ABSTRACT
Invasive meningococcal disease is a serious infection that is most often vaccine-preventable. Long-term protection relies on antibody persistence. Here we report the persistence of the immune response 2 y post-vaccination with a quadrivalent meningococcal serogroups A, C, W, Y tetanus toxoid conjugate vaccine (MenACWY-TT) compared with a MenACWY polysaccharide vaccine (Men-PS), in Asian adolescents aged 11–17 y. We also report a re-analysis of data from the primary vaccination study. This persistence study (NCT00974363) conducted in India and the Philippines included subjects who previously (study NCT00464815) received a single dose of MenACWY-TT or Men-PS. Persistence of functional antibodies was measured in 407 MenACWY-TT recipients and 132 Men-PS recipients (according-to-protocol cohort) using a rabbit complement serum bactericidal assay (rSBA, cut-off 1:8). Vaccine-related serious adverse events (SAEs) occurring since the end of the initial vaccination study were retrospectively recorded. Two y post-vaccination ≥99.3% of adolescents who received MenACWY-TT had persisting antibody titers ≥1:8 against each vaccine serogroup. Antibody persistence was higher (exploratory analysis) in the MenACWY-TT group than the Men-PS group in terms of rSBA titers ≥1:8 for serogroups W and Y; rSBA titers ≥1:128 for serogroups A, W and Y; and rSBA GMTs for serogroups A, W and Y; and was lower in the MenACWY-TT group for rSBA GMTs for serogroup C. No vaccine-related SAEs were reported. The results of this study indicated that antibodies persisted for at least 2 y in the majority of adolescents after vaccination with a single dose of MenACWY-TT.
Adolescents are at high risk of invasive meningococcal disease because of environmental and social behaviors that results in close contact, such as dormitory living and night club attendance, that promote transmission and increased carriage of Neisseria meningitidis.Citation1-4 Meningococcal infection can be prevented through vaccination. Meningococcal polysaccharide vaccines have been available for several decades but their effectiveness is limited because they do not stimulate T-cell dependent immune responses; therefore protection is not long-lasting, immune memory does not develop and repeated administration may result in hyporesponsiveness.Citation5,6 These limitations are avoided by meningococcal conjugate vaccines, which, through the coupling of a carrier protein to the meningococcal capsular polysaccharide, induce T-cell dependent responses with improved immunogenicity and development of immune memory.Citation7 Three quadrivalent meningococcal serogroups A, C, W and Y (MenACWY) meningococcal conjugate vaccines are available for use in adolescents as a single dose: MenACWY-diphtheria toxoid (DT) conjugate vaccine (Menactra™, Sanofi Pasteur, Lyon, France), MenACWY-CRM197 (non-toxic mutant diphtheria toxin) conjugate vaccine (Menveo™, GSK Vaccines, Wavre, Belgium) and MenACWY-tetanus toxoid conjugate vaccine (MenACWY-TT: Nimenrix™, Pfizer, New York, USA). In addition, 2 new N. meningitidis serogroup B vaccines that target non-capsular proteins are licensed for use in adolescents: Bexsero™ (GSK Vaccines, 2-dose schedule), and Trumenba™ (Pfizer; 3-dose schedule).
MenACWY-TT vaccine is licensed for use as a single dose from 12 months of age in more than 50 countries, including the European Union, Australia, Canada, India and the Philippines. Clinical trials have demonstrated that one dose of MenACWY-TT is immunogenic and well tolerated in children as of 12 months of age, and in adolescents and in adults.Citation8-18
Long-term protection against meningococcal disease is thought to rely on the presence of circulating antibodies.Citation19 As yet, the duration of clinical protection following vaccination with meningococcal conjugate vaccines is not known and booster recommendations continue to evolve. Although 3 MenACWY conjugate vaccines are available for use as a single dose in adolescents, data from the United States (US) indicate that immunity begins to wane 3 to 5 y after immunization.Citation20,21 In 2010, a booster dose of MenACWY conjugate vaccine was recommended in the US due to evidence of waning immunity and concerns about ongoing protection. Reporting on long-term persistence adds important information to the understanding of the kinetics of the antibody response to MenACWY-TT as compared to licensed quadrivalent meningococcal polysaccharide vaccines (Men-PS), and will assist in understanding whether booster doses are needed.
