ABSTRACT
Objective: To determine the effectiveness of existing school entry and education mandates on HPV vaccination coverage, we compared coverage among girls residing in states and jurisdictions with and without education and school-entry mandates. Virginia and the District of Columbia enacted school entry mandates, though both laws included liberal opt-out provisions. Ten additional states had mandates requiring distribution of education to parents or provision of education within school curricula. Methods: Using data from the National Immunization Survey-Teen from 2009–2013, we estimated multilevel logistic regression models to compare coverage with HPV vaccines for girls ages 13–17 residing in states and jurisdictions with and without school entry and education mandates, adjusting for demographic factors, healthcare access, and provider recommendation. Results: Girls residing in states and jurisdictions with HPV vaccine school entry mandates (DC and VA) and education mandates (LA, MI, CO, IN, IA, IL, NJ, NC, TX, and WA) did not have higher HPV vaccine series initiation or completion than those living in states without mandates for any year (2009–2013). Similar results were seen when comparing girls ages 13–14 to those ages 15–17, and after adjustment for known covariates of vaccination. Conclusions: States and jurisdictions with school-entry and education mandates do not currently have higher HPV vaccination coverage than states without such legislation. Liberal opt-out language in existing school entry mandates may weaken their impact. Policy-makers contemplating legislation to improve vaccination coverage should be aware of the limitations of existing mandates.
Introduction
HPV vaccine initiation and completion coverage in the United States are currently 60% initiation/40% completion for girls and 42% initiation/22% completion for the boys below national targets of 80% complete vaccination rates for both female and male adolescents, and HPV vaccination coverage among girls has improved little since 2011.Citation1 Raising HPV vaccination coverage is a priority of the Centers for Disease Control and Prevention, American Academy of Pediatrics, and President's Cancer Panel.Citation1-5 Vaccine mandates are widely considered effective for raising vaccination coverage in children and adolescents,Citation6,7 and school-entry mandates for other vaccines have historically been associated with increased vaccine use, decreased disease prevalence, and reduced racial disparities in disease rates in states with mandates compared to those without.Citation8,9
Because school-entry mandates have been successful in raising coverage for other vaccinations, policymakers have suggested mandating HPV vaccination to increase coverage.Citation10 After HPV vaccination was recommended by the Advisory Committee on Immunization Practices in 2006,Citation11 24 states and regions initiated legislation to mandate HPV vaccination.Citation12 Parents indicated only modest support for school entry mandates for HPV vaccination,Citation13,14 and the introduction of this legislation met with substantial public backlash.Citation15,16. By 2008, only Virginia and the District of Columbia had enacted school-entry mandates, and these included liberal opt-out provisions for HPV vaccine that did not apply to other vaccinations.Citation12 An additional 10 states enacted mandatory HPV vaccine education, including both parental education and school curricula.Citation12 We used national data to compare HPV vaccination coverage among girls residing in states and the District of Columbia (hereafter referred to as “states and jurisdictions” for ease of reading) with legislation requiring HPV vaccination for school entry (school-entry mandates), legislation requiring distribution of educational materials about HPV vaccination to parents or including HPV vaccination in mandated school curricula (education mandates), and no mandates to determine the impact of existing legislation.
Results
A total of 47,845 parents/guardians of girls ages 13–17 participated in the National Immunization Survey-Teen between 2009–2013 (). Of that total, 1,649 (3.4%) girls resided in states and jurisdictions with school-entry mandates for HPV vaccines, and 12,579 (26.9%) resided in states with education mandates. Girls living in states and jurisdictions with school-entry or education HPV vaccine mandates had similar levels of HPV vaccine series initiation and completion as those living in areas without mandates for each year individually as well as all years combined (, ).
Figure 1. HPV vaccine series initiation and completion were similar for 13–17 year old girls living in states and jurisdictions with and without education and school-entry mandates. The top panel depicts unadjusted coverage levels, and the bottom panel depicts coverage levels after adjustment for individual and state-level factors.
