We read with interest the letter by Brown and colleagues, which focuses on our model's assumptions regarding duration of cross-protection against non-vaccine HPV types (20, 30, 50 y and lifetime).
Brown et al. begin by highlighting an article reviewing cross protection in different vaccine trials by Malagón et al.,Citation1 which suggests that AS04-adjuvanted HPV16/18 (bHPV, Cervarix™) vaccine-mediated protection against non-vaccine HPV types 31, 33, and 45 is higher than that of the quadrivalent vaccine (Gardasil™), but that this protection may wane after 4 y, based on 6-month persistent infection data from the Phase III study PATRICIA (NCT00122681) (total vaccinated HPV-naïve cohort (TVC-naïve) n = 11,644) and the Phase II studies GSK HPV-001/007 (NCT00689741 and NCT00120848) (according-to-protocol efficacy (ATP-E) cohort n = 919) and GSK HPV-001/007/023 (NCT00518336) (ATP-E cohort n = 395) with up to 4, 6.4 and 9.4 y follow-up, respectively.Citation2,3 They mention as additional evidence the analyses by Naud et al. and Saah et al. which, we would like to clarify, are in fact analyses of the same Phase II data (studies HPV 001/007/023) analyzed by Malagón et al. In reviewing these data, it appears to us that several key aspects, as explained in detail in a recently accepted review of the bHPV vaccineCitation4 should be considered as follows:
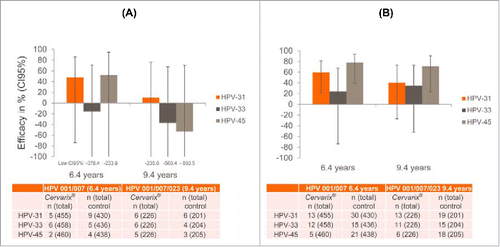
First, in the Phase II studies, the number of persistent infections was very small (<10 subjects in the vaccine and control arms), resulting in very large confidence intervals that have lower limits reaching substantially below −100% for nearly all the estimates (). Second, Malagón et al. base their conclusion of waning cross protection on the heterogeneity in vaccine efficacy between the 3 studies that seemed to decrease with increased follow-up, but it is unclear whether their heterogeneity analysis considered that HPV-001/007/023 was a follow-up of a sub-cohort of HPV-001/007 and not independent data. Third, the analyses do not consider the trials' incident infection data that are also publicly available in the clinical trial reports and show consistent efficacy at 6.4 and 9.4 y ().Citation2,3 These data are important to consider in addition to persistent infection data as: 1) incident infections occurred more frequently in the trials resulting in more robust efficacy estimates; and 2) efficacies against incident and persistent infections are expected to provide similar estimates if large enough populations are analyzed.Citation5 Finally, sustained efficacy against incident infections is supported by long-term immunogenicity data from HPV-001/007/023 showing sustained antibody titers against HPV31 and 45 up to 9.4 y after vaccination,Citation6 the longest follow-up of any HPV vaccine study to date. This raises the question as to what biologically plausible mechanism could explain such differences in the efficacies against incident and persistent infections while maintaining stable underlying antibody titres throughout the 9.4 y.
Figure 1. HPV-007 /023 persistent vs. incident infection data (A) 6-month persistent infectionsCitation2,3; (B) Incident infectionsCitation2,3
Furthermore, data from the 7 y follow-up of 5752 women aged > 25 y enrolled in the Phase III bHPV vaccine efficacy study VIVIANE (HPV-015, NCT00294047) were recently presented.Citation7 Despite being an older age group with consequently less vigorous immune responses, efficacy in the ATP-E cohort against persistent HPV31 and 45 infections after 7 y was significant and consistent with the 4 y follow-up: 65.8% (96.2% CI 24.9–85.8) and 79.1% (97.7% CI 27.6–95.9), respectively, for 31; 70.7% (96.2% CI 34.2–88.4) and 76.9% (97.7% CI 18.5–95.6), respectively, for 45.Citation8 No effect was demonstrated for persistent HPV33 infections at 7 and 4 y (−32.0, 96.2% CI −275.2–51.5, and −31.9%, 97.7% CI −460.8–66.3, respectively). Cross-protection has also been borne out in the real-world following introduction of universal mass vaccination with the bHPV vaccine in the United Kingdom from September 2008 until September 2012, with at least 2 doses of the bHPV vaccine being associated with > 50% reductions in the prevalence of HPV 31/33/45 among 20 to 21-year olds attending cervical cancer screening in Scotland (adjusted Odds Ratios: 0.46, 95% CI 0.21–0.94, for 2 doses; 0.45, 95% CI 0.29–0.68, for 3 doses).Citation9 Approximately 45% of the vaccinated girls were vaccinated at least 4 y earlier at age 15 to 16 y.