Here we report antibody persistence 2 y after vaccination of adolescents with MenACWY-TT or Men-PS (Mencevax™ ACWY, GSK Vaccines) (www.clinicatrials.gov NCT00974363). A protocol summary is available at www.gsk-clinicalstudyregister.com (study ID: 112148). We also report results of a re-analysis of clinical trial data from the original vaccination study (NCT00464815)Citation14 which was performed after identification of deviations in Good Clinical Practice (GCP) procedures that occurred during the study conduct.
Healthy adolescents randomized to receive a single dose of MenACWY-TT or Men-PS in the previous vaccination study were invited to return for the evaluation of antibody persistence 2 y after vaccination (y 2), and yearly thereafter until y 5. Here we report persistence data until y 2, after which time the rSBA assay used to test persistence samples was changed. Results from the 3 subsequent time points (y 3 to 5), which were generated with this different SBA assay, will be reported elsewhere. Subjects were not allowed to participate in the extension study if they had developed meningococcal disease or received any meningococcal vaccination since the primary vaccination study. The primary vaccination study was conducted in the Philippines, India and Taiwan. Based on the enrollment in the primary study, participation of the Indian and Filipino sites was deemed sufficient in terms of the sample size to be followed for antibody persistence. The persistence study was conducted from 08 September 2009 to 01 May 2010 and was open in design.
The studies were to be conducted in accordance with GCP and the Declaration of Helsinki. After publication of the initial vaccination study,Citation14 GSK Vaccines became aware of deviations from GCP at the study centers in the Philippines and India affecting the primary vaccination and y 2 follow-up phases. The identified issues included the following: the assent form, required for participants who are minors to convey their own independent decision to participate in the study, was not signed by subjects at one Indian site. In addition, although informed consent (an agreement or permission form that only adults are legally allowed to sign) was obtained for all subjects at all centers, the guardian giving consent for some minors in the Philippines was not the parent or legally authorized representative due to the local cultural norm that adult workers are sometimes employed outside of the Philippines, and therefore spend extended periods of time outside of the country.
From a subject rights perspective, the immunogenicity data of subjects without proper informed consent and/or assent should not be used. Therefore, GSK performed a re-analysis of the data obtained during the primary vaccination study, excluding all subjects with improper consent (112 subjects out of 1025 who participated in the primary vaccination study: 10.9%). However, in order not to omit any safety events (including events that occurred in the subjects with improper consent/assent), and to be as complete as possible in safety reporting, data from all study participants were taken into account for the safety analysis post-primary vaccination.
GCP deviations were also identified after completion of the y 2 persistence study. The identified issues included the following: the assent form was not signed by some subjects at one Indian site, and documentation confirming the appropriate storage of serum samples was lacking at one Indian site. In total 55 participants (8.0%) were excluded from the According-to-protocol persistence cohort due to GCP deviations. These findings necessitated a re-analysis of the persistence data.
A single blood sample was collected at y 2 for the assessment of antibody persistence. Serum bactericidal activity antibody dilution titers against each polysaccharide (A, C, W and Y) were measured by a serum bactericidal activity assay using baby rabbit complement (rSBA).Citation22 The cut-off of the assay was a 1:8 dilution. An antibody titer ≥1:8 is considered indicative of seroprotection for rSBA-MenCCitation23 and was also applied to the other serogroups.Citation24 In addition, data were analyzed according to a more conservative estimate using a threshold of 1:128.Citation25
The primary study objective was to assess persistence in terms of percentage of subjects with rSBA titers ≥ 1:8 for each of the 4 serogroups at y 2. The secondary objective was to assess persistence in terms of rSBA geometric mean titers (GMTs) for each serogroup at y 2.
