnostics; Salem, NH), a semi-quantitative determination of IgG-class antibodies to Bordetella pertussis and Bordetella pertussis toxin in plasma, intended for in vitro diagnostic use. Results are reported as a collective IgG response to pertussis antigens. There is no established correlate of antibody response to multiple or single antigens and protection from pertussis, however, low antibody level increase the susceptibility to infection.Citation31-34 In the literature it is established that a ‘positive’ diagnostic test may be consistent with some measure of protection.Citation35 Pertussis titers of >11 DU IgG/mL in plasma were regarded as protective level, the diagnostic specificity and sensitivity of >11 DU IgG/mL is 95.4% and 95.5% respectively. TT specific IgG were measured by ELISA kits (VaccZyme, The Binding Site; San Degio, CA) and >0.15 IU/mL in plasma were classified as providing sufficient protection by World Health Organization.Citation36
Statistical analysis
Statistical analyses were conducted using SigmaStat for Windows Version 3.5 (Systat Software, Inc.) and GraphPad Prism 5 for Windows (GraphPad Software). Distributions of study variables were analyzed and test assumptions of normality and equal variance. Data were transformed to produce normal distributions as needed. “Fold-change” in vaccine response was calculated as the ratio of plasma antibody level for each individual infant's pre- to post- vaccination. Repeated-measures one-way ANOVA using the Holm-Sidak post hoc comparison procedure was used to compare vaccine-specific plasma IgG level at 6 wk, 15 wk and 1 y of age. One-way ANOVA was used to compare the three sets of infants grouped according to centiles. Spearman's correlation co-efficient was used for tests of correlation. We found significant negative association between “interval between vaccination and blood collection for ALS assay” and ALS antibody level for both TT (r = −0.639, p < 0.01) and PT (r = −0.404, p = 0.01). Multivariate linear regression analysis was used to adjust confounding factor “interval between vaccination and blood collection for ALS/plasma antibody assay” and also other factors known to influence vaccine specific IgG responses in plasma and ALS; such as mode of delivery, sex, birth weight, gestational age and plasma vaccine specific IgG level at 6 wk of age (pre- vaccination). In this analysis, plasma/ALS antibody levels were log transformed to make normal distribution and equal variance. In these analysis variance inflation factor (VIF) were <2.0. Two-sided p ≤ 0.05 was considered significant.
Abbreviations
| ALS | = | Antibody in Lymphocyte Supernatant |
| ASC | = | Antibody Secreting Cells |
| BCG | = | Bacillus Calmette-Guérin |
| DTwP-HepB-Hib | = | Diphtheria/Tetanus/whole cell Pertussis-Hepatitis B-Haemophilus influenzae type b vaccine |
| OPV | = | oral polio vaccine |
| PBMC | = | Peripheral Blood Mononuclear Cells |
| PT | = | Pertussis antigen |
| TT | = | Tetanus Toxoid |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors gratefully acknowledge the participation of all cohort infants and their families. We also acknowledge Dr. Md. Sirajul Islam and Dr. Chinmoy K. Das at the collaborating clinic Maternal & Child Health Training Institute (MCHTI), Azimpur, Dhaka for the logistic and technical support.
Funding
This research protocol/activity/study was funded by icddr,b's core donors and World Health Organization (WHO) project 2010168947, funded by the Bill and Melinda Gates Foundation. icddr,b acknowledges with gratitude the commitment of US Department of Agriculture–Agricultural Research Service to its research efforts. icddr,b also gratefully acknowledges the following core donors which provide unrestricted support: Australian Agency for International Development (AusAID), Government of the People's Republic of Bangladesh, Canadian International Development Agency (CIDA), Swedish International Development Cooperation Agency (Sida), and the Department for International Development, UK (DFID).