Brown et al. also mention reduced efficacy against persistent HPV31, 33 and 45 infections in women who received 2 vs. 3 doses in a combined analysis of PATRICIA and the Costa Rica Vaccine Trial.Citation5 However, this was a secondary analysis of a subset of women who had accidentally missed 1 or 2 doses and therefore probably unrepresentative of the total study sample. Interestingly this study observed a 1-dose vaccine efficacy against persistent HPV31, 33, and 45 infections that was higher than the 2-dose efficacy and similar in range to the 3-dose efficacy for these types. Furthermore, unlike vaccine-type efficacy, efficacy against HPV31, 33 and 45 was assessed only in the total vaccinated cohort, which included baseline positive women. It is well documented in various HPV vaccine efficacy trials that the inclusion of baseline HPV positive subjects results in lower efficacy estimates as the available prophylactic HPV vaccines have no therapeutic effect. In a corresponding Phase IIb/III study population including all women who received at least 1 dose of the 9-valent HPV (9vHPV) vaccine, the 9vHPV vaccine failed to show any efficacy against high grade lesions compared to the quadrivalent vaccine (Gardasil™), highlighting the complexity of efficacy analyses in cohorts that include HPV exposed individuals.Citation10
Brown et al. further present data from their own model, where the 9vHPV vaccine manages to achieve 100% elimination of CIN1, CIN2/3, and cancer at post-vaccination steady state even though it does not contain VLPs for at least 6 other oncogenic HPV types and data has shown that the 9vHPV vaccine does not prevent infection and disease related to HPV types beyond the 9 types covered by the vaccine.Citation10 As presented in a recent systematic review of the efficacy of HPV vaccines against cervical lesions, efficacy irrespective of HPV type is one of the most important parameters from a public health perspective.Citation11 Efficacy irrespective of type captures all vaccine effects against vaccine and non-vaccine HPV types and is not confounded by co-infections or general limitations of HPV testing.Citation12 The bHPV vaccine's efficacy irrespective of type against CIN2+ is reported between 65% and 80% in HPV-naïve women in 4 different trials with up to 6.4 y of follow up.Citation13-16 Efficacy against CIN3+ demonstrated in the Phase III study PATRICIA was 93.2% (CI 95% 78.9–98.7). In all trials, data has shown that the bHPV vaccine efficacy irrespective of type was higher than the contribution of HPV16/18 to lesions in the respective control groups, demonstrating consistent cross protection beyond the vaccine types. Reported efficacy estimates against CIN2+ irrespective of type for the quadrivalent HPV vaccine in HPV naïve women were 42.7%,Citation17 and 62.8% for the 9vHPV vaccine (against historical placebo group),Citation18 which is lower than in effectiveness projections based on epidemiological type distribution.Citation19 The projected elimination of CIN1, CIN2/3 and cancer in the long-term for the 9vHPV vaccine is therefore unexpected, however the methodological details, in particular the assumptions about efficacies and vaccination coverage, have not been presented in the Brown et al. letter.
Finally Brown et al. question the potentially lower quality of cross reactive antibodies vs. antibodies specific to vaccine HPV VLPs. Existing clinical data has shown that the cross-protective effects of the bHPV vaccine are in fact induced by the vaccine HPV VLPs (i.e., HPV16/18 VLPs), with the broader immune response, including a broad T-cell response, being likely attributable to the AS04 adjuvant contained in the bHPV vaccine.Citation20 Nonetheless, from a clinical point of view, what is most important is not the antigens themselves but the immune-response the vaccines induce and the effect they produce in terms of disease prevention. Regarding the cross-reactive antibody response, indeed the persistence of the cross-reactive antibody titers against HPV31 and 45 after bHPV vaccination has been evidenced up to 9.4 yCitation6 and the avidity of these cross-reactive antibodies has been tested for up to 4 y.Citation21 Until the duration and the quality of the immune-response induced by the 9vHPV vaccine is clinically evidenced for equivalent or longer follow-up periods, any projection on these aspects based on experience with other vaccines and on the manufacturing processes of the antigens seems speculative.
Taken together these data suggest that the demonstrated cross-protective efficacy of the bHPV vaccine is sustained in the long-term and should not be discarded as a “short-lived epiphenomenon.”