For each vaccine serogroup, an exploratory evaluation of the difference in the immune response at each time point was performed in terms of the percentage of subjects with rSBA antibody titers ≥1:8 and ≥1:128 (with standardized asymptotic 95% confidence intervals [CIs]) and the ratio of the GMTs (with 95% CIs) between the MenACWY-TT and Men-PS groups. A potential difference was indicated if the value ‘1’ was excluded from the 95% CI on the GMT ratios, or, if the value ‘0%’ was excluded from the 95% CI on differences in proportions of subjects above an antibody threshold between groups. Note that potential differences should be interpreted with caution considering that there was no adjustment for multiplicity for these comparisons and that potentially significant findings may have occurred by chance alone.
Serious adverse events considered by the investigator to be related to study procedures were captured retrospectively at the y 2 study visit. To comply with worldwide safety reporting requirements, all serious adverse events considered to be related to any GSK medicine were also recorded.
Statistical analyses were performed using SAS® software version 9.22 for Windows (SAS Institute Inc., Cary, NC, US).
The results of the updated immunogenicity analysis for the previously published study time points are included in this report (). The composition of the according-to-protocol cohort for the re-analysis is provided in . The conclusions of the initial vaccination study were not impacted by the re-analysis: the statistical criteria for the non-inferiority of the vaccine response induced by the MenACWY-TT conjugate vaccine as compared to Men-PS 1 month after vaccination were met ().
Figure 1. Study flow. The according-to-protocol (ATP) cohort for the primary vaccination study represents the cohort included in the re-analysis of data after elimination of subjects (protocol violations: N=112: 83 in the MenACWY-TT group and 29 in the Men-PS group) due to GCP violations.
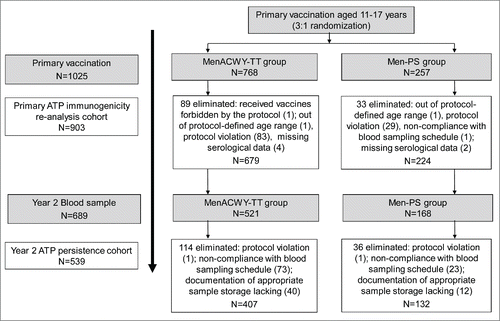
Table 1. rSBA antibody persistence 1 month and 2 y after vaccination with MenACWY-TT or Men-PS at 11–17 y of age (According to protocol cohort as defined for each time point).
Table 2. Primary vaccination study (NCT00464815): Difference between groups in percentage of subjects with rSBA vaccine response one month after vaccination (Primary ATP immunogenicity re-analysis cohort).
Based on review of the source documents, 2 previously unreported unsolicited adverse events (non-serious episodes of grade 1 headache and grade 1 vertigo) that occurred in the 31-days follow-up after vaccination were identified in 2 subjects in India. GSK's study database was updated to include these reports. No re-analysis of safety was conducted as neither event was considered by the investigator to be vaccine related and there would be no impact of these data on the study co-primary safety objective or on the Prescribing Information if they were considered as part of a re-analysis.
In the initial study on the safety and immunogenicity of MenACWT-TT in adolescents, 1025 subjects were enrolled in the Total vaccinated cohort (768 in the MenACWY-TT group and 257 in the Men-PS group). Citation14 Among these, 689 subjects returned at y 2 (521 in the MenACWY-TT group and 168 in the Men-PS group). The median time since the primary vaccination dose was 25 months (range 22 −25 months). There were 150 subjects eliminated from the According-to-protocol persistence cohort (). The mean age of subjects (According-to-protocol persistence cohort) in each study group and overall was 16.4 y (range 13–20 y) and 53.4% of subjects were female.
Results from a post hoc analysis on the According-to-protocol persistence cohort showed that 2 y after primary vaccination, at least 99.26% of subjects in the MenACWY-TT group and at least 93.02% in the Men-PS group had persisting antibody titers ≥1:8 for each vaccine serogroup, and hence were seroprotected to all 4 serogroups (Table S1). The percentage of subjects with titers ≥1:128 for each vaccine serogroup was at least 96.79% in the MenACWY-TT group and at least 79.84% in the Men-PS group.