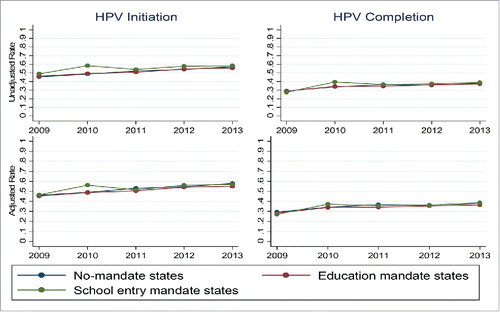
Table 1. Listing of states by type of mandate, year legislation took effect, and legislation content.
Table 2. Description of populations included in NIS-teen surveys 2009–2013 by mandate type (n = 47,845).
Table 3. Adjusted and unadjusted HPV vaccination coverage rates by year for females aged 13–17.
In 2009, HPV vaccine initiation coverage was 45% (95% CI 42–49%) for girls ages 13–17 in states without mandates, 46% (95% CI 40–53%) for girls in states with education mandates, and 49% (95% CI 34–63%) for girls in states and jurisdictions with school-entry mandates. By 2013, coverage had risen to 57% (95% CI 55–60%) for girls in states without mandates, 56% (95% CI 51–60%) for girls in states with education mandates, and 58% (95% CI 48–69%) for girls in states and jurisdictions with school-entry mandates. Patterns of completion were similar. In 2009, HPV vaccine completion levels were 29% (95% CI 26–32%) for girls in states without mandates, 29% (95% CI 23–35%) for girls in states with education mandates, and 28% (95% CI 15–40%) for girls in states and jurisdictions with school-entry mandates. By 2013, three dose coverage had risen to 38% (95% CI 36–40%) for girls in states without mandates, 37% (95% CI 33–42%) for girls in states with education mandates, and 39% (95% CI 28–50%) for girls in states and jurisdictions with school-entry mandates. Adjustment for known covariates of vaccination did not change the results (, ). Because mandate legislation largely affected girls ages 11–13 and their parents,Citation12 we performed separate analyses for girls ages 13 and 14 and those aged 15–17 (Appendix ). Although the NIS-Teen collects data only on 13–17 year olds, those who were 11–12 when legislation was implemented in 2008–2009 would be expected to age into the cohort by 2010–2011. Patterns of HPV vaccine initiation appeared similar for younger compared to older girls, regardless of the presence or absence of mandates. Older girls were more likely to complete the series than younger girls, but the presence or absence of mandates was not associated with series completion in either age group.
Discussion
School-entry mandates, although at times considered controversial, are widely considered to be effective tools for achieving and maintaining high coverage with recommended childhood vaccines.Citation6,8,17 NIS data examined previously indicated that daycare and school entry requirements led to significantly higher varicella vaccination rates in states that implemented mandates compared to those that did not, despite those states having similar vaccination rates at baseline.Citation18 Yet, we found no difference in HPV vaccination coverage between girls living in states and jurisdictions with and without education or school-entry mandates. HPV vaccine mandate laws were passed soon after HPV vaccine introduction in these states and jurisdictions, which limited our ability to compare vaccination coverage in the same states and jurisdictions prior to and following mandate enactment. However, the passage of legislation did not result in higher vaccine coverage in states and jurisdictions with mandates compared to those without. Thus, understanding factors contributing to persistently low vaccination coverage despite legislative mandates, including potential differences in the way laws designed and enacted, is important prior to enacting new laws aimed at raising vaccination coverage.Citation12,19
Compulsory vaccination is part of a larger spectrum of political, policy, and socio-cultural factors, and public opinion can either facilitate or undermine legislation effectiveness.Citation20 After the quadrivalent HPV vaccine was approved by the FDA, the pharmaceutical industry engaged in substantial lobbying of political groups, like Women in Government, and individuals, like Gov. Rick Perry of Texas to pass vaccine mandates.Citation21 In the case of HPV vaccine mandates in Virginia and DC, legislation moved rapidly through the legislature without enlisting adequate input from key stakeholders such as healthcare providers, public health policymakers, educators, and parents. In the absence of public consensus about the vaccine's benefits, there were widely publicized debates about concerns that HPV vaccines were too new to be considered safe and effective, that pharmaceutical companies were untrustworthy that the media had exaggerated the worries that the HPV vaccine would promote promiscuity,Citation22 and that mandates were impinging on parental rights to make decisions for their children and forcing them to have conversations about sexuality before they believed their children were ready. Citation23,24 Interviews with Virginia parents indicated that many parents did “opt-out” of vaccinating their daughters,Citation24 and the data in this study corroborate low-levels of compliance with mandates. All girls who were aged 13–14 in 2012 and 2013 would have been affected by the school entry mandate laws, yet HPV vaccine initiation rates in Virginia in 2012 and 2013 were 54% and 57% respectively, suggesting that nearly half of parents may have been opting out.