References
- Leuridan E, Van Damme P. Passive transmission and persistence of naturally acquired or vaccine-induced maternal antibodies against measles in newborns. Vaccine 2007; 25:6296-304; PMID:17629601; http://dx.doi.org/10.1016/j.vaccine.2007.06.020
- Gans H, Yasukawa L, Rinki M, DeHovitz R, Forghani B, Beeler J, Audet S, Maldonado Y, Arvin AM. Immune responses to measles and mumps vaccination of infants at 6, 9, and 12 months. J Infect Dis 2001; 184:817-26; PMID:11528592; http://dx.doi.org/10.1086/323346
- Klinge J, Lugauer S, Korn K, Heininger U, Stehr K. Comparison of immunogenicity and reactogenicity of a measles, mumps and rubella (MMR) vaccine in German children vaccinated at 9–11, 12–14 or 15–17 months of age. Vaccine 2000; 18:3134-40; PMID:10856793; http://dx.doi.org/10.1016/S0264-410X(00)00096-7
- Wang Z, Zhang S, Luo C, Wu Q, Liu Q, Zhou YH, Hu Y. Transplacentally acquired maternal antibody against hepatitis B surface antigen in infants and its influence on the response to hepatitis B vaccine. PLoS One 2011; 6:e25130; PMID:21966434; http://dx.doi.org/10.1371/journal.pone.0025130
- Englund JA, Anderson EL, Reed GF, Decker MD, Edwards KM, Pichichero ME, Steinhoff MC, Rennels MB, Deforest A, Meade BD. The effect of maternal antibody on the serologic response and the incidence of adverse reactions after primary immunization with acellular and whole-cell pertussis vaccines combined with diphtheria and tetanus toxoids. Pediatrics 1995; 96:580-4; PMID:7659480
- Booy R, Aitken SJ, Taylor S, Tudor-Williams G, Macfarlane JA, Moxon ER, Ashworth LA, Mayon-White RT, Griffiths H, Chapel HM. Immunogenicity of combined diphtheria, tetanus, and pertussis vaccine given at 2, 3, and 4 months versus 3, 5, and 9 months of age. Lancet 1992; 339:507-10; PMID:1346876; http://dx.doi.org/10.1016/0140-6736(92)90336-2
- Chang HS, Sack DA. Development of a novel in vitro assay (ALS assay) for evaluation of vaccine-induced antibody secretion from circulating mucosal lymphocytes. Clin Diagn Lab Immunol 2001; 8:482-8; PMID:11329444
- Kirkpatrick BD, Bentley MD, Thern AM, Larsson CJ, Ventrone C, Sreenivasan MV, Bourgeois L. Comparison of the antibodies in lymphocyte supernatant and antibody-secreting cell assays for measuring intestinal mucosal immune response to a novel oral typhoid vaccine (M01ZH09). Clin Diagn Lab Immunol 2005; 12:1127-9; PMID:16148184
- Bhuiyan TR, Choudhury FK, Khanam F, Saha A, Sayeed MA, Salma U, Lundgren A, Sack DA, Svennerholm AM, Qadri F. Evaluation of immune responses to an oral typhoid vaccine, Ty21a, in children from 2 to 5 years of age in Bangladesh. Vaccine 2014; 32:1055-60; PMID:24440210; http://dx.doi.org/10.1016/j.vaccine.2014.01.001
- Ahmad SM, Haskell MJ, Raqib R, Stephensen CB. Men with low vitamin A stores respond adequately to primary yellow fever and secondary tetanus toxoid vaccination. J Nutr 2008; 138:2276-83; PMID:18936231; http://dx.doi.org/10.3945/jn.108.092056
- Ahmad SM, Raqib R, Qadri F, Stephensen CB. The effect of newborn vitamin A supplementation on infant immune functions: Trial design, interventions, and baseline data. Contemp Clin Trials 2014; 39:269-79; PMID:25269669; http://dx.doi.org/10.1016/j.cct.2014.09.004
- Healy CM, Munoz FM, Rench MA, Halasa NB, Edwards KM, Baker CJ. Prevalence of pertussis antibodies in maternal delivery, cord, and infant serum. J Infect Dis 2004; 190:335-40; PMID:15216470; http://dx.doi.org/10.1086/421033
- Shakib JH, Ralston S, Raissy HH, Stoddard GJ, Edwards KM, Byington CL. Pertussis antibodies in postpartum women and their newborns. J Perinatol 2010; 30:93-7; PMID:19812588; http://dx.doi.org/10.1038/jp.2009.138
- Van Savage J, Decker MD, Edwards KM, Sell SH, Karzon DT. Natural history of pertussis antibody in the infant and effect on vaccine response. J Infect Dis 1990; 161:487-92; PMID:2313127; http://dx.doi.org/10.1093/infdis/161.3.487
- Barkoff AM, Grondahl-Yli-Hannuksela K, Vuononvirta J, Mertsola J, Kallonen T, He Q. Differences in avidity of IgG antibodies to pertussis toxin after acellular pertussis booster vaccination and natural infection. Vaccine 2012; 30:6897-902; PMID:22981763; http://dx.doi.org/10.1016/j.vaccine.2012.09.003
- Lennon JL, Black FL. Maternally derived measles immunity in era of vaccine-protected mothers. J Pediatr 1986; 108:671-6; PMID:3701511; http://dx.doi.org/10.1016/S0022-3476(86)81039-3