Abbreviations
| 9vHPV | = | 9-valent HPV vaccine |
| ATP-E | = | According-to-protocol efficacy |
| bHPV | = | AS04-adjuvanted HPV16/18 vaccine (Cervarix™) |
| CI | = | Confidence Interval |
| CIN2+ | = | High-grade cervical intraepithelial neoplasia |
| HPV | = | Human papillomavirus |
| TVC | = | Total vaccinated cohort |
| VLP | = | Virus-like particle |
Disclosure of potential conflicts of interest
S Taylor, M Ryser, A Mihalyi and T Van Effelterre are all employees of the GSK group of companies and hold shares in GSK Vaccines as part of their employee remuneration.
Author contributions
TVE and ST were authors of the original manuscript to which the letter refers. ST prepared draft letter. All authors reviewed and commented on draft and gave final approval for it to be submitted for publication.
Trademark statement
CERVARIX is a trademark of the GSK group of companies. GARDASIL is a trademark of Merck & Co.
Acknowledgments
The authors thank Denis Sohy, GSK Vaccines, Wavre for critical review of draft, and Veronique Delpire and Mandy Payne, Words & Science, Brussels, for editorial and co-ordination support.
References
- Malagón T, Drolet M, Boily MC, Franco EL, Jit M, Brisson J, Brisson M. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012 Oct; 12(10):781-9; PMID:22920953; http://dx.doi.org/10.1016/S1473-3099(12)70187-1. Epub 2012 Aug 22.
- GSK Clinical Study Register. Study of the efficacy of candidate HPV 16/18 VLP vaccine in the prevention of HPV-16 and/or HPV-18 cervical infection in adolescent & young adult women in North America and Brazil vaccinated in primary study 580299/001. Available from: http://www.gsk-clinicalstudyregister.com/study/580299/007?study_ids=580299/007#ps. Updated 2011 Oct 27; accessed 2015 Sep 1. GSK Study ID 580299/007.
- GSK Clinical Study Register. Follow-up study to evaluate the long-term efficacy of a HPV vaccine (580299) in healthy young adult women in Brazil. In: GSK Clinical Study Register [Internet]. Available from: http://www.gsk-clinicalstudyregister.com/study/109616%20%28Y7%29#rs. Updated 2014 Nov 20; accessed 2015 Sep 1. GSK Clinical Study Register Identifier: 109616 (Y7).
- Skinner SR, Apter D, De Carvalho N, Harper DM, Konno R, Paavonen J, Romanowski B, Roteli-Martins C, Burlet N, Mihalyi A and Frank Struyf. Human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for the prevention of cervical cancer and HPV-related diseases. Exp Rev Vacc 15(3):367-387. http://dx.doi.org/10.1586/14760584.2016.1124763. Epub 2016 Feb 22.
- Kreimer AR, Struyf F, Del Rosario-Raymundo MR, Hildesheim A, Skinner SR, Wacholder S, Garland SM, Herrero R, David MP, Wheeler CM et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA trials. Lancet Oncol 2015 Jul; 16(7):775-86; PMID:26071347; http://dx.doi.org/10.1016/S1470-2045(15)00047-9; Epub 2015 Jun 9
- Moscicki A-B, Harper D, Naud P, Wheeler C, Blatter M, De Carvalho N, Ferris D, Roteli-Martins C, Teixeira J, Tyring S et al. HPV-16/18 AS04-adjuvanted vaccine: sustained immunogenicity against HPV-31 and HPV-45 non-vaccine oncogenic types up to 9.4 years follow-up [Abstract HPV15-0620]. In Proceedings of the International Papillomavirus Society - 30th International Papillomavirus Conference. Lisbon, Portugal: 2015 Sep 17-21.
- GSK Clinical Study Register. A study to evaluate safety, immunogenicity and efficacy of GSK Biologicals HPV-16/18 L1/AS04 vaccine administered intramuscularly according to a three-dose schedule (0, 1, 6 month) in healthy adult female subjects aged 26 years and above. Available from: http://www.gsk-clinicalstudyregister.com/study/104820#rs. Updated 2015 Dec 7; accessed 2015 Dec 7. GSK Clinical Study Register Identifier: 104820.