The public controversy surrounding mandate development in both Virginia and DC led to the inclusion of liberal opt-out language in HPV vaccine mandates that did not apply to other mandated vaccines and substantially weakened the enacted legislation.Citation25,26 Parents could simply elect to not vaccinate their daughters after reviewing vaccine materials;Citation12 thus, these mandates functioned more as education requirements than school-entry requirements. Education and understanding around vaccine benefits are believed to be important components of vaccine acceptance. However, as shown in this study and others, the current implementation of existing legislative mandates requiring education have limited impact.Citation17
The Association of Immunization Managers believes that school-entry mandates must be enacted in the setting of adequate physician and public support as well as high vaccination coverage prior to mandate enforcement to reduce the compliance burden on schools and to limit public backlash that could risk loss of support for other immunization programs.Citation27 Other criteria used to evaluate whether or not vaccines should be mandated for school entry include: Advisory Committee on Immunization Practices recommendation, demonstrated effectiveness, cost-effectiveness, adequate track record of safety, prevention of a disease with significant morbidity and mortality, reduction in the risk of transmission from person-to-person, vaccine acceptability to the community and the public, reasonable administrative burdens for schools and health care providers required to track vaccines, and reasonable burdens on parents/adolescents to comply with requirements.Citation28 While HPV vaccines meet the effectiveness, safety, and prevention of transmission and morbidity requirements described above,Citation29-32 public acceptance is variable, and currently low coverage would result in substantial administrative burdens for school systems trying to enforce mandates in many regions. Thus, careful examination of public opinion and large-scale public education campaigns may be necessary to improve public opinion and to raise vaccination coverage substantially prior to enacting HPV mandates if the mandates are to succeed.Citation20,33 Rhode Island was the first state to enact a school-entry requirement for HPV vaccination that did not allow special exemptions and that applies to both males and females. Their mandate took effect on August 1, 2015. Rhode Island is well positioned for this challenge as they lead the nation in HPV vaccination rates: 77% initiation for girls and 69% for boys in 2013.Citation34 By enacting legislation after achieving high vaccination rates and broad public support, including both males and females in the requirements, and not allowing opt-out provisions that do not apply to other vaccines, the Rhode Island HPV vaccine legislation has the potential to succeed where other legislation failed. Other states with high vaccine coverage for males and females may observe the outcomes of the Rhode Island legislation when considering implementing their own legislative efforts.
Few other countries require HPV vaccination for school entry. The countries with the highest population coverage for HPV vaccines and largest declines in HPV-related disease outcomes (UK, Australia, and New Zealand) do not require HPV vaccination for school entry, but provide vaccines free-of-charge in schools to all eligible students.Citation35 Thus, school-located vaccination may be considered as an alternative to school-entry requirements.