- Jenks PJ, Caul EO, Roome AP. Maternally derived measles immunity in children of naturally infected and vaccinated mothers. Epidemiol Infect 1988; 101:473-6; PMID:3053223; http://dx.doi.org/10.1017/S095026880005442X
- Pabst HF, Spady DW, Marusyk RG, Carson MM, Chui LW, Joffres MR, Grimsrud KM. Reduced measles immunity in infants in a well-vaccinated population. Pediatr Infect Dis J 1992; 11:525-9; PMID:1528642; http://dx.doi.org/10.1097/00006454-199207000-00004
- Heyman B, Wigzell H. Immunoregulation by monoclonal sheep erythrocyte-specific IgG antibodies: suppression is correlated to level of antigen binding and not to isotype. J Immunol 1984; 132:1136-43; PMID:6363534
- Kim D, Huey D, Oglesbee M, Niewiesk S. Insights into the regulatory mechanism controlling the inhibition of vaccine-induced seroconversion by maternal antibodies. Blood 2011; 117:6143-51; PMID:21357766; http://dx.doi.org/10.1182/blood-2010-11-320317
- Kim D, Niewiesk S. Synergistic induction of interferon α through TLR-3 and TLR-9 agonists identifies CD21 as interferon α receptor for the B cell response. PLoS Pathog 2013; 9:e1003233; PMID:23516365 http://dx.doi.org/10.1371/journal.ppat.1003233
- Munoz FM, Bond NH, Maccato M, Pinell P, Hammill HA, Swamy GK, Walter EB, Jackson LA, Englund JA, Edwards MS, et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. JAMA 2014; 311:1760-9; PMID:24794369; http://dx.doi.org/; http://dx.doi.org/10.1001/jama.2014.3633
- Gervassi AL, Horton H. Is Infant Immunity Actively Suppressed or Immature? Virology (Auckl) 2014; 2014:1-9; PMID:25429207
- Lin A, Bik EM, Costello EK, Dethlefsen L, Haque R, Relman DA, Singh U. Distinct distal gut microbiome diversity and composition in healthy children from Bangladesh and the United States. PLoS One 2013; 8:e53838; PMID:23349750; http://dx.doi.org/10.1371/journal.pone.0053838
- Lundell AC, Bjornsson V, Ljung A, Ceder M, Johansen S, Lindhagen G, Tornhage CJ, Adlerberth I, Wold AE, Rudin A. Infant B cell memory differentiation and early gut bacterial colonization. J Immunol 2012; 188:4315-22; PMID:22490441; http://dx.doi.org/10.4049/jimmunol.1103223
- Huda MN, Lewis Z, Kalanetra KM, Rashid M, Ahmad SM, Raqib R, Qadri F, Underwood MA, Mills DA, Stephensen CB. Stool microbiota and vaccine responses of infants. Pediatrics 2014; 134:e362-72; PMID:25002669; http://dx.doi.org/10.1542/peds.2013-3937
- Ward ES, Zhou J, Ghetie V, Ober RJ. Evidence to support the cellular mechanism involved in serum IgG homeostasis in humans. Int Immunol 2003; 15:187-95; PMID:12578848; http://dx.doi.org/10.1093/intimm/dxg018
- Kuo TT, Baker K, Yoshida M, Qiao SW, Aveson VG, Lencer WI, Blumberg RS. Neonatal Fc receptor: from immunity to therapeutics. J Clin Immunol 2010; 30:777-89; PMID:20886282; http://dx.doi.org/10.1007/s10875-010-9468-4
- Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis 2010; 10:338-49; PMID:20417416; http://dx.doi.org/10.1016/S1473-3099(10)70049-9
- BDHS. Bangladesh demographic and health survey 2011: National Institute of Population Research and Training, Mitra and Associates, ICF International Calverton, Maryland, USA, 2013
- Cherry JD, Gornbein J, Heininger U, Stehr K. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine 1998; 16:1901-6; PMID:9796041; http://dx.doi.org/10.1016/S0264-410X(98)00226-6
- Storsaeter J, Hallander HO, Gustafsson L, Olin P. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 1998; 16:1907-16; PMID:9796042; http://dx.doi.org/10.1016/S0264-410X(98)00227-8
- Taranger J, Trollfors B, Lagergard T, Sundh V, Bryla DA, Schneerson R, Robbins JB. Correlation between pertussis toxin IgG antibodies in postvaccination sera and subsequent protection against pertussis. J Infect Dis 2000; 181:1010-3; PMID:10720524; http://dx.doi.org/10.1086/315318
- Storsaeter J, Hallander HO, Gustafsson L, Olin P. Low levels of antipertussis antibodies plus lack of history of pertussis correlate with susceptibility after household exposure to Bordetella pertussis. Vaccine 2003; 21:3542-9; PMID:12922081; http://dx.doi.org/10.1016/S0264-410X(03)00407-9
- Gall SA, Myers J, Pichichero M. Maternal immunization with tetanus-diphtheria-pertussis vaccine: effect on maternal and neonatal serum antibody levels. Am J Obstet Gynecol 2011; 204:334e1-5; PMID:21272845
- WHO. World Health Organization. Tetanus vaccine. Wkly Epidemiol Rec 2006; 81:198-208; PMID:16710950