For each serogroup, rSBA GMTs were reduced by year 2 but remained higher than pre-vaccination levels ().
Exploratory analyses indicated that antibody persistence was higher in the MenACWY-TT group than in the Men-PS group in terms of the percentage of subjects with rSBA titers ≥1:8 for serogroups W and Y, the percentage with rSBA titers ≥1:128 for serogroups A, W and Y, and in terms of rSBA GMTs for serogroups A, W and Y (). The rSBA GMT for serogroup C was higher in the Men-PS group than the MenACWY-TT group at y 2. A similar trend was observed in another study of MenACWY-TT conducted in adolescents and adults,Citation26 although the study was not designed or powered to detect differences in persistence so the data should be interpreted cautiously – particularly since there are other studies where the same trend has not been observed.Citation27 Despite the lower GMT, seroprotection rates in both groups were similar in our study. Of note, some countries, such as the United States, now recommend a booster dose approximately 5 y after primary vaccination for those at continued increased risk for meningococcal disease.
No vaccine-related serious adverse events were reported from the end of the primary vaccination study until y 2.
Evaluation of antibody persistence in a large cohort of adolescents vaccinated with a single dose of quadrivalent MenACWY-TT showed that vaccine serogroup-specific antibodies persist in almost all subjects until 2 y after vaccination. Exploratory analyses conducted after the primary vaccination dose indicated that the post-vaccination rSBA GMTs, adjusted for pre-vaccination measurements and age strata, were higher for all serogroups in the MenACWY-TT group compared to the Men-PS group (). Consistent with these results, antibody GMTs at y 2 were observed to be higher in the MenACWY-TT group than the Men-PS group in an exploratory analysis, for 3 out of 4 serogroups (A, W and Y).
The results of our study using the GSK rSBA are in-line with other studies of MenACWY-TT persistence in adolescents and children that employed the same assay.Citation16,27,28,26 Antibody persistence in the majority of MenACWY-TT recipients up until 42 months after vaccination of adolescents with MenACWY-TT has been demonstrated, with persisting antibody levels ≥ 1:8 that were similar or higher than after vaccination with licensed Men-PS vaccine.Citation27
While limited comparisons can be made with other studies using different rSBA assays, our results suggest that antibody persistence after a single dose of MenACWY-TT in adolescents is at least as good as that of other licensed MenACWY conjugate vaccines after 2 y follow-up.Citation29 Two y after vaccination of 11–18 y olds with Menactra™ or Menveo™, the percentage of adolescents with rSBA ≥ 1:8 for each vaccine serogroup was 25%−74% and 36%−84%, respectively.Citation29 In a study of antibody persistence 3 y after vaccination with Menactra™, the percentage of adolescents with rSBA ≥ 1:128 for serogroup C, W and Y was 75%−89%.Citation30
In conclusion, 2 y after vaccination with MenACWY-TT, the majority of subjects retained rSBA titers ≥1:8 for all vaccine serogroups, indicating that immunogenicity following a single dose in adolescents persists for at least 2 y.
Trademarks
MENVEO and BEXSERO are registered trademarks of the GSK group of companies. MENCEVAX and TRUMENBA are registered trademarks of Pfizer. MENACTRA is registered trademark of Sanofi Pasteur. NIMENRIX is a registered trademark of the GSK group of companies, licensed to Pfizer.
Abbreviations
| CI | = | confidence interval |
| GCP | = | Good Clinical Practice guidelines |
| GMT | = | geometric mean antibody titer |
| MenACWY-TT | = | quadrivalent meningococcal serogroups A, C, W and Y vaccine conjugated to tetanus toxoid |
| Men-PS | = | Quadrivalent meningococcal serogroups A, C, W and Y polysaccharide vaccine |
| rSBA | = | serum bactericidal assay using rabbit complement |
| TT | = | tetanus toxoid |
Disclosure of potential conflicts of interest
BPQ declares personal consulting fees, support for meetings, travel or accommodation expenses for the study from GSK group of companies; BPQ reports also personal consulting fees from Sanofi Pasteur for speaker's bureau.