Strengths and limitations
Strengths of this study include the use of provider-verified HPV vaccination data from a nationally representative sample of parents in the United States. Different methods of vaccine delivery, payment structures, and additional factors in other countries limit the applicability of this study outside the United States. Additional limitations include the relatively small number of girls living in states and jurisdictions with school-entry mandates, survey response rates under 60%, possible selection bias between teens whose parents completed the survey and those who did not, lack of information on parental beliefs from survey data, and inability to examine gender differences due to limited data on males. An additional limitation is the lack of data on vaccination coverage prior to mandate enactment because most legislation was passed within one year of HPV vaccine data availability in NIS-teen. Finally, the NIS-teen data do not differentiate whether teens received the bivalent or quadrivalent vaccine, nor their views on the effectiveness of these vaccines, or whether availability of the 9-valent HPV vaccine, with improved protection, would increase utilization.
Conclusion
Current school-entry and education mandate legislation around HPV vaccination have had limited impacts. States considering the use of mandates to raise HPV vaccination coverage must consider whether public climate will allow for strongly worded laws requiring vaccination for school entry, because laws requiring education only or including liberal opt-out language in school-entry requirements may have limited impacts on vaccine coverage.Citation36 Focusing HPV vaccine policies on promoting voluntary uptake and enlisting providers to give strong, consistent recommendations to prevent skepticism and hesitancy among parents and adolescents may be an important step prior to considering legislation.Citation10
Methods
Data were obtained from the publicly accessible files of the annual National Immunization Survey – Teen (NIS-Teen) surveys for 2009–2013.Citation37 NIS-teen has been conducted by the Centers for Disease Control and Prevention since 2008. The surveys collect data from a nationally representative survey of households with adolescents aged 13 to 17.Citation38 Information on eligible adolescents' vaccination status is obtained via a stratified random-digit-dialing survey of parents/guardians,Footnote1 then vaccination status is confirmed with healthcare providers.Citation39 Similar to previous studies using this database, we included girls for whom vaccination was reported by the parent/guardian and confirmed by the provider.Citation38,40 National data collected in 2009–2013 were released with a one-year delay (i.e., 2013 data were released in 2014); data were analyzed in 2015. The Boston University School of Medicine institutional review board deemed this project exempt.
Our primary outcomes of interest were initiation (receipt of at least one dose) and completion (receipt of 3 doses) of the HPV vaccine series among girls aged 13 to 17 on or prior to the date of the survey interview. Data on HPV vaccine legislation was obtained from the National Conference of State Legislatures,Citation12 and confirmed by correspondence with the website manager. NIS-teen data for HPV vaccination was first measured in 2007, and HPV vaccination mandate laws were passed during 2007–2008. Because mandates were enacted soon after vaccine availability, there was no pre-mandate time period to analyze to determine whether states and jurisdictions' individual levels of HPV vaccine coverage changed after mandate implementation. We therefore analyzed data beginning in 2009 to allow time for legislation implementation effect, and thereafter compared coverage in states and jurisdictions with and without school entry and education mandates. We adjusted results for covariates known to correlate with vaccination coverage: child's and parent's age, race, parent's education level, household income, parent's marital status, parent's primary language, receipt of vaccine in a private or public health facility, insurance status, number of medical visits in the past year, and provider recommendation.Citation41-43 Because HPV vaccination coverage changed over time, we considered each year separately as well as pooling data from all years together. Random effects logistic regression models were used to estimate adjusted HPV vaccination coverage rates adjusting for these covariates, year, and the systematic unobserved variation arising from clustering of observations at the state level. State level factors were controlled for by including state level random effects in all models. Analyses of pooled data and data for each year were performed separately, and weights provided in the data source files to adjust for the multi-stage stratified sampling design and response rates were used to obtain nationally representative estimates.Citation39 Using pooled data, we also estimated a model of annual linear trend in vaccination rates. Because most mandates were aimed at middle school girls, we also performed analyses stratified by age (13–14 and 15–17) to see if a greater impact was seen in younger girls. The distribution of states and jurisdictions with HPV legislation and the years in which legislation was enacted are detailed in . We considered states and jurisdictions to have school-entry mandates if their laws included language requiring HPV vaccination for school attendance. We considered states and jurisdictions to have education mandates if their laws included language requiring distribution of educational materials to parents, or requiring education on HPV and HPV vaccination in sexual education in schools.