- Skinner SR, Szarewski A, Romanowski B, Garland SM, Lazcano-Ponce E, Salmerón J, Del Rosario-Raymundo MR, Verheijen RH, Quek SC, da Silva DP, et al. Efficacy, safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in women over 25 years: 4-year interim follow-up of the double-blind, randomised VIVIANE study. Lancet 2014 Dec 20; 384(9961):2213-27; http://dx.doi.org/10.1016/S0140-6736(14)60920-X. Epub 2014 Sep 1
- Cameron RL, Kavanagh K, Pan J, Love J, Cuschieri K, Robertson C, Ahmed S, Palmer T, Pollock KG. Human papillomavirus prevalence and herd immunity after introduction of vaccination program, Scotland, 2009-2013. Emerg Infect Dis 2016 Jan; 22(1):56-64; PMID:26692336; http://dx.doi.org/10.3201/eid2201.150736
- Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, Moreira ED Jr, Ngan Y, Petersen LK, Lazcano-Ponce E, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 2015 Feb 19;372(8):711-23; PMID: 25693011; http://dx.doi.org/10.1056/NEJMoa1405044
- Di Mario S, Basevi V, Lopalco PL, Balduzzi S, D'Amico R, Magrini N. Are the two human papillomavirus vaccines really similar? A systematic review of available evidence: efficacy of the two vaccines against HPV. J Immunol Res 2015; 2015:435141. doi: 10.1155/2015/435141. Epub 2015 Aug 25. Review. PubMed PMID: 26380321; PubMed Central PMCID: PMC4562171.
- Vaccines and Related Biological Products Advisory Committee (VRBPAC). Cervarix human papillomavirus bivalent (types 16 and 18): briefing document [2009 Sep 9]; p64. In: US Food and Drug Administration [Internet]. Available from: http://www.fda.gov/downloads/Adviso...uctsAdvisoryCommittee/UCM181371.pdf.
- Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsagué X, Skinner SR, Apter D, Naud P, Salmerón J, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012 Jan; 13(1):89-99; Epub 2011 Nov 8. Erratum in: Lancet Oncol 2012 Jan;13(1):e1. PMID: 22075171; http://dx.doi.org/10.1016/S1470-2045(11)70286-8
- Lang Kuhs KA, Porras C, Schiller JT, Rodriguez AC, Schiffman M, Gonzalez P, Wacholder S, Ghosh A, Li Y, Lowy DR, et al. Effect of different human papillomavirus serological and DNA criteria on vaccine efficacy estimates. Am J Epidemiol 2014 Sep 15; 180(6):599-607; Epub 2014 Aug 19. PMID: 25139208; PubMed Central PMCID: PMC4157699; http://dx.doi.org/10.1093/aje/kwu168
- Konno R, Yoshikawa H, Okutani M, Quint W, V Suryakiran P, Lin L, Struyf F. Efficacy of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical intraepithelial neoplasia and cervical infection in young Japanese women. Hum Vaccin Immunother 2014; 10(7):1781-94; PubMed Central PMCID: PMC4186043; PMID:25424783; http://dx.doi.org/10.4161/hv.28712
- GlaxoSmithKline Vaccine HPV-007 Study Group, Romanowski B, de Borba PC, Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, Aoki F, Ramjattan B, Shier RM, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet 2009 Dec 12; 374(9706):1975-85; PMID: 19962185; http://dx.doi.org/10.1016/S0140-6736(09)61567-1
- Muñoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Brown DR, Koutsky LA, Tay EH, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst 2010 Mar 3; 102(5):325-39; PMID: 20139221; http://dx.doi.org/10.1093/jnci/djp534. Epub 2010 Feb 5.
- Giuliano AR, Joura E, Iversen OE, Bautista O, Chen J, Moeller E, Ritter M, Luxembourg A. Efficacy of a novel 9-valent HPV L1 vaccine against disease irrespective of HPV type. In Proceedings of International Papillomavirus Society - 29th International Papillomavirus Conference: pg. 252 [Abstract PH.PD04.05]. Seattle, WA: 2014 Aug 21–25.
- Joura EA, Ault KA, Bosch FX, Brown D, Cuzick J, Ferris D, Garland SM, Giuliano AR, Hernandez-Avila M, Huh W, et al. Attribution of 12 high-risk human papillomavirus genotypes to infection and cervical disease. Cancer Epidemiol Biomarkers Prev 2014 Oct;23(10):1997-2008; PMID: 25274978; http://dx.doi.org/10.1158/1055-9965.EPI-14-0410
- Pinto LA, Viscidi R, Harro CD, Kemp TJ, García-Piñeres AJ, Trivett M, Demuth F, Lowy DR, Schiller JT, Berzofsky JA, et al. Cellular immune responses to HPV-18, −31, and −53 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. Virology 2006 Sep 30; 353(2):451-62. Epub 2006 Jul 24; http://dx.doi.org/10.1016/j.virol.2006.06.021
- Boxus M, Lockman L, Fochesato M, Lorin C, Thomas F, Giannini SL. Antibody avidity measurements in recipients of Cervarix vaccine following a two-dose schedule or a three-dose schedule. Vaccine 2014 May 30; 32(26):3232-6; Epub 2014 Apr 13; http://dx.doi.org/10.1016/j.vaccine.2014.04.005