APD and AB declare personal support for traveling to investigator meetings from GSK group of companies; AB declares personal support for congress, travel or accommodation expenses from GSK group of companies.
The institutions of BPQ, AB and APD received grants for conducting the present study from GSK group of companies. HJ declares no conflict of interest.
DK, VB, MVdW and JMM are employees of GSK group of companies. MVdW and JMM declare stock ownership in GSK group of companies.
Authors' contributions
BPQ, APD, AB and HJ were investigators involved in the supervision of the study, administrative, logistic and technical supports, the recruitment and the medical evaluation of subjects, the evaluation of any reported AEs/SAEs for severity and causality, the collection and interpretation of the data, and the drafting and approval of the manuscript. JMM, MVW (clinical development scientists) and VB (biostatistician) were involved in all stages of the study (study design, data analyses and interpretations, drafting and approval of the manuscript). DK (biostatistician) was involved in re-analysis of the data excluding all subjects with improper consent, data interpretation, drafting and approval of the manuscript.
Supplementary Table
Download MS Word (15.2 KB)Acknowledgments
The authors thank the individuals who participated in the study, and all investigators involved in conducting the study. We would also like to thank the following employees of GSK Vaccines for their valuable contributions: Shailesh Mehta and Seona Macgregor for assistance in coordination of the study; Emmanuel Aris for input into statistical analyses and Pascal Lestrate and Nathalie de Schrevel, for conducting the laboratory assays.
Writing services were provided by Joanne Wolter (on behalf of GSK Vaccines) and coordination and editorial assistance by Virginie Durbecq (XPE Pharma & Science on behalf of GSK Vaccines).
Funding
This work was supported by GlaxoSmithKline Biologicals SA. GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also funded all costs associated with the development and the publishing of the present manuscript. All authors had full access to the data and the corresponding author was responsible for submission of the publication.
References
- MacLennan J, Kafatos G, Neal K, Andrews N, Cameron JC, Roberts R, Evans MR, Cann K, Baxter DN, Maiden MCJ, et al. Social behavior and meningococcal carriage in British teenagers. Emerging Infect Dis 2006; 12:950-7; PMID:16707051
- Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10:853-61; PMID:21075057
- Soriano-Gabarró M, Wolter J, Hogea C, Vyse A. Carriage of Neisseria meningitidis in Europe: a review of studies undertaken in the region. Expert Rev Anti Infect Ther 2011; 9:761-74; PMID:21905785; http://dx.doi.org/10.1586/eri.11.89.
- Harrison LH, Kreiner CJ, Shutt KA, Messonnier NE, O'Leary M, Stefonek KR, Lin H, Lynfield R, Barrett NL, Arnold KE, et al. Risk factors for meningococcal disease in students in grades 9–12. Pediatr Infect Dis J 2008; 27:193-9; PMID:18277925; http://dx.doi.org/10.1097/INF.0b013e31815c1b3a.
- Poolman J, Borrow R. Hyporesponsiveness and its clinical implications after vaccination with polysaccharide or glycoconjugate vaccines. Expert Rev Vaccines 2011; 10:307-22.
- Granoff DM, Gupta RK, Belshe RB, Anderson EL. Induction of immunologic refractoriness in adults by meningococcal C polysaccharide vaccination. J Infect Dis 1998; 178:870-4; PMID:9728562.
- MacDonald NE, Halperin SA, Law BJ, Forrest B, Danzig LE, Granoff DM. Induction of immunologic memory by conjugated vs plain meningococcal C polysaccharide vaccine in toddlers: a randomized controlled trial. JAMA 1998; 280:1685-9; PMID:9832000.