Abbreviations
| HPV, | = | Human Papillomavirus; |
| NIS-Teen, | = | National Immunization Survey- Teen |
Disclosure of potential conflicts of interest
No author declares a conflict of interest or financial disclosure.
Acknowledgments
The authors would like to thank Susan Lett MD, Medical Director, Immunization Program, Division of Epidemiology and Immunization, Massachusetts Department of Public Health for her insights on this manuscript.
Notes
1 Cell phones were included in addition to landlines in 2011 and 2012; analysis including and excluding cell phones yielded similar results, therefore cell phone data are included in results from 2011 and 2012.
References
- Stokley S, Jeyarajah J, Yankey D, Cano M, Gee J, Roark J, Curtis RC, Markowitz L; Immunization Services Division, National Center for Immunization and Respiratory Diseases, CDC; Centers for Disease Control and Prevention (CDC). Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014 - United States MMWR Morb Mortal Wkly Rep Jul 25 2014; 63(29):620-4; PMID:25055185
- PresidentsCancerPanel. President's Cancer Panel Annual Report 2012–2013: Accelerating HPV vaccine uptake: urgency for action to prevent cancer. 2014; http://deainfo.nci.nih.gov/ADVISORY/pcp/annualReports/HPV/index.htm-sthash.VFWWaxYh.dpbs. Accessed February 24, 2014.
- AAP. HPV vaccination. 2013; http://www2.aap.org/immunization/illnesses/hpv/hpv.html. Accessed August 7, 2013.
- CDC. HPV vaccine resources for healthcare professionals. 2015; http://www.cdc.gov/vaccines/who/teens/for-hcp/hpv-resources.html. Accessed June 29, 2015.
- CDC. What CDC Is Doing About HPV and Cancer. 2013; http://www.cdc.gov/cancer/hpv/what_cdc_is_doing/. Accessed June 29, 2015.
- Orenstein WA, Hinman AR. The immunization system in the United States - the role of school immunization laws. Vaccine Oct 29 1999; 17 Suppl 3:S19-24; PMID:10559531
- Simpson JE, Hills RA, Allwes D, Rasmussen L. Uptake of meningococcal vaccine in Arizona schoolchildren after implementation of school-entry immunization requirements. Public Health Rep Jan-Feb 2013; 128(1):37-45
- Centers for Disease C, Prevention. Impact of vaccines universally recommended for children–United States, 1990–1998. MMWR Morb Mortal Wkly Rep Apr 2 1999; 48(12):243-8; PMID:10220251
- Morita JY, Ramirez E, Trick WE. Effect of a school-entry vaccination requirement on racial and ethnic disparities in hepatitis B immunization coverage levels among public school students. Pediatrics Mar 2008; 121(3):e547–52; PMID:18310176; http://dx.doi.org/10.1542/peds.2007-0799
- Osazuwa-Peters N. Human papillomavirus (HPV), HPV-associated oropharyngeal cancer, and HPV vaccine in the United States–do we need a broader vaccine policy? Vaccine Nov 12 2013; 31(47):5500-05; PMID:24095883; http://dx.doi.org/10.1016/j.vaccine.2013.09.031
- ACIP. Quadrivalent Human papillomavirus vaccine: recommendations of the advisory comitte on immunization practices. MMWR Morbidity and Mortality Weekly Report Marh 23, 2007 2007; 56(No RR-2)
- NCOSL. National conference of state legislatures HPV vaccine: state legislation. http://www.ncsl.org/IssuesResearch/Health/HPVVaccineStateLegislation/tabid/14381/Default.aspx. 2015; Accessed March 15, 2015.