- Ostergaard L, Lebacq E, Poolman J, Maechler G, Boutriau D. Immunogenicity, reactogenicity and persistence of meningococcal A, C, W-135 and Y-tetanus toxoid candidate conjugate (MenACWY-TT) vaccine formulations in adolescents aged 15–25 years. Vaccine 2009; 27:161-8; PMID:18834910.
- Knuf M, Kieninger-Baum D, Habermehl P, Muttonen P, Maurer H, Vink P, Poolman J, Boutriau D. A dose-range study assessing immunogenicity and safety of one dose of a new candidate meningococcal serogroups A, C, W-135, Y tetanus toxoid conjugate (MenACWY-TT) vaccine administered in the second year of life and in young children. Vaccine 2010; 28:744-53.
- Baxter R, Baine Y, Ensor K, Bianco V, Friedland LR, Miller JM. Immunogenicity and safety of an investigational quadrivalent meningococcal ACWY tetanus toxoid conjugate vaccine in healthy adolescents and young adults 10 to 25 years of age. Pediatr Infect Dis J 2011; 30:e41-8; PMID:21200360; http://dx.doi.org/10.1097/INF.0b013e3182054ab9.
- Vesikari T, Karvonen A, Bianco V, Van der Wielen M, Miller J. Tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine is well tolerated and immunogenic when co-administered with measles-mumps-rubella-varicella vaccine during the second year of life: An open, randomized controlled trial. Vaccine 2011; 29:4274-84; PMID:21443965.
- Memish ZA, Dbaibo G, Montellano M, Verghese VP, Jain H, Dubey AP, Bianco V, Van der Wielen M, Gatchalian S, Miller JM. Immunogenicity of a single dose of tetravalent meningococcal serogroups A, C, W-135, and Y conjugate vaccine administered to 2- to 10-year-olds is noninferior to a licensed-ACWY polysaccharide vaccine with an acceptable safety profile. Pediatr Infect Dis J 2011; 30:e56-62; PMID:21278617; http://dx.doi.org/10.1097/INF.0b013e31820e6e02
- Knuf M, Pantazi-Chatzikonstantinou A, Pfletschinger U, Tichmann-Schumann I, Maurer H, Maurer L, Fischbach T, Zinke H, Pankow-Culot H, Papaevangelou V, et al. An investigational tetravalent meningococcal serogroups A, C, W-135 and Y-tetanus toxoid conjugate vaccine co-administered with Infanrix™ hexa is immunogenic, with an acceptable safety profile in 12–23-month-old children. Vaccine 2011; 29:4264-73; PMID:21420417; http://dx.doi.org/10.1016/j.vaccine.2011.03.009.
- Bermal N, Huang L-M, Dubey A, Jain H, Bavdekar A, Lin T-Y, Bianco V, Baine Y, Miller JM. Safety and immunogenicity of a tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine in adolescents and adults. Hum Vaccin 2011; 7:239-47; PMID:21343698.
- Dbaibo G, Macalalad N, Aplasca-De Los Reyes MRA-DL, Dimaano E, Bianco V, Baine Y, Miller J. The immunogenicity and safety of an investigational meningococcal serogroups A, C, W-135, Y tetanus toxoid conjugate vaccine (ACWY-TT) compared with a licensed meningococcal tetravalent polysaccharide vaccine: A randomized, controlled non-inferiority study. Hum Vaccin Immunother 2012; 8:873-80;PMID:22485050; http://dx.doi.org/10.4161/hv.20211.
- Vesikari T, Forstén A, Boutriau D, Bianco V, Van der Wielen M, Miller JM. A randomized study to assess the immunogenicity, antibody persistence and safety of a tetravalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine in children aged 2–10 y. Hum Vaccin Immunother 2012; 8 (12):1882-91. doi: 10.4161/hv.22165
- Aplasca-De Los Reyes MR, Dimaano E, Macalalad N, Dbaibo G, Bianco V, Baine Y, Miller J. The investigational meningococcal serogroups A, C, W-135, Y tetanus toxoid conjugate vaccine (ACWY-TT) and the seasonal influenza virus vaccine are immunogenic and well-tolerated when co-administered in adults. Hum Vaccin Immunother 2012; 8:881-7; PMID:22485048; http://dx.doi.org/10.4161/hv.20212.
- Dbaibo G, El-Ayoubi N, Ghanem S, Hajar F, Bianco V, Miller JM, Mesaros N. Immunogenicity and safety of a quadrivalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine (MenACWY-TT) administered to adults aged 56 Years and older: results of an open-label, randomized, controlled trial. Drugs Aging 2013; 30:309-19; PMID:23494214; http://dx.doi.org/10.1007/s40266-013-0065-0.
- Auckland C, Gray S, Borrow R, Andrews N, Goldblatt D, Ramsay M, Miller E. Clinical and immunologic risk factors for meningococcal C conjugate vaccine failure in the United Kingdom. J Infect Dis 2006; 194:1745-52; PMID:17109348.
- Capua T, Katz JA, Bocchini JA. Update on adolescent immunizations: selected review of US recommendations and literature. Curr Opin Pediatr 2013; 25:397-406; PMID:23652687; http://dx.doi.org/10.1097/MOP.0b013e328360dc63.
- Baccarini C, Ternouth A, Wieffer H, Vyse A. The changing epidemiology of meningococcal disease in North America 1945–2010. Hum Vaccin Immunother 2013; 9:162-71; PMID:23108355; http://dx.doi.org/10.4161/hv.22302.
- Maslanka SE, Gheesling LL, Libutti DE, Donaldson KB, Harakeh HS, Dykes JK, Arhin FF, Devi SJ, Frasch CE, Huang JC, et al. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The Multilaboratory Study Group. Clin Diagn Lab Immunol 1997; 4:156-67; PMID:9067649.
- Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection-serum bactericidal antibody activity. Vaccine 2005; 23:2222-7; PMID:15755600.
- Centers for Disease Control and Prevention. Inadvertent misadministration of meningococcal conjugate vaccine-United States, June-August 2005. MMWR Morb Mortal Wkly Rep 2006; 55:1016-7; PMID:16988640.
- Andrews N, Borrow R, Miller E. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin Diagn Lab Immunol 2003; 10:780-6; PMID:12965904.
- Borja-Tabora C, Montalban C, Memish ZA, Van der Wielen M, Bianco V, Boutriau D, Miller J. Immune response, antibody persistence, and safety of a single dose of the quadrivalent meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine in adolescents and adults: results of an open, randomised, controlled study. BMC Infectious Diseases 2013; 13:116; PMID:23510357; http://dx.doi.org/10.1186/1471-2334-13-116.
- Ostergaard L, Van der Wielen M, Bianco V, Miller JM. Persistence of antibodies for 42 months following vaccination of adolescents with a meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine (MenACWY-TT). Int J Infect Dis 2013; 17(3):e173-6. doi: 10.1016/j.ijid.2012.10.001
- Knuf M, Baine Y, Bianco V, Boutriau D, Miller JM. Antibody persistence and immune memory 15 mo after priming with an investigational tetravalent meningococcal tetanus toxoid conjugate vaccine (menacwy-TT) in toddlers and young children. Hum Vaccin Immunother 2012; 8:866-72; PMID:22485049; http://dx.doi.org/10.4161/hv.20229.
- Gill CJ, Baxter R, Anemona A, Ciavarro G, Dull P. Persistence of immune responses after a single dose of Novartis meningococcal serogroup A, C, W-135 and Y CRM-197 conjugate vaccine (Menveo®) or Menactra® among healthy adolescents. Hum Vaccin 2010; 6:881-7; PMID:21339701.
- Vu DM, Welsch JA, Zuno-Mitchell P, Cruz JV Dela, Granoff DM. Antibody persistence 3 years after immunization of adolescents with quadrivalent meningococcal conjugate vaccine. J Infect Dis 2006; 193:821-8; PMID:16479517.