- Robitz R, Gottlieb SL, De Rosa CJ, Guerry SL, Liddon N, Zaidi A, Walker S, Smith JS, Brewer NT, Markowitz LE. Parent attitudes about school requirements for human papillomavirus vaccine in high-risk communities of Los Angeles, California Cancer Epidemiol Biomarkers Prev Jul 2011; 20(7):1421-9; PMID:21551243; http://dx.doi.org/10.1158/1055-9965.EPI-10-1236
- Perkins RB, Pierre-Joseph N, Marquez C, Iloka S, Clark JA. Parents' opinions of mandatory human papillomavirus vaccination: does ethnicity matter? Womens Health Issues Nov-Dec 2010; 20(6):420-6; PMID:21051001; http://dx.doi.org/10.1016/j.whi.2010.07.001
- Balog JE. The moral justification for a compulsory human papillomavirus vaccination program. Am J Public Health Apr 2009; 99(4):616-22; PMID:19197085; http://dx.doi.org/10.2105/AJPH.2007.131656
- Gollust SE, Dempsey AF, Lantz PM, Ubel PA, Fowler EF. Controversy undermines support for state mandates on the human papillomavirus vaccine. Health Aff (Millwood) Nov 2010; 29(11):2041-6; PMID:21041746; http://dx.doi.org/10.1377/hlthaff.2010.0174
- Bugenske E, Stokley S, Kennedy A, Dorell C. Middle school vaccination requirements and adolescent vaccination coverage. Pediatrics Jun 2012; 129(6):1056-63; PMID:22566425; http://dx.doi.org/10.1542/peds.2011-2641
- Davis MM, Gaglia MA. Associations of daycare and school entry vaccination requirements with varicella immunization rates. Vaccine Apr 27 2005; 23(23):3053-60; PMID:15811652; http://dx.doi.org/10.1016/j.vaccine.2004.10.047
- RIDPH. Rules and regulartions pertaining to immunization and communicable disease testing in preschool school colleges or universities 2014; http://sos.ri.gov/documents/archives/regdocs/released/pdf/DOH/7602.pdf. Accessed August 11, 2014
- Gostin LO. Mandatory HPV vaccination and political debate Jama Oct 19 2011; 306(15):1699-700; PMID:21979129; http://dx.doi.org/10.1001/jama.2011.1525
- Allen T. Merck's Murky Dealings: HPV Vaccine Lobby Backfires. 2007; http://www.corpwatch.org/article.php?id=14401. Accessed July 20, 2015.
- Forster A, Wardle J, Stephenson J, Waller J. Passport to promiscuity or lifesaver: press coverage of HPV vaccination and risky sexual behavior J Health Commun Mar 2010; 15(2):205-17; PMID:20390987; http://dx.doi.org/10.1080/10810730903528066
- Knox R. HPV vaccine, the science behind the controversy. 2009; http://www.npr.org/2011/09/19/140543977/hpv-vaccine-the-science-behind-the-controversy. Accessed May 5, 2014.
- Pitts MJ, Adams Tufts K. Implications of the Virginia human papillomavirus vaccine mandate for parental vaccine acceptance Qual Health Res May 2013; 23(5):605-17; PMID:23275459; http://dx.doi.org/10.1177/1049732312470871
- VALegislature. Wording of VA HPV vaccine mandate. 2008; http://leg1.state.va.us/cgi-bin/legp504.exe?071+ful+CHAP0858. Accessed April 8, 2014.
- DCLegislature. Wording of DC HPV vaccine mandate. 2008; http://dcclims1.dccouncil.us/images/00001/20090615095558.pdf. Accessed April 8, 2014.
- AssociationofImmunizationManagers. POSITION STATEMENT: School and Child Care Immunization Requirements. 2014; http://c.ymcdn.com/sites/www.immunizationmanagers.org/resource/resmgr/files/aimpositionstatement.pdf. Accessed August 21, 2014.
- Opel DJ, Diekema DS, Marcuse EK. A critique of criteria for evaluating vaccines for inclusion in mandatory school immunization programs Pediatrics Aug 2008; 122(2):e504-10; PMID:18676536; http://dx.doi.org/10.1542/peds.2007-3218
- Ali H, Donovan B, Wand H, Read TR, Regan DG, Grulich AE, Fairley CK, Guy RJ. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data Bmj 2013; 346:f2032; PMID:23599298; http://dx.doi.org/10.1136/bmj.f2032
- Brotherton JM, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet Jun 18 2011; 377(9783):2085-92; PMID:21684381; http://dx.doi.org/10.1016/S0140-6736(11)60551-5
- Labadie J. Postlicensure safety evaluation of human papilloma virus vaccines Int J Risk Saf Med. 2011; 23(2):103-12; PMID:21673418
- ACIP. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep May 28 2010; 59(20):626-9; PMID:20508593
- Colgrove J. The ethics and politics of compulsory HPV vaccination. N Engl J Med Dec 7 2006; 355(23):2389-91; PMID:17151362; http://dx.doi.org/10.1056/NEJMp068248
- CDC. 2013 NIS-Teen Vaccination Coverage Table Data. 2014; http://www.cdc.gov/vaccines/imz-managers/coverage/nis/teen/data/tables-2013.html. Accessed August 11, 2014.
- Drolet M, Benard E, Boily MC, Ali H, Baandrup L, Bauer H, Beddows S, Brisson J, Brotherton JM, Cummings T, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis Lancet Infect Dis May 2015; 15(5):565-80; PMID:25744474; http://dx.doi.org/10.1016/S1473-3099(14)71073-4
- AssociationofImmunizationManagers. AIM Position Statement on Personal Belief Exemptions from State Vaccination Mandates. 2014; http://c.ymcdn.com/sites/www.immunizationmanagers.org/resource/resmgr/policy/pbe_statement_final_1_4-25-1.pdf. Accessed August 21, 2014.
- Datasets for the National Immunization Survey - Teen. 2013. http://www.cdc.gov/nchs/nis/data_files_teen.htm. Accessed February 1.
- Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13-17 years–United States, 2011. MMWR. Morbidity and mortality weekly report Aug 31 2012; 61(34):671-7; PMID:22932301
- National Immunization Survey-Teen: A User's Guide for the 2011 Public-Use Data File. 2012. http://www.cdc.gov/nchs/nis/data_files_teen.htm. Accessed February 1.
- Dorell C, Yankey D, Kennedy A, Stokley S. Factors that influence parental vaccination decisions for adolescents, 13 to 17 years old: National Immunization Survey-Teen, 2010 Clin Pediatr (Phila) Feb 2013; 52(2):162-70; PMID:23221308; http://dx.doi.org/10.1177/0009922812468208
- Perkins RB, Brogly SB, Adams WG, Freund KM. Correlates of human papillomavirus vaccination rates in low-income, minority adolescents: a multicenter study. J Womens Health (Larchmt) Aug 2012; 21(8):813-20; PMID:22860770; http://dx.doi.org/10.1089/jwh.2011.3364
- Rosenthal SL, Rupp R, Zimet GD, Meza HM, Loza ML, Short MB, Succop PA. Uptake of HPV vaccine: demographics, sexual history and values, parenting style, and vaccine attitudes. J Adolesc Health Sep 2008; 43(3):239-45; PMID:18710678; http://dx.doi.org/10.1016/j.jadohealth.2008.06.009
- Verdenius I, Harper DM, Harris GD, Griffith RS, Wall J, Hempstead LK, Malnar GJ, Bekkers RL. Predictors of three dose on-time compliance with HPV4 vaccination in a disadvantaged, underserved, safety net population in the US Midwest PLoS One 2013; 8(8):e71295; PMID:23951123; http://dx.doi.org/10.1371/journal.pone.0071295
Appendix
Table A1. Adjusted and unadjusted HPV vaccination coverage rates by year for females aged 13–14.
Table A2. Adjusted and unadjusted HPV vaccination coverage rates by year for females aged 15–17
